INTRODUCTION
The family Conidae Fleming, 1822 is a group of tropical marine gastropods with a strong conical or biconical shell and a long aperture; their unusual attributes, as cited by Kohn (Reference Kohn2002), include the radula, the conotoxin, and the shape and structure of the shell. The family comprises over 550 species around the world (Filmer, Reference Filmer2001). In Brazilian waters, Rios (Reference Rios1994) listed 18 described species and 2 subspecies in the single genus Conus Linnaeus, 1758. In a recent review, Gomes (Reference Gomes2004) recognized 20 species of cone shells found off Brazil as valid.
Conus jaspideus Gmelin, 1791 is known from the Brazilian coast from Amapá to Rio de Janeiro, as well as Florida and the Caribbean region in the Western Atlantic. In his original description, Gmelin indicated a figure as the type; later, Clench (Reference Clench1942), in addition to redescribing the species, also designated as representation of a lectotype the figure illustration of Martini (Reference Martini1773: plate 55, figure 612c). In spite of the attempt of Vink (Reference Vink1991) to propose a neotype, the Clench lectotype indication has priority under the International Code of Zoological Nomenclature (ICZN). Only recently, Kohn & Vink (Reference Kohn and Vink2007) formally designated a neotype MHNG 16154, as well as a type locality, to avoid instabilities related to the nomenclature and biology of the species.
The present paper provides information on the shell, soft parts and radula of C. jaspideus. The soft parts of Brazilian specimens with imposex are illustrated for the first time, as well as the predation process in the laboratory.
MATERIALS AND METHODS
Shell
Shells stored at museums and specimens collected along the Brazilian coast were examined (see Examined Material). Shells and specimens (shell with soft parts) were measured and photographed with a Nikon Coolpix 4500 digital camera or by SEM. The main measurements used were total height, shell width, spire height, and body-whorl height (in mm). The number of whorls, coloration, sculpture, and the profile of the spire and body whorl were also examined.
Soft parts
Specimens collected along the Brazilian coast were preserved in 70% ethanol. The shell was broken and the visceral mass was observed. In the frontal view of the visceral mass, the anterior groove of the pedal gland and the foot sole are apparent. After removing the mantle, in the head–foot mass, the cephalic tentacles and the male genital system were studied. Because Gomes (Reference Gomes2004) observed that the penis morphology is a distinct character for discriminating the Brazilian species, a total of eight male and two imposex specimens stored at the MNRJ and available for anatomical study were dissected by standard techniques under a Zeiss SV 11 stereomicroscope. Drawings of the structures were made in order to supplement their description.
Radula
The radula was extracted from radular sac and preserved in 70% ethanol. The teeth were cleaned in KOH, washed in distilled water, isolated from each other on a glass slide, and covered with glycerol for later photography by optical microscopy (Zeiss Axiolab microscope). The same procedure was adopted for the SEM micrographs, except the teeth were placed on an aluminium stub and sputter-coated with gold. The photographs allowed the study of different parts of the tooth structure. Each tooth has three main parts: the apex, the anterior region of the tooth, which corresponds to the terminal end of the shaft that penetrates into the prey tissue; the base, the solid area of the posterior region of the tooth (Peile, Reference Peile1939); and the tooth shaft, the longest and cylindrical part of the tooth (Franklin et al., Reference Franklin, Fernando, Chalke and Krishnan2007)
Abbreviations: MHNG, Muséum d'Histoire Naturelle, Genève, Switzerland; MNRJ, Museu Nacional, Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ; MORG, Museu Oceanográfico ‘Prof. Eliézer de Carvalho Rios’, Fundação Universidade de Rio Grande (FURG), Rio Grande, RS; IB-UFRJ, Instituto de Biologia, Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil; collectors: NOAS, Navio Oceanográfico Almirante Saldanha, Marinha Brasileira; Eq. MORg, Museu Oceanográfico Prof. Eliézer de Carvalho Rios staff.
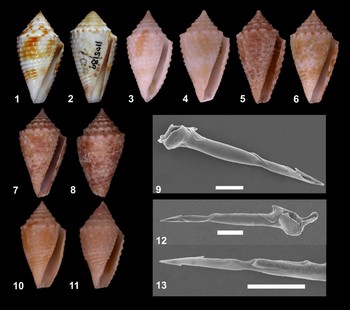
Plate 1. Fig. 1.Conus jaspideus Gmelin, 1791: neotype MHNG 16154. 25.0 × 13.0 mm. Fig. 2.Conus verrucosus Hwass in Bruguière, 1792: Type MHNG 1105/89. 26.8 × 14.0 mm. Fig. 3.Conus jaspideus from Brazil MNRJ 13732: 16.9 × 8.6 mm. Fig. 4.Conus jaspideus from Brazil MNRJ 13729: 15.2 mm. Fig. 5.Conus jaspideus from Brazil MNRJ 13730: 13.2 mm. Fig. 6.Conus jaspideus from Brazil MNRJ 13727: 19.6 × 11.5 mm. Figs 7–9.Conus jaspideus from Brazil MNRJ 13731: 18.5 × 10.0 mm. Fig. 7. Shell, ventral view. Fig. 8. Shell, dorsal view. Fig. 9. Radula tooth, SEM micrograph, scale bar: 50 µm.10–13: Conus jaspideus from Bahamas. Fig. 10. Shell MNRJ 13619: 14.6 × 7.8 mm. Figs 11–13. Shell MNRJ 13620: 12.7 × 6.3 mm. Fig. 12. Radula tooth, SEM micrograph, scale bar: 50 µm. Fig. 13. Radula tooth, SEM micrograph from first half showing both barbs and blade, scale bar: 50 µm.
Conus jaspideus Gmelin, 1791: 3387, No. 28; Kiener, Reference Kiener1848; Clench, Reference Clench1942; Warmke & Abbott, Reference Warmke and Abbott1961; Van Mol, Tursch & Kempf, Reference Van Mol, Tursch and Kempf1967; Abbott, Reference Abbott1974; Rios, Reference Rios1975; Domaneschi & Penna-Neme, Reference Domaneschi and Penna-Neme1984; Kohn, Reference Kohn1992; Rios, Reference Rios1994; Costa, Reference Costa1994; Rosenberg, Reference Rosenberg1996; Filmer, Reference Filmer2001; Redfern, Reference Redfern2001. Conus verrucosus Hwass in Bruguière, 1792: 708 (Figure 2); Reeve, Reference Reeve1842; Kiener, Reference Kiener1846; Kobelt, Reference Kobelt1878; Tryon, Reference Tryon and Tryon1884; Clench, Reference Clench1942; Lange-de-Morretes, Reference Lange-de-Morretes1949; Costa, Reference Costa1994; Filmer, Reference Filmer2001.
TYPE MATERIAL
Neotype MHNG 16.154. 25.0 × 13.0 mm (Figure 1) designated by Kohn & Vink (Reference Kohn and Vink2007).
TYPE LOCALITY
Off Mono Island, Trinidad (Kohn & Vink, Reference Kohn and Vink2007).
DISTRIBUTION
Florida, West Indies, Brazil, from Amapá to Rio de Janeiro; 60 m depth.
COMPARATIVE MATERIAL EXAMINED
Conus jaspideus Gmelin, 1791. Neotype MHNG 16.154. 25.0 × 13.0 mm.
Conus verrucosus Hwass in Bruguière, 1792. Type (probable) MHNG 1105/89. 26.8 × 14.0 mm.
Bahamas: MNRJ 13620, 1 specimen, 3 m depth, 4 July 1994, Colin Redfern don.; Treasure Cay: MNRJ 13621, 1 specimen, 0.3 m depth, 20 July 1999, Colin Redfern don.
Brazil, Amapá: Off, MORG 21601, 2 shells, ‘Riobaldo’ col., February 1981, 80 m depth; MORG 14364, 4 shells, NOAS col., 21 September 1968, 76 m depth; MORG 14828, 7 shells, 4 May 1968, NOAS col., 86 m depth; Caviana, Off, MORG 19415, 6 shells, NOAS col., November 1968, 47 m depth; Cassiporé, Off, MORG 19433, 5 shells, NOAS col., November 1968, 47 m depth. Maranhão: Off, MNRJ 13730, 2 specimens, 9 November 2008, 75 m depth; MNRJ 13725, 2 shells, 21 November 2008, 51 m depth; MNRJ 13727, 1 specimen, 9 December 2008, 75 m depth; MNRJ 13728, 2 shells, 21 November 2008, 46–48 m depth; MNRJ 13729, 4 specimens, 22 November 2008, 62–64 m depth; MNRJ 13731, 2 specimens, 9 November 2008, 75 m depth; MNRJ 13732, 2 shells, 22 November 2008, 33 m depth; Apiu, Off, MORG 14687, 4 shells, NOAS col., 6 November 1967, 51 m depth; Parcel Manuel Luís, MNRJ 13726, 2 shells, 21 November 2008, 49 m depth. Rio Grande do Norte: Rio do Fogo, MORG 19312, 2 shells, J.J. Frota–Pacamon col., 45 m prof., May 1977; Cabo de São Roque, Off, MORG 13070, NOAS col., 13 April 1968, 39 m depth, 2 shells. Ceará: Fortaleza, MNRJ 3589, 5 shells, 1964; Off, MORG 34598, 1shell. Atol das Rocas, MNRJ 4305, 3 shells, J.H. Leal col., C.B. Castro don. Pernambuco: MORG 24730, 2 shell, Maurício col., 1985; Olinda: MORG 5719, 3 shells. Alagoas: MORG 11118, 2 shells, barco de pesca ‘Akaroa’ col., L. Pontes don., 7 December 1965, 22–40 m depth; Off: MORG 11117, 5 shells, ‘Akaroa’ col., 7 December1965, 22–40 m depth; Maragogi, MORG 31451, 5 shells, March 1985; Paripueira, MNRJ HSL5904, 1 shell, H.S. Lopes col., November 1958. Bahia: MORG 34596, 1 shell; MORG 7320, 2 shell, Dr Bryan col., 1960, Abbott don.; MORG 7321, 9 shells, Dr Bryan col., 1960; MNRJ 8482, 4 shells; MORG 8018, 6 shells, 1962, 23 m depth; Arembepe, MORG 13759, 2 shells, S. Paes col., November 1968; Garapuá, MNRJ 9754, 6 specimens, P.M.S. Costa col., 2003; MNRJ 15020, 18 specimens, P.M.S. Costa col., December 2008; Boipeba, MNRJ 9519, 20 specimens, Eq. Zoologia, July 1977; Salvador, MORG 12770, 5 shells, D. Mendonça col., November 1964; MORG 41427, 27 shells, G. Oliveira col., October 1983; MORG 13774, 9 shells, D. Mendonça col., 1965, E.C. Rios don.; MORG 10561, 8 shells, A. Camargo col., 1964, E.C. Rios don.; Itapuã, MNRJ 6634, 2 shells, Amâncio col.; MNRJ 8465, 12 shells, H.S. Lopes col., May 1951; MORG 15197, 8 shells, E.C. Rios col., 17 July 1967; MORG 12349, 1 shell, S.G. Paes & E.C. Rios col., 17 July 1967; MNRJ HSL5651, 16 shells, H.S. Lopes col., May 1951; Barra, MORG 24743, 9 shells, G.S. Pomponet col., September 1986; Itaparica, MORG 24217, 9 shells, 23 May 1986, Trinchão; Mar Grande, MNRJ HSL5654, 5 shells, 9 June 1951; Coroa da Penha, MORG 21939, G. Oliveira col., March 1982, 10 shells; Banco Panela, MORG 10116, 3 shells, Pierret–Tursch col., 1963, 20 m prof.; Ilha da Maré, MNRJ HSL4990, 2 shells, A. da Silva col., 1957; MORG 31926, 10 shells, G. Oliveira col., March 1982; MNRJ 8464, 3 shells, H.S. Lopes col., May 1951; Paraguassú, MNRJ HSL4950, 1 shell, De Fiore col., 2 February 1954; Belmonte, Off, MORG 13802, 4 shells, NOAS col., September 1968, 48 m depth; Salinópolis, Off, MORG 19477, 2 shells, NOAS col., April 1968, 33 m depth; MORG 13684, 7 shells, NOAS col., 26 April 1968, 27 m depth; MORG 13153, 4 shells, NOAS col., 26 April 1968, 50 m depth; MORG 13171, 6 shells, 26 April 1968, NOAS col., 31 m depth; MORG 13815, 10 shells, NOAS col., 26 April 1968, 27 m depth; Abrolhos, MORG 20118, 30 shells, Eq. MORG col., February 1978; Parcel das Paredes, MNRJ 9734, 2 shells, 5 February 2003; MNRJ 9699, 34 shells, 5 February 2003; MORG 23251, 1 shell, Eq. Morg col., January 1985; Coroa Vermelha, MORG 23296, 3 shells, Eq. MORG col., February 1985, 1 m depth; Ilha Guarita, MORG 26853, 4 shells, L. Laurino & A. Silveira col., February 1987, 5 m depth, Silveira & Laurino don. Espírito Santo, Rio Doce, Off: MORG 19510, 2 shells, NOAS col., September 1967, 57 m depth; Guarapari: MORG 12600, 5 shells, E.C. Rios col., January 1960; Aracruz, MORG 29041, 2 shells, V. Abud col., 1 August 1988, R. Cruz don.; MNRJ 978, 1 shell, M.H. Loureiro col., 22 October 1945; Santa Cruz, Off, MORG 14135, 4 shells, NOAS col., 12 September 1968, 42 m depth. Rio de Janeiro: Búzios, MORG 22628, 17 shells, L.C. Araújo col., 1973; MORG 23736, 4 shells, L.R. Tostes col., 15 August 1985; MORG 12807, 5 shells, D. Mendonça col., July 1966, E.C. Rios don.; MORG 19158, 5 shells, L.R. Tostes col., February 1997; Manguinhos, MNRJ 8466, 3 shells, A. Coelho & S. Ypiranga col., January 1960; Saco da Ferradura, MNRJ 2879, 3 shells, A. Coelho & S. Ypiranga col., January 1960; Cabo Frio, MNRJ 1153, 2 shells, E.A. Martins col., 28 July 1950; MORG 38986, 6 shells; MNRJ 1984, 2 shells, 8 July 1956; Praia do Forte: MNRJ 1973, 1shell, 8 July 1956; MNRJ HSL5905, 2 shells; Arraial do Cabo, MNRJ HSL5652, 12 shells, May 1951. 1°43′S 048°18′W: MORG 13207, 3 shells, NOAS col., 1 May 1968, 56 m depth 01°45′S 048°18′W, MORG 13206, NOAS col., 56 m depth, 3 shells. Norte-Nordeste No. 1906, IB-UFRJ 707, NOAS col., 1967, 1 shell.
RESULTS
Shell
Shell of medium size (28.0 × 15.0 mm/height × width), obconic (spire height 27.3% of total shell height), with 10 whorls. Protoconch translucent, globose, up to two whorls. Spire high, outline straight and shouldered (shoulders of spire downwards-directed); in apical view, spire whorls after protoconch, sculptured with curved axial lines. Colour white, orange, or brownish with long spiral dark-brown or violet spots, regularly distributed on each whorl. Body whorl outline concave, sculptured with spiral cords (granulated or not), interspaces narrower than cords and sculptured with axial lines; dark-brown spots may form a zigzag pattern or only alternate with the white spaces; and some shells have dark-brown spiral dots. Aperture wider near base; outer lip straight, with anterior portion descendent in relation to body whorl.
Soft parts
Head–foot complex: tonality grey with white or black dots that group to form white or black patches, in no defined pattern. Mantle border smooth and thickened. Foot sole with fold and ventral pedal gland in females, in mid-anterior region near groove of pedal gland. Cephalic tentacles short and thickened. Anterior digestive system: radular sac small and thin-walled; venom gland measuring around 22 mm; muscular bulb small, elongated, measuring around 3 mm. Setae of polychaetes present in rectum of one specimen. Genital system: male, penis long and large, slightly flattened, curved at base, basal region with evident fold; pleats present, and apex pointing toward dorsal region of animal (Figure 14); calibre of penis decreasing towards apex. Apex with penial papilla where the penial duct opens.

Plate 2. Fig. 14. MNRJ 9519 Conus jaspideus from Brazil. Head–foot mass, male, scale bar: 5 mm. Fig. 15. MNRJ 9519 Radula tooth, optical microscopy showing structures (400×). Fig. 16. MNRJ 9519, Conus jaspideus from Brazil. Head–foot mass with imposex, scale bar: 5 mm.
Imposex
Genital system: female, penis very short and narrow, curved at base, basal region with no evident fold. Apex rounded, pointing to posterior ventral region of the animal (Figure 16). No evidence of penial duct. Presence of albumen gland, capsule gland and oviduct.
DISCUSSION
Specimens of C. jaspideus Gmelin, 1791 were compared to the neotype MHNG 16.154 designated by Kohn & Vink (Reference Kohn and Vink2007). The specimens examined were collected along the coast of Brazil from the States of Amapá to Rio de Janeiro. The examined material included two types of shells, with the spiral cords granulated or not. The shell is obconic with 10 whorls, white, orange, or brownish with long spiral dark-brown or violet spots, regularly distributed on each whorl; body whorl sculptured with spiral cords and interspaces with axial lines. Gmelin's original description consisted of one line; later, Clench (Reference Clench1942: 10) provided a more consistent description including the shell outline, colour pattern, aperture, sculpture and periostracum. The shell description presented herein adds information on the protoconch (type and number of whorls), spire sculpture in apical view (curved axial lines), sculpture of body whorl (spiral cords granulated or not) and anterior portion of outer lip descendant in relation to body whorl.
It is usual to find both types of shells, with spiral cords granulated or not, in the same geographical area. Rios (Reference Rios1994) assigned to the Brazilian coast C. jaspideus Gmelin, 1791 and the subspecies Conus jaspideus verrucosus Hwass, 1792. Costa (Reference Costa1994) observed both smooth and granulated shells of C. jaspideus, and according to this author, the smooth pattern dominates in this species. Herein Conus verrucosus Hwass in Bruguière, 1792 is considered a synonym of C. jaspideus Gmelin, 1792, with a granulated pattern, as previously stated by Filmer (Reference Filmer2001). In any event, the smooth pattern is related to the wear of the shell surface. When two individuals were examined that represented the extremes of variation, i.e. one shell smooth and another completely granulated, the tendency was to consider them as distinct species. The Brazilian material included shells with both types of ornamentation, all smooth and all granulated; as well as shells that were partly smooth or granulated, which we understand to be an intermediate pattern. Abbott (Reference Abbott1958) observed that in shells of C. jaspideus from Grand Cayman Island, the granulated pattern was associated with turbulent environments, and the smooth pattern was associated with protected areas. Coomans (Reference Coomans1973) stated that the occurrence of granulated shells is not related to geographical province, but can occur in the Indo-Pacific, West Indies, or the Panamanian Province, and the granules must be more closely associated with genetic mutations than with environmental changes.
During the study of Brazilian specimens, I had the opportunity to examine the soft parts of two Bahamian male specimens (Figure 17). These had the same morphology of head–foot mass and penis as all C. jaspideus with soft parts examined from Brazil (Figure 14). The penis is long and large, curved at the base, with the apex pointing toward the dorsal region of the animal, and with a penial papilla. The examination of the anatomy of the head–foot mass in C. jaspideus revealed a distinct penis, differing in shape, size and width from that of C. pusio Hwass in Bruguière, 1792 (Figure 18), a species whose shell could be confused at first glance with the shell of C. jaspideus in Brazil. Although C. pusio occurs from the eastern Caribbean to the Brazilian coast, most of the material collected in Brazil came from Bahia, Espírito Santo and Rio de Janeiro. Costa (Reference Costa1994) studied the ‘C. jaspideus complex’ and illustrated differences in the two species C. jaspideus and C. pusio, based on shell and penis morphology. This latter character, distinct in the two species, is important because it contributes to reproductive isolation between co-occurring populations. Strong (Reference Strong2003) dissected an immature male of C. jaspideus for a study of Caenogastropoda phylogeny, and reported the position of the gonad, vas deferens, prostate and the penial duct opening at the tip of the penis, but did not provide information on penis morphology. Gomes (Reference Gomes2004) observed that the penis morphology is a distinct character in most Brazilian species; it has different lengths (in relation to the head-foot mass), calibres, apex and fold locations.
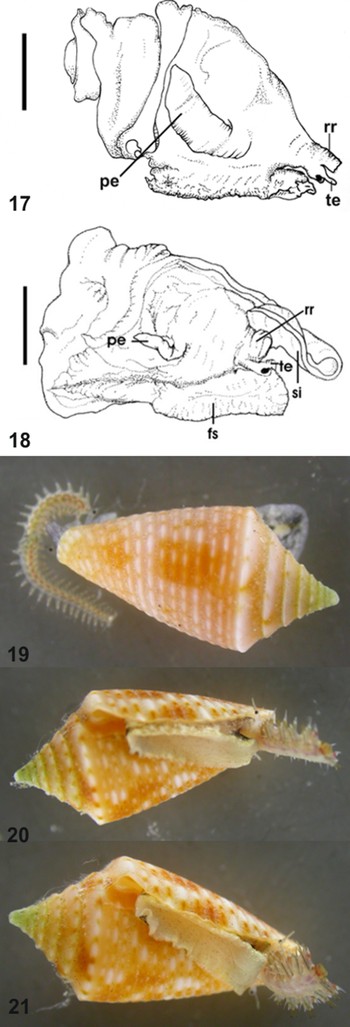
Plate 3. Fig. 17. MNRJ 13621 Conus jaspideus from Bahamas. Head–foot mass, male, scale bar: 1 mm. Fig. 18. MNRJ 9749 Conus pusio Hwass in Bruguière, 1792. Head–foot mass, male, scale bar: 2.5 mm. Figs 19–21. MNRJ 9519 specimen alive: 16.1 mm (orange shell).

Plate 4. Figs 22–25. MNRJ 9519 specimen alive: 18.5 mm (dark brown spots shell). fs, foot sole; pe, penis; pg, anterior groove of foot gland; rr, rostrum; si, siphon; te, cephalic tentacles. Photograph by Paulo Márcio Costa.
The importance of the radula was reported by Peile (Reference Peile1939) as a good guide for classification because of the specific character of the structure. Lim (Reference Lim1969) associated the food habit (vermivorous, molluscivorous or piscivorous) to the radula morphology, as did Rolán & Röckel (Reference Rolán and Röckel2000) for endemic Angolan species of Conus. Franklin et al. (Reference Franklin, Fernando, Chalke and Krishnan2007) studied Conus from India and noticed that even species showing the same food habit also show fine differences in radular structures, and proposed that these structures would be useful to discriminate species in case of ambiguity in other characteristics. The radula tooth of C. jaspideus is characterized by the presence of a first and second barb on the apex, a blade at the beginning of the posterior shaft region, and the base oval with sharp spur. In the case of C. jaspideus, the radula morphological pattern (short tooth, apex with barb, presence of waist, absence of well-developed blades) indicates the vermivorous habit. The radula tooth was photographed by SEM and optical microscopy. In addition to the usual micrographs, the photographs made by optical microscopy allowed the examination of the interior parts of the radula because of its transparency. Both techniques are important to visualize the structures, improving the descriptions as previously reported by Franklin et al. (Reference Franklin, Fernando, Chalke and Krishnan2007), studying the radula of Indian Conus. Rios (Reference Rios2009) attempted to illustrate a C. jaspideus radula tooth, but there was no evidence of the first barb, which emphasizes the importance of using more than one photographic method to record morphological data.
As well as the character of the male genitals, the radula morphology of Brazilian C. jaspideus (Figures 9 & 15) is the same as that of the Bahamian radula illustrated (Figures 12 & 13).
Two specimens with imposex (masculinization of the female) were examined, from two different locations in the State of Bahia: one collected at Boipeba in 1977 (MNRJ 9519) and another at Garapuá in 2003 (MNRJ 9754). The masculinization of females can lead to reproductive failure and to the extinction of populations (Gibbs & Bryan, Reference Gibbs and Bryan1986); sterility in Conidae was reported by Shi et al. (Reference Shi, Huang, Zhu, Yu and Xie2005).
The first evidence of imposex in the family was reported by Kohn & Almasi (Reference Kohn and Almasi1993) for six species from Western Australia. These authors considered that the imposex was caused by tributyltin (TBT) in anti-fouling paint, which was allowed in Western Australia at the time of the study. Additional evidence of the influence of TBT on masculinization of females in molluscs was reported by Axiak et al. (Reference Axiak, Vella, Micallef, Chircop and Mintof1995), Castro et al. (Reference Castro, Meirelles, Matthews-Cascon and Fernandez2004) and Horiguchi (Reference Horiguchi2006). Most species in which imposex have been observed belong to the family Muricidae Rafinesque, 1815, which makes this group the one most commonly used as bioindicators to evaluate contamination by organotin compounds worldwide (Castro et al., Reference Castro, Braga and Rocha-Barreira2005). Despite evidence of imposex associated with marine pollution, in Brazil Caetano & Absalão (Reference Caetano and Absalão2003) also reported the phenomenon in unpolluted waters, for the family Olividae Latreille, 1825. In our case, the locations of the specimens at Boipeba and Garapuá, which are both areas that are isolated from shipping, suggest that the masculinization of the female was a natural occurrence.
Although the morphological expression of imposex shows diverse stages and types (Shi et al., Reference Shi, Huang, Zhu, Yu and Xie2005), this minor record represents the first evidence of imposex for the species and for the family in Brazilian waters. Fioroni et al. (Reference Fioroni, Oehlmam and Stroben1991) suggested an imposex classification to quantify the morphological diversity in different stages. Their proposal was later adapted by Oehlmann (Reference Oehlmann, Stroben and Fioroni1991) and Stroben (Reference Stroben, Oehlmann and Fioroni1992), and is now used for most prosobranch imposex descriptions (Shi et al., Reference Shi, Huang, Zhu, Yu and Xie2005).
The availability of two specimens with soft parts, one of them preserved for over 30 years, allowed me to verify the presence of an atrophied penis (Figure 16) in the head–foot mass; the foot sole with a ventral pedal gland; and the female genital system with an albumen gland, capsule gland, and oviduct. A greater number of specimens are required for further investigation, especially considering that the imposex character shows intra-specific, inter-specific and geographical differences (Fioroni et al., Reference Fioroni, Oehlmam and Stroben1991). Even the classification in four different stages mentioned by Shi et al. (Reference Shi, Huang, Zhu, Yu and Xie2005) for most prosobranchs, sometimes fails to distinguish the imposex stage in some species, which has led some authors to develop specific schemes for each species (Barreiro et al., Reference Barreiro, Quintella and Ruiz1999; Shi et al., Reference Shi, Huang, Zhu, Yu and Xie2005)
Observations of living specimens of Conus in aquaria, including photographs of feeding, began long ago (Kline, Reference Kline1956; Kohn, Reference Kohn1956), and even now still aid in feeding studies (Kantor, Reference Kantor2007). Recent experiments (Kohn, Reference Kohn, Wells and Jones2003, Reference Kohn2008; Kantor, Reference Kantor2007) have investigated the size of the prey that Conus can ingest, the possible sites of digestion, and the capacity for multiple radula attacks. Here, for the first time, Brazilian specimens of C. jaspideus captured and kept in an aquarium are illustrated. The specimens were isolated in Petri dishes with live polychaetes and photographed (Figures 19 & 20). The process of ingestion (from the moment of striking to when the prey was completely engulfed) lasted approximately 18 minutes for each specimen. The experiment was carried out under daylight, and Conus only responded to live polychaetes.
CONCLUSIONS
Considering that C. jaspideus is such a controversial taxon, partially because of the lack of information at the time of its description and of its wide distribution in the tropical western Atlantic and Caribbean region, the aim of this study was to add data for the species on the Brazilian coast. New observations of shell morphology improved the description of Clench (Reference Clench1942). Illustrations of the head–foot mass revealed differences in penis morphology of a C. jaspideus male and an imposex female, and also allowed the comparison of the structure in C. pusio. Different photographic techniques helped in the description of the radular morphology and in illustrating the radular tooth of a Brazilian specimen. Laboratory experiments provided photographs of the predation process in C. jaspideus.
The similarity of radula and penis morphology between the Brazilian and Bahamian specimens suggests that this taxon indeed has a continuous distribution. Further investigations are required to determine if C. jaspideus is a widely distributed species or a complex of species.
ACKNOWLEDGEMENTS
I thank Fred G. Thompson (FMNH) for the loan material; Dr Alan Kohn (University of Washington) for helpful comments and for sending important papers; Dr Paulo Márcio Costa (MNRJ) for shell pictures; Dr Arnaldo Campos dos Santos Coelho and Dr Norma Campos Salgado (both MNRJ) for advice; Dr Janet Reid for English review; Dr Yves Finet (MHNG) for type material photographs; Dr Franklin Noel dos Santos (UFPA) for providing material with soft parts; Colin Redfern for manuscript review and specimens donation; Elivaldo de Lima for SEM micrographies from ‘Centro de Microscopia Eletrônica de Varredura’, Museu Nacional/UFRJ (supported by CENPES/PETROBRAS). Financial support: CAPES and UFRJ.






