INTRODUCTION
Polychaete collections made between 1946 and 1958 at five atolls in the northern Marshalls (i.e. Bikini, Enewetak, Majuro, Rongelap and Rongerik atolls) contained over 130 species, including sixteen new species (Hartman, Reference Hartman1954; Reish, Reference Reish1961, Reference Reish1968; Woodwick, Reference Woodwick1964). Few serpulines were identified, among them Hydroides multispinosa from Bikini Atoll (Hartman, Reference Hartman1954), Hydroides albiceps from Enewetak Atoll (Reish, Reference Reish1968), Salmacina incrustans from Bikini, Enewetak, and Majuro atolls (Reish Reference Reish1968; as Salmacina sp. in Hartman, Reference Hartman1954), Serpula hartmanae from Bikini and Enewetak atolls (Reish, Reference Reish1968; as Serpula sp. in Hartman, Reference Hartman1954), Spirobranchus giganteus from Enewetak Atoll (Hartman, Reference Hartman1954; Reish, Reference Reish1968), and Vermiliopsis glandigerus from Enewetak Atoll (Reish, Reference Reish1968; as Vermiliopsis sp. in Hartman, Reference Hartman1954). Several other records of serpulines from Majuro Atoll are presented in Imajima & ten Hove (Reference Imajima and ten Hove1984). However, few attempts were made to identify the spirorbine tubeworms, although dextral and sinistral forms were recorded by Hartman (Reference Hartman1954) in Enewetak Atoll. Straughan (Reference Straughan1969a) identified two spirorbine tubeworms from Enewetak, the sinistral Vinearia koehleri as the new species Spirorbis (Pileolaria) polyoperculatus Straughan Reference Straughan1969a and the dextral Neodexiospira steueri as Spirorbis (Circeis) bellulus Bush (in Moore & Bush, Reference Moore and Bush1904).
Eight serpuline species representing seven genera and two spirorbines in two genera are included in a serpulid checklist from Enewetak Atoll, Marshall Islands (Devaney & Bailey-Brock, Reference Devaney, Bailey-Brock, Devaney, Reese, Butch and Helfrich1987). There are no records of tubeworms from Kwajalein, Rongelap and Utirik Atolls. Serpulids of west and south Pacific island groups are of interest to researchers evaluating faunal relationships of Indo-West Pacific regions including southern Japan and China (Imajima & ten Hove, Reference Imajima and ten Hove1984, Reference Imajima and ten Hove1986; Fiege & Sun, Reference Fiege and Sun1999), Hawaii, Fiji, Tonga and Guam (Bailey-Brock, Reference Bailey-Brock1985, Reference Bailey-Brock1987a, Reference Bailey-Brockb, Reference Bailey-Brock1999) and biodiversity studies. Polychaetes are consumed by coral reef fish, so identification of prey species is useful for predator–prey studies (Sano et al., Reference Sano, Shimizu and Nose1984). Serpulids are important components of fouling communities and could be troublesome by their rapid colonization and secretion of calcareous tubes. Therefore, the understanding of the life history of several serpulid species has practical importance and was reviewed in Kupriyanova et al. (Reference Kupriyanova, Nishi, ten Hove and Rzhavsky2001). Many species are brooders and have pelagic larvae that are transported by currents and in ballast water, and as adults serpulids may be dispersed on the hulls of ships and attached to flotsam (Allen, Reference Allen1953; Thorp et al., Reference Thorp, Pyne and West1987).
Distributional records for twenty-one serpulines and seven spirorbines from Marshallese atolls are included here. A new name, Neodexiospira turrita nom. nov. is proposed to replace Neodexiospira preacuta by being the earliest available name. Variations of opercula, tubes and chaetae that are useful for taxonomic identification are discussed. Such regional differences of these diagnostic structures have been noted before by researchers in tropical serpulid taxonomy (ten Hove, Reference Hove1970; Imajima & ten Hove, Reference Imajima and ten Hove1984, Reference Imajima and ten Hove1986; Fiege & Sun Reference Fiege and Sun1999; Pillai, Reference Pillai2009).
MATERIALS AND METHODS
Collections from Rongelap, Utirik and Kwajalein Atolls were made in July 1975 and Enewetak in September 1975 and January 1976 (Figure 1). Several localities were sampled on the south-east side of Enewetak Atoll. Enewetak, Elmer and Clyde islands represent respectively Stations 1, 2 and 3 (Figure 1). Station 1 represents samplings on nearby coral reefs or on pinnacles further in the lagoon side. Two sampling stations were selected in Kwajalein Atoll, Station 1 at the north side of Kwajalein Island and Station 2 in two Japanese pools at the south side of the same island (Figure 1). Three stations were selected on the south-east islands of Rongelap Atoll and three stations on the south-east islands of Utirik Atoll (Figure 1).
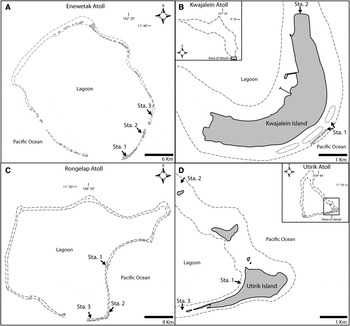
Fig. 1. Map showing collecting localities at: (A) Enewetak Atoll; (B) Kwajalein Atoll; (C) Rongelap Atoll; (D) Utirik Atoll.
Tube worms were scraped from hard substrates or chiselled from rock, preserved in 10% formalin and stored in 70% ethanol. Worms were extracted from their tubes by carefully chipping away tube fragments from the aperture towards the narrow end of the tube until the worm was free. Tubes, opercula and chaetae were viewed with dissecting and phase contrast microscopy. Spirorbine tubes and chaetae were prepared for scanning electron microscopy (SEM). Tubes and chaetae were air dried, mounted on stubs and coated with gold/palladium. SEM observations were carried out using the Hitachi S-4800 at the Biological Electron Microscopy Facility (BEMF), University of Hawaii at Manoa. Voucher specimens were deposited in the Bernice Pauahi Bishop Museum (BPBM), Honolulu, Hawaii, USA.
SYSTEMATICS
Twenty-one species of Serpulinae and seven of Spirorbinae were collected (Table 1). Brief descriptions and habitat and collection information are provided. Full descriptions were made for poorly known species and those with wide morphological variation among habitats. The geographical range, especially in the Indo-Pacific region, is given for each species.
Table 1. Distribution of the tubeworm species found in the Marshall Islands.

1Hartman (Reference Hartman1954); 2Reish (1964); 3Straughan (1969); 4Devaney & Bailey-Brock (Reference Devaney, Bailey-Brock, Devaney, Reese, Butch and Helfrich1987); 5this study; 6Imajima & ten Hove (Reference Imajima and ten Hove1984).
Dasynema chrysogyrus—Saint-Joseph Reference Saint-Joseph1894: 262, 264; Bush Reference Bush1905: 221–222; Imajima & ten Hove Reference Imajima and ten Hove1984: 55–58, figure 6a–t; ten Hove & Kupriyanova Reference Hove and Kupriyanova2009: 36–39, figure 14.
MATERIAL EXAMINED
Enewetak Atoll: Station 1, Lagoon Pinnacles, 15–20 m (4), Station 3, Clyde Island, 5–7 m, September 1975, coll. R. Brock (2, BPBM R3495).
DIAGNOSIS
Tubes elaborated with five longitudinal ridges of tubercles; pale dull yellow with white ridges (Figure 2B–E). Radioles with slender external stylodes in a single row; paired eyespots occur at the base of stylodes. Operculum with light-brown chitinous cap with two slightly off-centre calcareous tiers (Figure 2A & F–H). Thoracic uncini become more numerous in posterior chaetigers; uncinigerous tori broadly fan-shaped. For a full description see Imajima & ten Hove (Reference Imajima and ten Hove1984).

Fig. 2. Dasynema chrysogyrus: (A) operculum; (B) tube; (C) tube in dorso-lateral view; (D) tube in lateral view; (E) cross-section of tube; (F, G & H) opercula.
REMARKS
Dasynema chrysogyrus could be mistaken for Vermiliopsis glandigerus based on tube and opercular features. The external stylotes of D. chrysogyrus are diagnostic and have to be teased carefully away from the radiole to be seen in some preserved material.
DISTRIBUTION
Japan, Philippine Islands, Ponape and Indonesia (Imajima & ten Hove, Reference Imajima and ten Hove1984; ten Hove & Kupriyanova, Reference Hove and Kupriyanova2009) and newly recorded for the Marshall Islands at Clyde Island, Enewetak Atoll.
Hydroides diramphus—Bastida-Zavala & ten Hove Reference Bastida-Zavala and Hove2002 and references therein: 161–164, figure 34a–p; Bastida-Zavala & ten Hove Reference Bastida-Zavala and Hove2003 and references therein: 83–86, figure 10a–l; Bastida-Zavala Reference Bastida-Zavala2008: 25, figure 6g; Ben-Eliahu & ten Hove Reference Ben-Eliahu and Hove2011: 17–19, figures 4 & 5a–c.
MATERIAL EXAMINED
Enewetak Atoll: Station 1, Lagoon Pinnacles, 23 m, on a clam shell, January 1976, coll. J.H. Bailey-Brock (2, BPBM R3496).
DIAGNOSIS
Verticil with 12–14 chitinous T-shaped spines, each crescentric in shape (Figure 3A); a small spine at the base of each T-shaped spine present. Opercular funnel comprised 28–30 radii with strongly pointed and darkly pigmented tips (Figure 3A). For full descriptions see Bastida-Zavala & ten Hove (Reference Bastida-Zavala and Hove2002, Reference Bastida-Zavala and Hove2003).

Fig. 3. (A) Operculum of Hydroides diramphus; (B) operculum of Hydroides exaltatus; (C) tube of H. exaltatus; (D) operculum of Hydroides longispinosus; (E) operculum of Hydroides minax; (F & G) opercula of Hydroides spiculitubus; (H) tube of H. spiculitubus; (I) posterior end of H. spiculitubus showing inner tube with chitinous spicules.
REMARKS
Hydroides diramphus is most similar to Hydroides microtis Mörch, Reference Mörch1863 by having verticil spines with distal laterally expanded tips but the spines are T-shaped with flattened tip in H. diramphus and globular in H. microtis (Bastida-Zavala & ten Hove, Reference Bastida-Zavala and Hove2002).
The specimens from Enewetak Atoll fit well the descriptions presented from material of the western Atlantic (Bastida-Zavala & ten Hove, Reference Bastida-Zavala and Hove2002) and eastern Pacific including Hawaii (Bailey-Brock, Reference Bailey-Brock1987a; Bastida-Zavala & ten Hove, Reference Bastida-Zavala and Hove2003). Hydroides diramphus is considered to be introduced by ship to the Hawaiian Islands (Carlton & Eldredge, Reference Carlton and Eldredge2009) and it is quite possible that this species has spread westerly into the Pacific region by human-mediated dispersal processes.
DISTRIBUTION
Circum(sub)tropical (Bastida-Zavala & ten Hove, Reference Bastida-Zavala and Hove2002). In the western Pacific, Hydroides diramphus has been reported to occur in south Japan (Imajima, Reference Imajima1978) and the Hawaiian Islands (Hartman, Reference Hartman1966; Bailey-Brock, Reference Bailey-Brock1987a). The record of Enewetak Atoll in the Marshall Islands is new.
Eupomatus exaltatus—Willey Reference Willey and Herdman1905: 312–313, pl. VII, figure 182.
Hydroides exaltata—Imajima Reference Imajima1976a: 127–128, figure 4a–j; Imajima Reference Imajima1976b: 232–233; Imajima Reference Imajima1987: 77; Imajima & ten Hove Reference Imajima and ten Hove1984: 48; Imajima & ten Hove Reference Imajima and ten Hove1986: 4–5; Pillai Reference Pillai1960: 10–12, figure 4a–e; Mak Reference Mak, Morton and Tseng1982: 603; Wu & Chen Reference Wu and Chen1985: 62, figure 11a, b.
Hydroides exaltatus—Dew Reference Dew1959: 27–28, figure 6a; Fiege & Sun Reference Fiege and Sun1999: 116, figure 5d–f; Straughan Reference Straughan1967a: 220.
MATERIAL EXAMINED
Rongelap Atoll: Stations 1 (7, BPBM R3498) and 2 (1, BPBM R), July 1975, coll. R. Brock.
DESCRIPTION
Tube: white, thick, opaque, sub-trapezoidal in cross-section and with three low longitudinal ridges and several transverse ridges (Figure 3C).
Branchiae: 10–12 radioles per lobe; branchial eyes and inter-radiolar membrane absent.
Peduncle: from left or right sides; cylindrical, smooth, with oblique constriction before opercular funnel. Pseudoperculum present at the right or left side.
Operculum: funnel with 26–28 marginal radii with pointed tips and curved outwards (Figure 3B). Verticil with 6–9 spines curving outwards and a large dorsal hook curved inwards (Figure 3B); one basal internal spinule present on each verticil spine.
Collar and thoracic membranes: collar smooth, tri-lobed, extending to the second row of thoracic uncini; apron present.
Thorax: with collar chaetiger and 6 uncinigerous chaetigers; collar chaetae two types: four bayonets with one or two blunt to conical teeth and four capillaries. Thoracic chaetae 6–8 same-length limbate capillaries. Thoracic uncini saw-shaped with 6–7 teeth including a simple anterior and large tooth.
Abdomen: abdominal uncini similar to thoracic ones with 5–6 teeth including a large anterior tooth. Abdominal chaetae flat-trumpets with 20 or more minute teeth. Posterior capillaries present in the last 5 chaetigers. Abdomen of 63 or more chaetigers.
Size: 5–17 mm in length, up to 1.0 mm width of thorax, operculum 0.5–1.2 mm length.
Colour of preserved specimens: pale yellow.
REMARKS
Hydroides exaltatus is similar to H. brachyacanthus Rioja 1941 and H. deleoni Bastida-Zavala & ten Hove Reference Bastida-Zavala and Hove2003 by the presence of a large dorsal hook that is curved inwards; however, verticil spines are curved outwards in H. exaltatus and strongly curving inwards in H. brachyacanthus and H. deleoni (see Bastida-Zavala & ten Hove, Reference Bastida-Zavala and Hove2003). The specimens from Rongelap Atoll agree well with Imajima's (Reference Imajima1976a) description of material from south-west Japan. The internal verticil spinules are slender and slightly longer in the Rongelap material if compared to Imajima's illustrations (Imajima Reference Imajima1976a; figure 4a, b).
DISTRIBUTION
Japan, west and east coasts of Australia, Sri Lanka, Red Sea, Solomon Islands (Imajima, Reference Imajima1976a), south China Sea (Fiege & Sun, Reference Fiege and Sun1999) and newly recorded to Rongelap Atoll, Marshall Islands.
Hydroides longispinosus—Fiege & Sun Reference Fiege and Sun1999: 116–118, figure 6a, b; Imajima Reference Imajima1977: 95; Imajima Reference Imajima1982: 46; Imajima Reference Imajima1987: 78; Imajima & ten Hove Reference Imajima and ten Hove1984: 48; Imajima & ten Hove Reference Imajima and ten Hove1986: 3; Meng et al., Reference Meng, Hong and Wu1994: 46.
Hydroides centrospina Wu & Chen Reference Wu and Chen1981: 354–355, figure 1a–i. syn. by Fiege & Sun Reference Fiege and Sun1999: 116–118; Meng et al., Reference Meng, Hong and Wu1994: 46, figure 3, 1–9.
MATERIAL EXAMINED
Enewetak Atoll: Station 1, East Pinnacle Lagoon, 12–18 m, September 1975, coll. R. Brock (1, BPBM R3499); Station 3, Clyde Island, September 1975, coll. R. Brock (1, BPBM R3502). Utirik Atoll: Station 2, July 1975, coll. R. Brock (1, BPBM R3501). Rongelap Atoll: Station 3, July 1975, coll. R. Brock (1, BPBM R3500).
DESCRIPTION
Tube: white, circular in cross-section with growth marks and without longitudinal ridges.
Branchiae: 8–11 radioles per lobe with very long terminal filament; branchial eyes and inter-radiolar membrane absent.
Peduncle: cylindrical, smooth, without constriction before opercular funnel. Pseudoperculum present at the left side.
Operculum: funnel with 20–22 marginal radii with obtuse tips. Verticil with 14–16 peripheral spines bearing 5–6 long lateral spinules equally arranged and few, smaller external and internal spinules; one central spine slightly to twice longer than peripheral ones without spinules or with a few on its distal half (Figure 3D).
Collar and thoracic membranes: collar smooth, tri-lobed, extending to the second row of thoracic uncini. Thoracic membrane conspicuous; apron absent.
Thorax: with collar chaetiger and 6 uncinigerous chaetigers; collar chaetae two types: six bayonets with two conical and pointed teeth, blades with small subapical denticulate and six capillaries. Thoracic chaetae 6–8 same-length limbate capillaries. Thoracic uncini saw-shaped with 5–6 teeth including a main fang.
Abdomen: abdominal uncini rasp-shaped with 5–6 teeth including a main fang. Abdominal chaetae flat-trumpet with several minute teeth. Posterior capillaries present in the last 5 chaetigers. Abdomen of 24 or more chaetigers.
Size: 3.5–5 mm in length, up to 0.4 mm width of thorax, operculum 0.3–0.45 mm in length.
Colour of preserved specimens: pale yellow.
REMARKS
Specimens from Enewetak, Utirik and Rongelap atolls agree well with the original description by Imajima (Reference Imajima1976b) from Japanese specimens. However, the author did not describe the nature of the thoracic and abdominal uncini, which are saw-shaped and rasp-shaped respectively. The tubes of the Marshallese specimens did not present longitudinal ridges as did those from Japan (Imajima, Reference Imajima1976b), but Fiege & Sun (Reference Fiege and Sun1999) describe tubes with two low longitudinal ridges for the Chinese material.
One of the three specimens examined presented a shorter opercular central spine, just slightly longer than the peripheral ones. This central spine is smooth, without spinules and could be due to regeneration after predation or growth related as Fiege & Sun (Reference Fiege and Sun1999) also reported juveniles with a smooth central spine. For this reason, these authors synonimized Hydroides centrospina Wu & Chen, Reference Wu and Chen1981 with Hydroides longispinosus.
Hydroides longispinosus is most similar to H. multispinosus Marenzeller, 1884 by the overall appearance of the operculum; however, the opercular central spine is long and with lateral spinules in H. longispinosus and short and smooth in H. multispinosus.
DISTRIBUTION
Known from southern Japan (Imajima, Reference Imajima1976b), Palau Islands, Ponape Island, Australia, Solomon Islands, Gilbert Islands (ten Hove & Imajima, Reference Imajima and ten Hove1986), South China Sea (Fiege & Sun, Reference Fiege and Sun1999) and newly recorded to Enewetak, Rongelap and Utirik Atolls in the Marshall Islands.
Eupomatus minax—Willey Reference Willey and Herdman1905: 314.
Hydroides minax—Fauvel Reference Fauvel1939: 361–362; Fauvel Reference Fauvel1953: 460, figure 241 f; Fiege & Sun Reference Fiege and Sun1999: 119–120, figure 9a–c; Imajima Reference Imajima1976a: 129–130, figure 5a–j; Imajima Reference Imajima1976b: 233–234; Imajima Reference Imajima1982: 42; Imajima Reference Imajima1987: 77; Imajima & ten Hove Reference Imajima and ten Hove1984: 48; Imajima & ten Hove Reference Imajima and ten Hove1986: 3–4; ten Hove Reference Hove and Land1994: 108; ten Hove & Ben-Eliahu Reference Hove and Ben-Eliahu2005: figure 1h; Pillai Reference Pillai1960: 8–10, figure 3a–e; Pillai Reference Pillai1971: 110; Vine & Bailey-Brock Reference Vine and Bailey-Brock1984: 141.
MATERIAL EXAMINED
Enewetak Atoll: Station 1, Mid-Pacific Marine Laboratory, lagoon side, 5 m, September 1975, coll. R. Brock (1, BPBM R3503).
DIAGNOSIS
Funnel with several marginal radii with pointed tips and directed outwards. Verticil with 6–9 peripheral spines directed outwards and one very large and stout dorsal spine bent ventrally with two terminal hooks curved down. For a complete description see Imajima (Reference Imajima1976a).
REMARKS
The specimen from Enewetak agrees well with the description of material from Japan (Imajima, Reference Imajima1976a) and Sri Lanka (Pillai, Reference Pillai1960). Funnel of the specimen from Enewetak with 30 marginal radii and vertical with 6 peripheral spines (Figure 3E).
Hydroides minax resembles only superficially to Hydroides ancorispina Pillai, Reference Pillai1971, Hydroides albiceps (Grube, 1870), Hydroides bulbosus ten Hove, 1990 and Hydroides trivesiculosus by the presence of a bulbous dorsal spine. It differs from all these similar species because the large and stout dorsal spine is bent ventrally and has terminal hooks that are curved down.
DISTRIBUTION
Hydroides minax is widely distributed in the Indo-West Pacific (ten Hove & Kupriyanova, Reference Hove and Kupriyanova2009) including records from the Philippines, Red Sea, Mozambique, Southern Africa, Indian Ocean, Sri Lanka, Havannah and Heron Islands, Australia, Solomon Islands, Gambier Islands, French Polynesia, Japan (Imajima, Reference Imajima1976a), and newly recorded to Enewetak Atoll in the Marshall Islands.
Hydroides tambalagamensis Pillai 1961—Imajima Reference Imajima1976a: 123–126, figure 2a–j; Imajima Reference Imajima1976b: 231–232; Imajima Reference Imajima1979: 167; Imajima & ten Hove Reference Imajima and ten Hove1984: 49; Imajima & ten Hove Reference Imajima and ten Hove1986: 4; Straughan Reference Straughan1967b: 33, figure 3g; Sun & Yang Reference Sun and Yang2000: 128, figure 6k–s.
MATERIAL EXAMINED
Enewetak Atoll: Station 1, Henry Ocean Side, January 1976, 5–20 m, coll. R. Brock (1, BPBM R3504); Station 1, next to Oak crater, September 1975, 1–4 m, coll. R. Brock (1, BPBM R3506); Station 1, East Pinnacle Lagoon, January 1976, coll. R. Brock (1 loose operculum, BPBM R3505).
DIAGNOSIS
Funnel with 26–30 marginal radii with pointed tips and directed outwards (Figure 3F, G). Verticil with 8 peripheral spines each bearing on the middle 2 lateral spinules at each side, curved outwards, one superior internal spinule pointed down and one basal internal spinule also pointing to the base (Figure 3F, G); central tooth absent. For a complete description see Pillai (Reference Pillai2009).
REMARKS
The two specimens and the one loose operculum examined agree well with the original description of this species by Pillai (Reference Pillai2009) as well with Imajima's (Reference Imajima1976a) description of Hydroides tambalagamensis. The inner tube with chitinous fine spicules and brown pigment band present anteriorly to each thoracic uncinal torus described by Pillai (Reference Pillai2009) was also observed in the Enewetak specimens (see Figure 3H for tube of this species and Figure 3I for exposed inner tube). This species is most similar to H. tambalagamensis but differs on the position of the spinules from the peripheral spines; for instance, the pair of lateral spinules is external in H. spiculitubus and lateral in H. tambalagamensis (see also Pillai, Reference Pillai2009).
DISTRIBUTION
Indo-Pacific, originally described from Western Australia (Pillai, Reference Pillai2009) but reported before as Hydroides tambalagamensis Pillai, 1961 from southern Japan (Imajima, Reference Imajima1976a, Reference Imajima1979), Palau Islands and Majuro Atoll (Imajima & ten Hove, Reference Imajima and ten Hove1984), Solomon and Gilbert Islands (Imajima & ten Hove, Reference Imajima and ten Hove1986), China (Sun & Yang, Reference Sun and Yang2000) and newly recorded to Enewetak Atoll in the Marshall Islands.
MATERIAL EXAMINED
Enewetak Atoll: Station 2, Elmers Pinnacles, 12–18 m, September 1975, coll. R. Brock (1, BPBM R3508). Rongelap Atoll: Station 1, July 1975, coll. R. Brock (3, BPBM R3507).
DESCRIPTION
Tube: white, massive, quadrangular in cross-section, with indistinct longitudinal ridges and transverse thickenings.
Branchiae: 11–14 radioles per lobe with very long terminal filament; branchial eyes and inter-radiolar membrane absent.
Peduncle: cylindrical, smooth, with constriction before opercular funnel. Pseudoperculum present at the right or left side.
Operculum: funnel asymmetrical with 22–23 marginal radii with clear or pale yellow T-shaped tips. Verticil with 6 T-shaped spines curved out. A single lobate spine with two lateral shoulder-like projections giving a trilobed appearance (Figure 4A). Lobate spine narrow and elongate in two specimens and bulbous and massive in two others.

Fig. 4. (A) Operculum of Hydroides trivesiculosus; (B) operculum of Hydroides tuberculatus; (C) tube of Hydroides tuberculatus; (D) operculum of Janita fimbriata; (E) tube of Janita fimbriata.
Collar and thoracic membranes: collar smooth, tri-lobed. Thoracic membrane conspicuous; apron absent.
Thorax: with collar chaetiger and 6 uncinigerous chaetigers; collar chaetae two types: bayonets with two conical to blunt teeth, blades with apical denticulation and capillaries. Thoracic chaetae 8–10 same-length limbate capillaries. Thoracic uncini saw-shaped with 6–7 teeth including a main fang.
Abdomen: abdominal uncini saw-shaped with 4–5 teeth including a main fang. Abdominal chaetae flat-trumpets with a main fang and minute teeth on two thirds of length. Posterior capillaries present in the last 8 chaetigers. Abdomen of 60 or more chaetigers. Pygidium bilobed.
Size: 3–8 mm in length, up to 0.8 mm width of thorax, operculum 0.5–0.8 mm in length.
Colour of preserved specimens: pale yellow.
REMARKS
These specimens agree with Straughan's description (Reference Straughan1967b) except for 6 small spines in the verticil (4 or 5 according to Straughan) and 22 radioles (24 in Straughan's specimens). These differences may be different growth stages or age of the specimens, or are due to natural variability within species. Hydroides trivesiculosus resembles H. albiceps Imajima, Reference Imajima1976a, which has more radii and verticil spines in the operculum, and bifid spines differ in shape. There are similar numbers of opercular radii and spines in H. trivesiculosus and H. ancorispinus Pillai, Reference Pillai1971 (19 funnel and 7 verticil spines), but the massive spine is a single lobe without any projections in H. ancorispinus.
DISTRIBUTION
Hydroides trivesiculosus is known from Queensland, Australia (Straughan, Reference Straughan1967b), Tanzania, Red Sea (ten Hove & Kupriyanova, Reference Hove and Kupriyanova2009) and newly recorded to Enewetak and Rongelap Atolls in the Marshall Islands.
Hydroides tuberculata—Bailey-Brock Reference Bailey-Brock1985: 210–211, figure 12a–c; Imajima Reference Imajima1976b: 233; Imajima Reference Imajima1978: 53; Imajima Reference Imajima1979: 168; Imajima Reference Imajima1982: 44; Imajima Reference Imajima1987: 77; Imajima & ten Hove Reference Imajima and ten Hove1984: 44–45; Imajima & ten Hove Reference Imajima and ten Hove1986: 5.
Hydroides tuberculatus—Fiege & Sun Reference Fiege and Sun1999: 121–123, figure 11a–c; Ishaq & Mustaquim Reference Ishaq and Mustaquim1996: 170–172, figure 6a–h; ten Hove Reference Hove and Land1994: 108.
MATERIAL EXAMINED
Rongelap Atoll: Station 1, July 1975, coll. R. Brock (1, BPBM R3509). Utirik Atoll: Station 2, July 1975, coll. R. Brock (2, BPBM R3510).
DESCRIPTION
Tube: white, sub-trapezoidal in cross-section, with longitudinal ridges and a dark membranous lining (Figure 4C). One specimen with large eggs in the tube.
Branchiae: 7–8 radioles per lobe with very long terminal filament; branchial eyes and inter-radiolar membrane absent.
Peduncle: cylindrical with constriction before opercular funnel. Pseudoperculum short, rudimentary and present at the left side.
Operculum: funnel with 20–22 marginal radii with brown pointed tips (Figure 4B). Verticil with four inflated spines and one large incurved dorsal spine, all with a dorsal blunt tubercle (Figure 4B).
Collar and thoracic membranes: darkly pigmented with brown speckles; collar smooth, tri-lobed. Thoracic membrane conspicuous; apron present.
Thorax: with collar chaetiger and 6 uncinigerous chaetigers; collar chaetae two types: bayonets with two conical to blunt teeth and 2–3 small accessory teeth and capillaries. Thoracic chaetae 8 same-length limbate capillaries. Thoracic uncini saw-shaped with 6–7 teeth including a main fang.
Abdomen: abdominal uncini saw-shaped with 4–5 teeth including a main fang. Abdominal chaetae flat-trumpets with a large tooth and several small serrations. Posterior capillaries present in the last chaetigers. Abdomen of 70 or more chaetigers.
Size: 7–13 mm in length, up to 0.5 mm width of thorax, operculum 0.5–0.7 mm in length.
Colour of preserved specimens: pale yellow with brown speckled on collar and thoracic membranes.
REMARKS
One specimen from Rongelap Atoll resembles H. tuberculatus except the operculum is incompletely developed. The massive opercular lobe is very thin, fragile and pale, and the smaller lobes inside it are visible through the wall. This specimen may have been regenerating so the opercular structures are not fully developed.
Hydroides tuberculatus superficially resembles H. brachyacantus on the shape of operculum, but the verticil spines are broad valves in H. tuberculatus without subapical hooks as in H. brachyacanthus. Hydroides inermis Monro, 1933 from the Galapagos also have inflated verticil spines but they lack dorsal blunt tubercles.
DISTRIBUTION
This species is widely distributed in the Indo-West Pacific (ten Hove & Kupriyanova Reference Hove and Kupriyanova2009) including southern Japan, Ponape, Truk, Palau, Yap and Queensland, Australia (Imajima, Reference Imajima1976a; Imajima & ten Hove, Reference Imajima and ten Hove1984) and is newly recorded for Rongelap and Utirik Atolls in the Marshall Islands.
Omphalopomopsis fimbriata—Zibrowius Reference Zibrowius1968: 149–151, pl. 6, figures 13–21 and references therein.
Janita fimbriata—Zibrowius Reference Zibrowius1972: 122, figures 19 & 20; Zibrowius Reference Zibrowius1973: 59–61; Imajima Reference Imajima1979: 174–176, figure 7a–o; ten Hove & Kupriyanova Reference Hove and Kupriyanova2009: 55–57, figure 24a–f.
MATERIAL EXAMINED
Enewetak Atoll: Station 1, Pinnacle Lagoon, removed from clam shell, September 1975, 23 m, coll. R. Brock (1, BPBM R3511).
DIAGNOSIS
Elaborate tube with five longitudinal ridges raised into fins and spines (Figure 4E); tube light tan, raised ridges white (Figure 4E). Operculum golden-brown, cup-shaped plate with a hollow talon extending into the base (Figure 4D); the latter joins the wrinkled opercular peduncle and comprises three fleshy projections, two round and one angular (Figure 4D). For a full description see Imajima (Reference Imajima1979).
REMARKS
The presence of distinct collar chaetae (Spirobranchus type, acicular and/or limbate) among and within populations has been discussed by several authors (e.g. Ben-Eliahu & Fiege, Reference Ben-Eliahu and Fiege1996; ten Hove & Kupriyanova, Reference Hove and Kupriyanova2009) and might be of ontogenetic effect (ten Hove personal communication in Ben-Eliahu & Fiege, Reference Ben-Eliahu and Fiege1996). For instance, the single specimen collected from Enewetak Atoll presented two kinds of collar chaetae: five aciculars with proximal rasps and fine longitudinal striations and five limbate chaetae, lacking the Spirobranchus type.
DISTRIBUTION
Subtropical Atlantic, Mediterranean, Indo-West Pacific (ten Hove & Kupriyanova, Reference Hove and Kupriyanova2009). In the western Pacific this species has been found in southern Japan (Imajima, Reference Imajima1979), Solomon Islands (Imajima & ten Hove, Reference Imajima and ten Hove1986) and newly recorded to Enewetak Atoll in the Marshall Islands.
Pomatostegus actinoceros—Willey Reference Willey and Herdman1905: 314–316, pl. VIII, figures 3 & 4; Pillai Reference Pillai2009: 109–112, figures 9a–e & 10a–e.
Pomatostegus stellatus (Abildgaard, 1789)—Dew Reference Dew1959: 41–42, figure 14a–g; Fiege & Sun Reference Fiege and Sun1999: 131–133, figure 19a–f; Imajima Reference Imajima1977: 101–102, figure 7a–k; Imajima Reference Imajima1982: 51; Imajima Reference Imajima1987: 80; Imajima & ten Hove Reference Imajima and ten Hove1984: 54; Imajima & ten Hove Reference Imajima and ten Hove1986: 9; Mak Reference Mak, Morton and Tseng1982: 608; Nishi Reference Nishi1995: 29–31, figure 1h; Pillai Reference Pillai1960: 23–25, figure 9a–d; Pillai Reference Pillai1971: 94; Straughan Reference Straughan1967a: 238; Straughan Reference Straughan1967b: 38; Straughan Reference Straughan1967c: 224.
MATERIAL EXAMINED
Enewetak Atoll: Station 1, on coral rock and mollusc shells from lagoon pinnacles, 15–20 m, September 1975, coll. R. Brock (12, BPBM R3514; 5, BPBM R3515; 1, BPPM R3513); Station 3, Clyde Island, 0.5–6 m, September 1975, coll. R. Brock (7, BPBM R3512).
DESCRIPTION
Tube: thick, opaque, white, triangular in section (Figure 5B–D); a strong median keel with blunt teeth and two or more lateral rows of well-defined teeth present (Figure 5B, C).
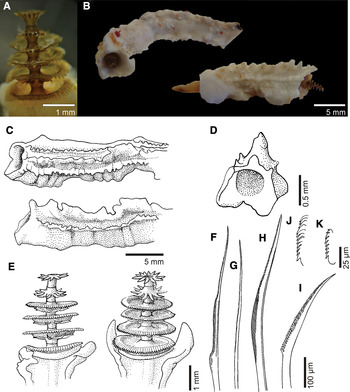
Fig. 5. Pomatostegus actinoceras: (A) operculum; (B) tubes in frontal and lateral view; (C) tubes in dorso-lateral and lateral views; (D) cross-section of tube; (E) opercula; (F) collar chaeta; (G) thoracic capillary chaeta; (H) Apomatus chaeta; (I) abdominal chaeta; (J) thoracic uncinus; (K) abdominal uncinus.
Branchiae: each lobe with 20–22 radioles, inter-radiolar membrane present.
Peduncle: muscular peduncle broad and flat with lateral wings terminating in pointed tips.
Operculum: a column with 2–6 chitinous plates with frilled margins stacked one above the other (Figure 5A, E); diameter of plates decreases upwards (Figure 5A, E). Circlets of 10–12 spines present applied to the basis of each disk, except for the first chitinous plate (Figure 5A, E); spines curved downwards on first plates and then becoming straight on the distal whorls.
Collar and thoracic membranes: collar well developed, smooth, tri-lobed, continuing into short thoracic membranes extending to the second row of thoracic uncini. Apron present.
Thorax: with collar chaetiger and 6 uncinigerous chaetigers; collar chaetae two types: bayonets with serrated base (Figure 5F) and capillaries. Thoracic chaetae include simple blades (Figure 5G) and Apomatus chaetae (Figure 5H). Thoracic uncini saw-shaped with 11 teeth including a simple anterior tooth (Figure 5J).
Abdomen: abdominal uncini saw-shaped with 8 teeth including a simple anterior tooth (Figure 5K). Abdominal chaetae all flat geniculate with narrow blades and 36 or more rounded teeth (Figure 5I). Abdomen of 60 or more chaetigers.
Size: 8–30 mm in length, up to 2.0 mm width of thorax, operculum up to 3.2 mm length.
Colour of preserved specimens: white with a bronze coloured operculum.
REMARKS
Pomatostegus actinoceras opercula filled the stomachs of butterfly fish, Chaetodon auriga, collected from Enewetak lagoon patch reefs by E. Reese (Bailey-Brock, unpublished data). This species has been regarded as synonymous with P. stellatus, but the latter seems to be restricted to the tropical Atlantic (ten Hove & Kupriyanova, Reference Hove and Kupriyanova2009).
DISTRIBUTION
This species has a broad Indo-West Pacific distribution (ten Hove & Kupriyanova, Reference Hove and Kupriyanova2009), which includes southern Japan (Imajima, Reference Imajima1977, Reference Imajima1987; Nishi, Reference Nishi1995), Micronesia and Australia (Dew, Reference Dew1959; Imajima Reference Imajima1982; Imajima & ten Hove, Reference Imajima and ten Hove1984, Reference Imajima and ten Hove1986; Straughan, Reference Straughan1967a, Reference Straughanb, Reference Straughanc; Pillai, Reference Pillai2009), China (Mak, Reference Mak, Morton and Tseng1982; Fiege & Sun Reference Fiege and Sun1999) and Ceylon (Pillai, Reference Pillai1960, Reference Pillai1971).
MATERIAL EXAMINED
Kwajalein Atoll: Station 1, July 1975, coll. R. Brock (1). Utirik Atoll: Station 1, Utirik harbour, July 1975, coll. R. Brock (23, BPBM R3516); Station 2, July 1975, 1–2 m, coll. R. Brock (7, BPBM R3517).
DESCRIPTION
Tube: white, opaque, fragile, irregularly coiled, circular in cross section with 2 well-developed longitudinal ridges and transversal growths (Figure 6A).

Fig. 6. (A) Tube of Protula sp.; (B) tubes of Salmacina sp.; (C) operculum of Serpula hartmanae; (D) operculum of Serpula cf. watsoni; (E) operculum of Serpula willeyi.
Branchiae: pectiniform paired lobes number 12–15 on each side with long terminal filament; branchial eyes absent; inter-radiolar membrane present.
Collar and thoracic membranes: collar smooth and tri-lobed. Thoracic membrane well developed, overlapping dorsally, ending posterior to last thoracic uncinigerous segment.
Thorax: with collar chaetiger and 6 uncinigerous chaetigers; collar and thoracic chaetae all limbate capillaries of two lengths organized in two rows of 10–12 short and 10–12 long capillaries. Apomatus chaetae present numbers 1–2 on chaetigers 5 to 7. Thoracic uncini rasp-shaped with very long basal process and about 20 or more teeth.
Abdomen: abdominal uncini also rasp-shaped; anterior abdominal uncini similar in shape and size to thoracic ones then become slightly longer and with more teeth. Anterior abdominal chaetae 3–4 geniculate blades, sickle-shaped, coarsely denticulate (teeth easily seen at 400×). Posterior abdominal chaetae 3–4 long and fine capillaries with curved tips.
Size: 3–9 mm in length, up to 2 mm width of thorax.
Colour of preserved specimens: pale yellow.
REMARKS
The specimens from Kwajalein and Utirik Atolls fit the description of Imajima (Reference Imajima1977) for Protula tubularia caeca. However, the number of branchial radioles in the Utirik material is smaller (12) than in the Japanese material (15–19) but this could be age related, since the material analysed is also very small (up to 9 mm) and the type material analysed by Imajima (Reference Imajima1977) was 25 mm in length.
DISTRIBUTION
Kwajalein and Utirik Atolls in the Marshall Islands.
Pseudovermilia pacifica—Imajima Reference Imajima1979: 170; Imajima & ten Hove Reference Imajima and ten Hove1984: 58; ten Hove Reference Hove and Land1994: 110.
MATERIAL EXAMINED
Enewetak Atoll: Station 1, reef 8, 20 m, September 1975, coll. R. Brock (2, BPBM R3523); Station 1, 30–35 m, September 1975, coll. R. Brock (1, BPBM R3522). Utirik Atoll: Station 2, July 1975, coll. R. Brock (1, BPBM R3519; 1, BPBM R3520; 5, BPBM R3518); Station 3, July 1975, coll. R. Brock (1, BPBM R3521).
DESCRIPTION
Tube: white, triangular in section with median and two lateral ridges (Figure 7A–C); median ridge with a finely toothed margin (Figure 7A, B). One specimen with a raised transverse ridge (Figure 7A, B). Tube with lateral flanges where it meets the substrate (Figure 7A, B).

Fig. 7. Pseudovermilia pacifica: (A) tubes in dorsal view; (B) tubes in lateral view; (C) cross-section of tube; (D) opercula with flat plate and bell-shaped ampulla; (E) operculum with diabolo-like tiers; (F) Apomatus chaeta; (G) collar chaeta; (H) geniculate abdominal chaeta; (I) posterior abdominal capillary chaeta; (J) abdominal chaeta; (K) bifid anterior tooth of thoracic uncini; (L & M) abdominal uncini with bifid anterior tooth.
Branchiae: 10–12 radioles per lobe with long terminal filament; branchial eyes and inter-radiolar membrane absent.
Peduncle: with asymmetrical swelling at the distal end of opercular peduncle best seen in lateral view; peduncles of single plate specimens slightly wrinkled, but not conspicuously swollen. Pseudoperculum absent.
Operculum: three specimens with a flat plate and bell-shaped ampulla (Figure 7D), one specimen a rimmed plate and two others a stack of diabolo-like tiers with distinct margins (Figure 7E); all contained an opaque inclusion in ampulla (Figure 7D, E). Tiered and rimmed opercular plates faintly yellow and chitinous in appearance.
Collar and thoracic membranes: collar smooth, tri-lobed, with laciniate margin. Thoracic membranes ending posterior to first row of abdominal uncini.
Thorax: with collar chaetiger and 6 uncinigerous chaetigers (5 uncinigerous in the small specimen with a flat plate); collar chaetae two types: 3–4 simple blades (Figure 7G) and 1–2 capillaries. Chaetiger 2–3 with seven simple blades and chaetigers 3–7 each with 3–4 simple blades and 1–2 Apomatus chaetae (Figure 7F). Thoracic uncini with gouged bifid anterior tooth and single row of teeth (Figure 7K).
Abdomen: abdominal uncini shorter than thoracics and with bifid anterior tooth and multiple rows of teeth (Figure 7L, M). Achaetigerous region between thorax and abdomen present. Abdominal chaetae geniculate blades (Figure 8H) and capillary chaetae (Figure 8I). Anterior abdominal chaetigers with a pair of flat geniculate chaetae (Figure 8J), more posterior chaetigers a pair of capillaries. Abdomen of single plate specimen with 11 chaetigers.

Fig. 8. Spirobranchus decoratus: (A) operculum; (B) tubes in dorsal and lateral views; (C) tube in lateral view; (D) tube in cross-section; (E) operculum; (F) opercular crown with branching spines; (G) collar and thoracic region in lateral view; (H) inter-radiolar membranes.
Size: 3–3.5 mm in length, up to 0.2 mm width of thorax, operculum up to 0.8 mm in length.
Colour of preserved specimens: pale yellow.
REMARKS
Pseudovermilia pacifica resembles Semivermilia uchidai Imajima & ten Hove, Reference Imajima and ten Hove1986 and P. fucostriata ten Hove, 1975. All three species are small (less than 12 mm in length), have diabolo-tiered opercula, but the tubes differ. P. fucostriata has tubes with pits and spinous ridges marked by transverse brown bands. S. uchidai has a white tube with a median and two oblique lateral keels that are serrated. Pseudovermilia pacifica has white tubes, triangular in section, with a distinct median keel.
DISTRIBUTION
Indo-West Pacific (ten Hove & Kupriyanova, Reference Hove and Kupriyanova2009) including southern Japan (Izu Islands), Ponape (Imajima, Reference Imajima1978; Imajima & ten Hove, Reference Imajima and ten Hove1984) and newly recorded from Enewetak and Utirik Atolls in the Marshall Islands.
MATERIAL EXAMINED
Enewetak Atoll: Station 1, next to Oak crater, September 1975, 1–4 m, coll. R. Brock (3, BPBM R3551).
DESCRIPTION
Tube: white, slender, with fine transverse markings (Figure 6B).
Branchiae: 3–4 radioles per lobe; branchial eyes and inter-radiolar membrane absent.
Collar and thoracic membranes: collar smooth, tri-lobed. Thoracic membrane forming apron.
Thorax: with collar chaetiger and 6 uncinigerous chaetigers; collar chaetae two types: fin-and-blade and limbate capillaries. Chaetiger 2 with 1–2 Apomatus chaetae and 3–4 limbate chaetae. Thoracic uncini rasp-shaped with 8–10 teeth including anterior pointed fang.
Abdomen: abdominal uncini similar to thoracic ones but with more teeth, about 10–12. Achaetigerous region between thorax and abdomen as long as 2 thoracic segments. Abdominal chaetae 1–2 flat geniculate blades.
Size: 1.8–2.0 mm in length, up to 0.3 mm width of thorax and up to 20 abdominal chaetigers.
Colour of preserved specimens: pale yellow.
REMARKS
Hartman (Reference Hartman1954) reports the occurrence of Salmacina sp. from Enewetak Atoll and Reish (1964) and Devaney & Bailey-Brock (Reference Devaney, Bailey-Brock, Devaney, Reese, Butch and Helfrich1987) assign it to Salmacina incrustans and expand the occurrence of this species to Majuro and Bikini Atolls. The specimens described herein probably belong to the same species as that reported by the authors above but it would be unwise to name it because a revision of the Pacific records is needed. The occurrence of Salmacina incrustans in the Pacific Ocean is questionable and it belongs to a complex of species (ten Hove & Kupriyanova, Reference Hove and Kupriyanova2009).
DISTRIBUTION
Bikini, Enewetak and Majuro Atolls in the Marshall Islands.
Serpula hartmanae Reish Reference Reish1968: 228–229, figures 5 & 11–16.
Serpula hartmanae—Ben-Eliahu & ten Hove Reference Ben-Eliahu and Hove2011: 72–83, figures 28–32; Gibbs Reference Gibbs1971: 203; Imajima & ten Hove Reference Imajima and ten Hove1984: 36–38, figure 1a–d; Rullier Reference Rullier1972: 154.
MATERIAL EXAMINED
Enewetak Atoll: Station 1, Quarry on Fred, September 1975, coll. R. Brock (1, BPBM R3524).
DIAGNOSIS
Long opercular funnel with shallow cup, 12–18 blunt marginal radii and with a constriction between basal part of the funnel and peduncle (Figure 6C). For a complete description see the original description (Reish, Reference Reish1968) and redescriptions (Imajima & ten Hove, Reference Imajima and ten Hove1984).
REMARKS
The single specimen collected has 18 blunt marginal radii (Figure 6C) and agrees with the descriptions by Reish (Reference Reish1968), Imajima & ten Hove (Reference Imajima and ten Hove1984) and Ben-Eliahu & ten Hove (Reference Ben-Eliahu and Hove2011).
DISTRIBUTION
Indo-West Pacific (ten Hove & Kupriyanova, Reference Hove and Kupriyanova2009) reported to occur in the Solomon Islands, New Caledonia, Palau Islands, Truk Islands, Ponape Islands (Imajima & ten Hove, Reference Imajima and ten Hove1986) but originally described from Bikini Atoll in the Marshall Islands (Reish, Reference Reish1968) and also occurs in Majuro (Imajima & ten Hove, Reference Imajima and ten Hove1984) and Enewetak atolls. Ben-Eliahu & ten Hove (Reference Ben-Eliahu and Hove2011) report this species in the Red Sea.
Serpula watsoni Willey Reference Willey and Herdman1905: 317, pl. VII, figure 187, pl. VIII, figure 6.
Serpula watsoni—Imajima Reference Imajima1977: 91–92, figure 2a–j; Imajima Reference Imajima1982: 40; Imajima Reference Imajima1987: 78; Imajima & ten Hove Reference Imajima and ten Hove1984: 38–39, figure 2; Imajima & ten Hove Reference Imajima and ten Hove1986: 2; Mak Reference Mak, Morton and Tseng1982: 609; Pillai Reference Pillai2009: 140–143, figures 32a–n & 33a–n; Straughan Reference Straughan1967a: 207, figure 3b; Sun & Yang 2001: 194, figure 6g–m.
MATERIAL EXAMINED
Enewetak Atoll: Station 3, Clyde Patch, January 1976, 4–6 m, coll. R. Brock (1, BPBM R3525).
DIAGNOSIS
Long opercular funnel with deep hollow and 44–46 blunt marginal radii with a few tubercles on distal margin (Figure 6D). See Imajima (Reference Imajima1977) and Pillai (Reference Pillai2009) for complete description of this species.
REMARKS
The specimens from Enewetak Atoll agree very well with the descriptions of Imajima (Reference Imajima1977) and Pillai (Reference Pillai2009) of the operculum and thoracic and abdominal chaetae and uncini. However, the tube of Enewetak's specimen has two longitudinal ridges as described in Pillai (Reference Pillai2009) and not 5 longitudinal ridges as illustrated and described by Imajima (Reference Imajima1977).
DISTRIBUTION
This species has an Indo-West Pacific distribution (ten Hove & Kupriyanova, Reference Hove and Kupriyanova2009), which includes Sri Lanka (Willey, Reference Willey and Herdman1905), southern Japan (Imajima, Reference Imajima1977), Australia (Straughan, Reference Straughan1967a; Pillai, Reference Pillai2009), Palau, Truk and Ponape Islands (Imajima & ten Hove, Reference Imajima and ten Hove1984), Solomon Islands (Imajima & ten Hove, Reference Imajima and ten Hove1986) and Majuro (Imajima & ten Hove, Reference Imajima and ten Hove1984) and Enewetak Atolls in the Marshall Islands.
MATERIAL EXAMINED
Enewetak Atoll: Station 1, from dead Helmet shell, September 1975, 10–20 m, coll. R. Brock (2, BPBM R3527). Utirik Atoll: Station 3, July 1975, coll. R. Brock (1, BPBM R3526).
DIAGNOSIS
Long opercular funnel with deep hollow, 13 blunt marginal radii with a constriction between basal part of the funnel and peduncle (Figure 6E). For a complete description see Pillai (Reference Pillai1971).
REMARKS
The single specimen sampled agrees well with Pillai's description.
DISTRIBUTION
Pearl Banks, Sri Lanka (Pillai, Reference Pillai1971) and newly recorded to Enewetak and Utirik Atolls, Marshall Islands.
Spirobranchus tricornigerus decoratus Imajima Reference Imajima1982: 48–50, figure 5a–m.
Spirobranchus tricornigerus var. decoratus Pillai Reference Pillai1971: 99–100, figure 3e.
Spirobranchus decoratus—Fiege & Sun Reference Fiege and Sun1999: 125–126, figure 14a–d; Imajima Reference Imajima1987: 79; Imajima & ten Hove Reference Imajima and ten Hove1984: 52–53, figure 5d; Imajima & ten Hove Reference Imajima and ten Hove1986: 8.
MATERIAL EXAMINED
Enewetak Atoll: Station 1, reef near Oak crater on coral rubble and live coral, September 1975, 1–4 m, coll. R. Brock (4, BPBM R3528).
DIAGNOSIS
Tubes pink or pink and white, broadly triangular in section with lateral flanges and a median keel divided into coarse spines (Figure 8B–D). A spine protrudes over the mouth and one or two rows of circular holes and four rows of pits occur on either side of keel, sometimes occluded by encrusting foraminiferans and coralline algae (Figure 8B–D). Operculum with a long flattened stalk with elaborate wings each ending in a single process (Figure 8A, E, F). Opercular ampula globular, calcareous plate thick and flat bearing a crown of 5–6 branching spines (Figure 8A, E, F). Spines branch dichotomously from centre and bear small paired denticles along their length (Figure 8F). Spines all in same plane giving a finely pinnate, stellate appearance to the opercular plate (Figure 8F). For a complete description see Imajima (Reference Imajima1982) and Imajima & ten Hove (Reference Imajima and ten Hove1984).
REMARKS
These specimens agree with Imajima's description except that one small specimen had five spine bases on the opercula ampulla instead of six.
DISTRIBUTION
Spirobranchus decoratus is known from Palau, Ponape, Truk (Imajima & ten Hove, Reference Imajima and ten Hove1984), the Gilbert and Solomon Islands, Majuro, Marshall Islands and South China Sea (Fiege & Sun, Reference Fiege and Sun1999).
Spirobranchus gaymardi—Fiege & ten Hove Reference Fiege and Hove1999 and references therein: 356–362, figures 1–3.
Spirobranchus giganteus (Pallas 1766)—Hartman Reference Hartman1954: 629; Reish Reference Reish1968: 229.
MATERIAL EXAMINED
Enewetak Atoll: north-western reefs near Mike and Koa craters, September 1975, 1–2 m, coll. R. Brock (1, BPBM R3554; 3, BPBM R3555). Recorded from Rongelap and Utirik Atolls on live corals.
DIAGNOSIS
See Fiege & ten Hove (Reference Fiege and Hove1999) for a full description of this species.
REMARKS
Although numerous individuals of this species were observed on pinnacles and live coral heads in the lagoon at Enewetak, Rongelap and Utirik only a few were collected to avoid destroying live coral. Opercula of this species were found in the guts of chaetodontid fish speared in the lagoon during an analysis of chaetodon diets (Bailey-Brock, unpublished data). Spirobranchus gaymardi was recorded from Enewetak by Hartman (Reference Hartman1954) and Reish (Reference Reish1968) as S. giganteus.
DISTRIBUTION
This species is widely distributed in the Indo-West Pacific (ten Hove & Kupriyanova, Reference Hove and Kupriyanova2009), including Japan, South China Sea, Philippines, Indonesia, India, New Caledonia, Australia (Fiege & ten Hove, Reference Fiege and Hove1999) and Enewetak, Rongelap and Utirik Atolls in the Marshall Islands.
MATERIAL EXAMINED
Utirik Atoll: Station 3, shallow reefs near Lone Palm Island, July 1975, coll. R. Brock (5, BPBM R3529).
DIAGNOSIS
Tubes white to pink with a pink interior, triangular in section with a median ridge forming a tooth over the mouth; with a row of perforations on either side of the ridge. Operculum with ampulla and calcareous cap which may be a tall cone or dome with a flared distal part with two lapel-shaped knobs (Figure 9B). Opercular peduncle flattened, finely tapered with single wings that do not bifurcate (Figure 9B). For a complete description see Imajima (Reference Imajima1977).

Fig. 9. (A) Operculum of Spirobranchus gaymardi; (B) operculum of Spirobranchus polytrema type B; (C & D) opercula of Vermiliopsis glandigerus; (E) tube of Vermiliopsis glandigerus; (F) operculum of Vermiliopsis torquata.
REMARKS
These specimens agree with Imajima's description of Spirobranchus cf. polytrema type B (Imajima, Reference Imajima1977; Fiege & Sun, Reference Fiege and Sun1999). The smallest specimen (probably juvenile) has a calcareous cap without any ornamentation.
DISTRIBUTION
Spirobranchus polytrema type B is known from southern Japan (Imajima, Reference Imajima1977), south China Sea (Fiege & Sun, Reference Fiege and Sun1999), Cyprus and Israel (Ben-Eliahu & Payiatas, Reference Ben-Eliahu and Payiatas1999) and newly recorded to Utirik Atoll in the Marshall Islands.
Vermiliopsis glandigerus Gravier Reference Gravier1906: 121, pl. VIII, figures 290 & 291, text-figures 476–481.
Vermiliopsis infundibulum/glandigera-complex—Fiege & Sun Reference Fiege and Sun1999 and references therein: 133–135, figure 21a–3.
Vermiliopsis glandigera—Pillai Reference Pillai2009: 103–108, figures 5a–e, 6a-l & 7 a–n.
MATERIAL EXAMINED
Enewetak Atoll: Station 3, Clyde Patch, January 1976, coll. R. Brock (1, BPBM R3535); Station 1, from dead Helmet, September 1975, 8–12 m, coll. R. Brock (4, BPBM R3533); Station 1, next to Oak crater, September 1975, 1–4 m, coll R. Brock (14, BPBM R3534). Utirik Atoll: Station 2, July 1975, coll. R. Brock (3, BPBM R3530; 2, BPBM R3531); Station 1, Utirik harbour, July 1975, 2 m, coll. R. Brock (16, BPBM R3532).
DIAGNOSIS
Tubes massive with five ill-defined longitudinal ridges (Figure 9E). Operculum with a fleshy bulbous part basally and chitinized terminal cap with 6–12 dark brown rings (Figure 9C, D); shape of terminal cap very variable but usually conical in Enewetak's material. See Pillai (Reference Pillai2009) for a full description of this species.
REMARKS
Pillai (Reference Pillai2009) provides a great description of this species and also clarifies the differences between V. glandigerus and V. infundibulum (Philippi, 1844).
DISTRIBUTION
Red Sea, Indo-West Pacific (ten Hove & Kupriyanova, Reference Hove and Kupriyanova2009) including Australia (Pillai, Reference Pillai2009), southern Japan (Imajima, Reference Imajima1976a, Reference Imajima1977, Reference Imajima1979), Micronesia (Imajima, Reference Imajima1982), Gilbert and Solomon Islands (Imajima & ten Hove, Reference Imajima and ten Hove1986), Majuro and Ponape Atolls and Truk Islands (Imajima & ten Hove, Reference Imajima and ten Hove1984), newly recorded to Utirik and Enewetak Atolls in the Marshall Islands.
Vermiliopsis torquata—Straughan, Reference Straughan1969b: 233, 235, figure 2b; Bailey-Brock, Reference Bailey-Brock1987a: 426, figure 3.II.200.
Vermiliopsis hawaiiensis Treadwell, Reference Treadwell1943: 3, 4, figures 14 & 15.
Vermiliopsis multiannulata—Hartman Reference Hartman1966: 239.
MATERIAL EXAMINED
Kwajalein Atoll: Station 1, July 1975, coll. R. Brock (4, BPBM R3536).
DIAGNOSIS
Tubes massive, white and longitudinally ridged. Peduncle wrinkled, slightly swollen at base of ampulla; operculum with a ringed and brown chitinous cap (Figure 9F). See Treadwell (Reference Treadwell1943), Straughan (Reference Straughan1969b) and Bailey-Brock (Reference Bailey-Brock1987a) for description of this species.
REMARKS
The unique specimen is similar to Hawaiian material and agrees well with descriptions in Treadwell (Reference Treadwell1943), Straughan (Reference Straughan1969b) and Bailey-Brock (Reference Bailey-Brock1987a). Hartman (Reference Hartman1966) synonymized this species with V. multiannulata (Moore, 1923) because both species have brown chitinous opercula but Straughan (Reference Straughan1969b) clarifies that the tips of the branchiae are free in V. multiannulata only. However, the specimen from Kwajalein Atoll and also comparative material from Hawaii (Mamala Bay, Oahu) have long and free radiole tips. Type material of both species should be closely compared.
DISTRIBUTION
This species was believed to be endemic to the Hawaiian Islands but now seems to have a north-western Pacific distribution occurring also in the Marshall Islands at Kwajalein Atoll.
Eulaeospira orientalis—Pillai Reference Pillai1970: 138, Vine Reference Vine1972a: 178–180, figure 1a–j; Bailey-Brock Reference Bailey-Brock1985: 213–214, figure 17a, b; Vine & Bailey-Brock Reference Vine and Bailey-Brock1984: 147; Bailey-Brock Reference Bailey-Brock1987a: 429, figure 3.II.201.
MATERIAL EXAMINED
Kwajalein Atoll: Station 2, July 1975, coll. R. Brock (2, BPBM R3552; 1, BPBM R3553).
DIAGNOSIS
Tubes with sinistral coiling and a single longitudinal ridge (Figure 10B); ascending portion lacks longitudinal ridge, circular in cross-section and with peristomial marks (Figure 10A). Operculum an inverted cone with a transparent collar-like rim. See Pillai (Reference Pillai1960) and Vine (Reference Vine1972a) for a full description and illustration of this species.

Fig. 10. (A & B) Tubes of Eulaeospira orientalis; (C) tube of Neodexiospira nipponica; (D) mature operculum of N. nipponica; (E) collar chaetae of N. nipponica; (F) tube of Neodexiospira steueri.
REMARKS
Specimens agree well with the Pillai (Reference Pillai1960) description. Several tubes presented ascending portions with 1–2 peristomial marks.
DISTRIBUTION
Known from Ceylon (Pillai, Reference Pillai1960), Red Sea (Vine, Reference Vine1972a; Vine & Bailey-Brock, Reference Vine and Bailey-Brock1984), Fiji (Bailey-Brock, Reference Bailey-Brock1985), Hawaii (Bailey-Brock, Reference Bailey-Brock1987a) and newly recorded to the Marshall Islands at Kwajalein Atoll.
Janua (Dexiospira) nipponica—Knight-Jones et al., 1979: 433–434, figure 4d; Knight-Jones et al., Reference Knight-Jones, Knight-Jones and Kawahara1975: 110–111, figures 4c & 5a; Vine et al., Reference Vine, Bailey-Brock and Straughan1972: 170–172, figure 12a–j.
Neodexiospira nipponica—Bailey-Brock Reference Bailey-Brock1987a: 431, figure 3.II.215 i, j; Bailey-Brock Reference Bailey-Brock1999: 189.
MATERIAL EXAMINED
Utirik Atoll: Station 2, July 1975, coll. R. Brock (4, BPBM R3537; 6, BPBM R3538).
DIAGNOSIS
Tubes with dextral coiling, 3 longitudinal ridges and transverse bars between them, appearing to be indentations in the surface of the tube (Figure 10C). Opercular plate flat to concave with a wedge-shaped peripheral talon (Figure 10D). See Vine et al. (Reference Vine, Bailey-Brock and Straughan1972) and Bailey-Brock (Reference Bailey-Brock1987a) for a full description and illustration of this species.
REMARKS
The material analysed agrees well with the Hawaiian specimens described by Vine et al. (Reference Vine, Bailey-Brock and Straughan1972) and Bailey-Brock (Reference Bailey-Brock1987a).
DISTRIBUTION
This species is known from Japan (Okuda, Reference Okuda1934), South Africa as D. foraminosus (Vine et al., Reference Vine, Bailey-Brock and Straughan1972), Hawaii (Vine et al., Reference Vine, Bailey-Brock and Straughan1972; Bailey-Brock, Reference Bailey-Brock1987a) and newly recorded to Utirik Atoll in the Marshall Islands.
Janua (Dexiospira) steueri— Knight-Jones et al., Reference Knight-Jones, Knight-Jones and Llewellyn1974: 140–141, figure 14a–p; Vine et al., Reference Vine, Bailey-Brock and Straughan1972: 168–170, 11a–j.
Janua (Dexiospira) steueri var. steueri—Vine Reference Vine1972b: 188–189, figure 6a–j.
Neodexiospira steueri—Bailey-Brock Reference Bailey-Brock1999: 189.
MATERIAL EXAMINED
Enewetak Atoll: Station 3, Clyde patch, 1–6 m, January 1976, coll. R. Brock (1, BPBM R3539; 5, BPBM R3540).
DIAGNOSIS
Tubes dextral with 3 longitudinal ridges on the top and one on the side forming blunt projections and with deep indentations between the ridges (Figure 10F). Opercular plate slightly concave with a peripheral and bifid talon bearing lateral wings. See Vine et al. (Reference Vine, Bailey-Brock and Straughan1972) and Bailey-Brock (Reference Bailey-Brock1987a) for a full description and illustration of this species.
REMARKS
The records referred to Neodexiospira foraminosa (Moore & Bush, Reference Moore and Bush1904) in the north-western Pacific (Bailey-Brock, Reference Bailey-Brock1987a for Hawaii; Bailey-Brock, Reference Bailey-Brock1976 for Johnston Atoll; Bailey-Brock, Reference Bailey-Brock1985 for Fiji; Bailey-Brock, Reference Bailey-Brock1987b for Tonga) actually belong to this species.
DISTRIBUTION
This species is widely distributed in the western Pacific, including Australia (Knight-Jones et al., Reference Knight-Jones, Knight-Jones and Llewellyn1974), Hawaii (Vine et al., Reference Vine, Bailey-Brock and Straughan1972; Bailey-Brock, Reference Bailey-Brock1976, Reference Bailey-Brock1987a), Fiji (Bailey-Brock, Reference Bailey-Brock1985), Tonga (Bailey-Brock, Reference Bailey-Brock1987b), Guam and Saipan (Bailey-Brock, Reference Bailey-Brock1999, Reference Bailey-Brock2003) and newly recorded to Enewetak Atoll in the Marshall Islands.
Janua (Dexiospira) turrita Vine Reference Vine1972a: 145–147, figure 4a–p.
Janua (Dexiospira) preacuta Vine Reference Vine1972b: 186–188, figure 5a–k.
Neodexiospira preacuta—Bailey-Brock Reference Bailey-Brock1987a: 431–432, figure 3.II.206.
MATERIAL EXAMINED
Utirik Atoll: Station 2, from Tridacna shell, July 1975, coll. R. Brock (9, BPBM R3541).
DIAGNOSIS
Tubes with 3 longitudinal ridges, transversal growths and dextral coiling (Figure 11A). Opercular plate slightly concave with a long talon with an asymmetrically pointed end (Figure 11B). See Vine (Reference Vine1972a, Reference Vineb) and Bailey-Brock (Reference Bailey-Brock1987a) for a full description and illustration of this species.

Fig. 11. (A) Tube of Neodexiospira turrita; (B) primary operculum of N. turrita in lateral view; (C) tube of Pileolaria pettiboneae; (D) primary operculum of P. pettiboneae; (E) mature operculum of P. pettiboneae; (F) collar chaeta of P. pettiboneae.
REMARKS
Drs E.W. Knight-Jones and P. Knight-Jones in correspondence dated 15 May 1973 to J.H.B-B., stated that after close comparison of Jania (D.) preacuta from the Red Sea and J. (D.) turrita from Hawaii, they could not find significant differences and considered J. (D.) preacuta to be the senior synonym. However, the species J. (D.) turrita is the earliest available name being published in April 1972 (Vine, Reference Vine1972a), while J. (D.) preacuta was published in May 1972 (Vine, Reference Vine1972b). Therefore, J. (D.) turrita, now accepted as Neodexiospira turrita nom. nov. should be considered to be the senior synonym.
DISTRIBUTION
This species is widely distributed in the Indo-West Pacific, including in the Red Sea (Vine, Reference Vine1972b), Hawaii (Vine, Reference Vine1972a; Bailey-Brock, Reference Bailey-Brock1976, Reference Bailey-Brock1987a), Fiji (Bailey-Brock, Reference Bailey-Brock1985), Tonga (Bailey-Brock, Reference Bailey-Brock1987b), Guam and Saipan (Bailey-Brock Reference Bailey-Brock1999, Reference Bailey-Brock2003) and newly recorded to Utirik Atoll in the Marshall Islands.
MATERIAL EXAMINED
Enewetak Atoll: Station 1, reef 8, September 1975, 30–35 m, coll. R. Brock (1, BPBM R3544; 3, BPBM R3542); same locality 1–5 m (2, BPBM R3545; 2, BPBM R3543); Station 2, Elmers Pinnacles, September 1975, 12–18 m, coll. R. Brock (3, BPBM R3556).
DIAGNOSIS
Tubes with 3 longitudinal ridges, transversal growths and sinistral coiling (Figure 11C). Primary opercula with delicate concave plates with a smooth and slender peg talon extending from the base of the cup-shaped plate (Figure 11D). Mature opercula domed calcareous chambers, open on one side and ornamented with spines (Figure 11E). See Bailey-Brock (Reference Bailey-Brock1987b) for a full description and illustration of this species.
REMARKS
These specimens analysed here comprised part of those reported in Bailey-Brock (Reference Bailey-Brock1987b) from Enewetak, Utirik and Rongelap Atolls in the Marshall Islands as unpublished notes.
DISTRIBUTION
Tonga, Enewetak, Utirik and Rongelap Atolls (Bailey-Brock, Reference Bailey-Brock1987b).
Pileolaria pseudomilitaris (Thiriot-Quiévreux, Reference Thiriot-Quiévreux1965)
Pileolaria (Laeospira) pseudomilitaris Thiriot-Quiévreux, 1965: 495–502, figures 1a–c, 2a–c & 3.
Pileolaria (Laeospira) pseudomilitaris—Zibrowius Reference Zibrowius1968: 207.
Spirorbis (Pileolaria) pseudomilitaris—Bailey 1969a: 369–371, figure 5a–d.
Pileolaria (Simplicaria) pseudomilitaris—Vine et al., Reference Vine, Bailey-Brock and Straughan1972: 158, 160–161, figure 6a–i; Knight-Jones et al., Reference Knight-Jones, Knight-Jones and Llewellyn1974: 126–128, figure 9a–m; Knight-Jones et al., Reference Knight-Jones, Knight-Jones and Dales1979: 441.
Spirorbis regalis Bailey & Harris Reference Bailey and Harris1968: 172–174, figure 11a–j.
MATERIAL EXAMINED
Enewetak Atoll: Station 1, reef 8, January 1976, 30–35 m, coll. R. Brock (2 juveniles, BPBM R3546).
DIAGNOSIS
Tubes with transverse growth lines and sinistral coiling. Primary opercula with concave plates and small eccentric talon. Mature opercula with helmet-shaped calcified chamber with convex plate bearing several spines and incomplete rim with short spines. For a complete description see Vine et al. (Reference Vine, Bailey-Brock and Straughan1972) and Knight-Jones et al. (Reference Knight-Jones, Knight-Jones and Llewellyn1974).
REMARKS
The primary opercula of the two juveniles found agree well with descriptions of material from Hawaii (Vine et al., Reference Vine, Bailey-Brock and Straughan1972) and Australia (Knight-Jones et al., Reference Knight-Jones, Knight-Jones and Llewellyn1974).
DISTRIBUTION
Described from the Mediterranean (Thiriot-Quiévreux, Reference Thiriot-Quiévreux1965; Zibrowius, Reference Zibrowius1968) but reported to occur in the West Indies (Bailey, Reference Bailey1970), Malta, Mozambique, Angola, Cape Verde Islands (Knight-Jones et al., Reference Knight-Jones, Knight-Jones and Llewellyn1974). This species is broadly distributed in the Pacific Ocean including the Galapagos Islands (Bailey & Harris, Reference Bailey and Harris1968), Hawaii (Vine et al., Reference Vine, Bailey-Brock and Straughan1972), New Zealand (Vine, Reference Vine1977), Australia and Japan (Knight-Jones et al., Reference Knight-Jones, Knight-Jones and Llewellyn1974).
Spirorbis (Laeospira) koehleri—Zibrowius Reference Zibrowius1968: 197–198, pl. 12, figures 15–20, pl. 14, figure h.
Spirorbis (Pileolaria) koehleri—Bailey Reference Bailey1969: 373, figure 8a–l.
Pileolaria (Duplicaria) koehleri—Vine et al., Reference Vine, Bailey-Brock and Straughan1972: 161–163, figure 7a–j.
Pileolaria koehleri—Vine & Bailey-Brock Reference Vine and Bailey-Brock1984: 147.
Vinearia koehleri—Bailey-Brock Reference Bailey-Brock1987a: 436–437, figure 3.II.214a–c, figure 3.II.215a; Bailey-Brock Reference Bailey-Brock1987b: 288; Bailey-Brock Reference Bailey-Brock1985: 213, figure 15.
Spirorbis (Pileolaria) polyoperculatus Straughan Reference Straughan1969a: 151–153, figure 1a–c.
MATERIAL EXAMINED
Enewetak Atoll: Station 2, Elmers Pinnacle, September 1975, 10–15 m, coll. R. Brock (28, BPBM R3550). Kwajalein Atoll: Station 2, July 1975, coll. R. Brock (13, BPBM R3549). Rongelap Atoll: Station 1, July 1975, coll. R. Brock (34, BPBM R3547). Utirik Atoll: Station 3, near leeward reef on pinnacle, July 1975, 10-12 m, coll. R. Brock (12, BPBM R3548).
DIAGNOSIS
Tubes with one well-defined longitudinal ridge, transverse ridges and sinistral coiling (Figure 12A). Operculum composed of 2–4 concave plates interlocking by a peg-and-socket arrangement and also supported by lateral wings on talon (Figure 12B, C); brood chambers develop underneath opercular plates (Figure 12B). See Vine et al. (Reference Vine, Bailey-Brock and Straughan1972) as Pileolaria (Duplicaria) koehleri and Bailey-Brock (Reference Bailey-Brock1987a) for a full description and illustration of this species.

Fig. 12. Vinearia koehleri: (A) tube; (B & C) opercula with interlocking concave plates; (D) collar chaeta; (E) thoracic uncini; (F) abdominal uncini.
REMARKS
A few specimens from Rongelap Atoll presented up to 4 opercular plates but description from material found elsewhere reports the presence of only 2–3 opercular plates.
DISTRIBUTION
This species is widely distributed in the Mediterranean and Indo-West Pacific, including Hawaii (Vine et al., Reference Vine, Bailey-Brock and Straughan1972; Bailey-Brock, Reference Bailey-Brock1987a), Fiji (Bailey-Brock, Reference Bailey-Brock1985), Tonga (Bailey-Brock, Reference Bailey-Brock1987b) and newly recorded from Kwajalein, Utirik and Rongelap Atolls in the Marshall Islands.
DISCUSSION
Serpulid tubeworms are conspicuous components of polychaete faunas of west Pacific Islands. Twenty-five species are known from Palau and Yap, 24 from the Solomon Islands, 18 from Ponape, 13 from Truk, 13 from Majuro Atolls (Marshall Islands) and nine from the Gilbert Islands (Imajima & ten Hove Reference Imajima and ten Hove1984, 1986). We report 20 species from Enewetak (16 serpulines and four spirorbines), four from Kwajalein (two serpulines and two spirorbines), seven from Rongelap (five serpulines and two spirorbines) and 12 from Utirik Atolls (eight serpulines and four spirorbines). From countries further south, eight species were collected from both Fiji and Guam, 12 from Tonga (Bailey-Brock Reference Bailey-Brock1985, Reference Bailey-Brock1987b, Reference Bailey-Brock1999), although these records do not reflect a sampling effort solely for serpulids. A general trend of decreasing species richness from west to east, with increasing distance from the centre of the Indo-West Pacific region appears typical for serpulids as it is for other marine fauna (Kay & Palumbi, Reference Kay and Palumbi1987). The relationship of serpulids from southern Japan with the Indo-Polynesian province has been well documented (Imajima & ten Hove, Reference Imajima and ten Hove1984). Differences between serpulid faunas of Guam, New Caledonia and Marshallese atolls need to be substantiated with more collections from these locations.
The ornate tubes of many serpulids are conspicuous on reef rock, harbour structures and shallow bench habitats. The brightly coloured branchial crowns of the Christmas tree worm (Spirobranchus gardineri), emerge from tubes overgrown by live coral. Tubeworms are part of the diet of coral reef fish. Gut contents of chaetodontids (7 spp.), labrids (2 spp.) and a pseudochromid species from Okinawa contained 12–15% serpulid parts (Sano et al., Reference Sano, Shimizu and Nose1984). Guts of 6% of Canthigaster solandri (sharp-backed puffer) from Bikini and Enewetak contained serpulids (Hiatt & Strasburg, Reference Hiatt and Strasburg1960). E. Reese collected Chaetodon auriga guts at Enewetak which were full of Pomatostegus actinoceras opercula (Bailey-Brock, unpublished data). Operculate serpulids associated with live corals may deter coral predators. The coral eating sea star, Acanthaster planci avoids eating polyps adjacent to the tube openings of S. giganteus corniculatus (De Vantier et al., Reference De Vantier, Reichelt and Bradbury1986). These authors suggest that tube and opercular spines irritate the sea star causing it to move away and so preventing predation on the tubeworm and adjacent coral polyps. Tubeworms associated with live corals may have a competitive advantage over those living in cryptic habitats and being in a superficial position on coral heads is more beneficial for suspension filter feeding. In some cases these genera have evolved well-developed eyes and respond to shadows from fish passing over them, most likely predators, and spiny tubes and opercula that deter predators from feeding on their tissues.
ACKOWLEDGEMENTS
Dr R.E. Brock collected most of the specimens at all locations. He is grateful to the Energy Research and Development Administration (formerly Atomic Energy Commission) for travel funds. We are grateful to Sue Monden for the line drawings based on the sketches of J.H.B-B. Tina M. Carvalho helped with scanning electron microscopy analyses in the Biological Electron Microscope facility (University of Hawaii at Manoa). Holly Bolick (BPBM) kindly coordinated the deposition of voucher material. Three anonymous referees made valuable comments to this manuscript.















