INTRODUCTION
The biological consequences of global warming are already affecting species physiology, distribution and phenology, and this will inevitably alter competitive and other interactions between species, which in turn will affect local abundance and geographic ranges (Hughes, Reference Hughes2000). During the 20th century, increasing [CO2]atm has driven an increase in the global oceans’ average temperature of 0.74°C and in acidity by 0.1 pH unit (Solomon, Reference Solomon2007). Ocean acidification (OA) is increasingly recognized as a major global problem and is rapidly changing the carbonate system of the world's oceans. Past mass extinction events have been linked to OA and the current rate of change in seawater chemistry is unprecedented (Beerling & Berner, Reference Beerling and Berner2002). The combustion of fossil fuels has enriched levels of CO2 in the world's oceans and decreased ocean pH (Talmage & Gobler, Reference Talmage and Gobler2010).
This altered seawater chemistry has been far more rapid than the Earth has previously experienced (Kurihara, Reference Kurihara2008). The CO2-driven OA leads to a decrease in the calcium carbonate (CaCO3) saturation state of the ocean surface waters and has potentially negative impacts on marine taxa, particularly those that build skeletons, shells and biogenic calcium carbonate tests, commonly known as calcifiers (Guinotte & Fabry, Reference Guinotte and Fabry2008; Kurihara, Reference Kurihara2008). Several analyses have used molluscs as a study model to understand the effects of ocean acidification (Narita et al., Reference Narita, Rehdanz and Tol2012), yet these studies are still scant and results cannot be generalized. In this respect, OA may be inhibiting the development and survival of larval shellfish and contributing to global declines of some bivalve populations (Talmage & Gobler, Reference Talmage and Gobler2010). In addition, several experiments have shown a decrease in growth, calcification, survival, development and abundance of organisms at decreased pH levels, in response to OA across a broad range of marine organisms (Gazeau et al., Reference Gazeau, Gattuso, Dawber, Pronker, Peene, Peene, Heip and Middelburg2010; Kroeker et al., Reference Kroeker, Kordas, Crim, Hendriks, Ramajo, Singh, Duarte and Gattuso2013).
Body size is probably the most studied trait in ecological and evolutionary literature, because it has profound consequences on the structure and function of natural systems (Schmidt-Nielsen, Reference Schmidt-Nielsen1984). Studying spatial and temporal patterns of variation in body size is therefore crucial to understanding biotas on ecological and evolutionary timescales. Bergmann's rule is a classic conceptual framework for geographic variations in body size, and predicts that body size increases towards polar regions (Meiri, Reference Meiri2011). In a recent review of Bergmann's rule by Watt et al. (Reference Watt, Mitchell and Salewski2010), an overview of 57 studies was carried out. Twenty of the 57 studies were on ectothermic organisms (vertebrates, invertebrates, protozoans and plants) and only nine of these 20 corresponded to invertebrates, of which only one marine invertebrate, a snow crab from the northern hemisphere, was included. Of these nine studies, four followed Bergmann's rule and the other five did not. Indeed, Bergmann's rule remains poorly described for marine invertebrates (Berke et al., Reference Berke, Jablonski, Krug, Roy and Tomasovych2013). Given that Bergmann's rule was originally proposed for endotherms, its use with ectotherms has been criticized; yet the name still prevails, either by inertia or tradition (Heinze et al., Reference Heinze, Foitzik, Fischer, Wanke and Kipyatkov2003; Fisher et al., Reference Fisher, Frank and Leggett2010; Berke et al., Reference Berke, Jablonski, Krug, Roy and Tomasovych2013). Here we use the term ‘Bergmann's pattern’ to refer to the existence of a latitudinal increase in body size towards higher latitudes, irrespective of the driving mechanisms.
As size and temperature increased, growth became increasingly constrained due to oxygen shortage in aquatic and water-saturated habitats (Atkinson, Reference Atkinson1994). In this respect, high temperatures may impose constraints on growth that only arise later on during ontogeny; this simple and potentially general explanation is supported by the fact that thermal optima for growth efficiency and growth rate decrease as individuals grow (Angilletta & Dunham, Reference Angilletta and Dunham2003). Ultimately, higher temperatures result in faster growth to a smaller final size. In other words, most ectotherms mature at a larger size at lower developmental temperatures. This pattern of wide taxonomic prevalence has been coined the temperature-size rule.
Trophon geversianus (Pallas, 1774) is a common and widespread inhabitant of intertidal and shallow subtidal environments. On the south-west Atlantic coast this species is reported from 35°S to 56°S (including the Malvinas/Falkland Islands), while on the south-east Pacific coast it ranges from 42°S to 56°S (Griffin & Pastorino, Reference Griffin and Pastorino2005; Pastorino, Reference Pastorino2005). This species is dioecious, with internal fertilization and intra-capsular embryonic development (Zaixso, Reference Zaixso1973; Penchaszadeh, Reference Penchaszadeh1976). For this genus, planktonic larvae are not known (Pastorino, Reference Pastorino2005), neither is there evidence of external sexual dimorphism, and the sex ratio is different from 1:1 (with a bias towards females) (Cumplido et al., Reference Cumplido, Averbuj and Bigatti2010). The shell size (up to 100 mm) is extremely variable, as can be appreciated from the large number of names proposed for the different morphological variants of this species (Pastorino, Reference Pastorino2005). In Southern Chile, this carnivorous gastropod is also one of the commercially harvested benthic species because productivity is significantly correlated with body size (P/B ratio) (Andrade et al., Reference Andrade, Montiel and Quiroga2009).
Here, we used death assemblages (sets of dead, taxonomically identifiable organic remains that are encountered in still largely unburied form on landscapes and seafloors, Kidwell & Tomasovych, Reference Kidwell and Tomasovych2013) as a source of biological information whose most important feature is that they are time-averaged, temporally coarse accumulations in which non-contemporaneous individuals co-occur. The living assemblages can vary from year to year due to vagaries of recruitment and mortality, so the death assemblages provide a better record of the organisms that usually inhabit an environment. Therefore, molluscan death assemblages capture a strong signal of relative abundance of living species (Kidwell, Reference Kidwell2002).
The aim of this study is to assess patterns and possible processes driving latitudinal variation of body size in the gastropod Trophon geversianus, and in particular to: (a) evaluate the existence of a poleward increase in body size and identify conformance to Bergmann’s rule or the temperature-size rule and (b) determine the role of oceanographic variables as drivers of the latitudinal trends in body size.
MATERIALS AND METHODS
Study area
The Argentinean coast is an interesting case study given its extension towards polar latitudes and the presence of two different biogeographic provinces: the Argentine and the Magellanic. The different physiographic characteristics allow the distinction of movable sandy bottoms in the former, and gravel bottoms where large algae grow in the latter. Climatic differences explain the prevalence of northern winds in the Argentine province, where warm and temperate-cold coastal waters alternate, while in the Magellanic province there are strong westerly winds with a predominance of subantarctic waters from the Malvinas Current. These factors explain the faunal composition differences between provinces: the Argentine is characterized by the considerable heterogeneity of its components and the Magellanic by its homogeneity and endemic taxa (Balech & Ehrlich, Reference Balech and Ehrlich2008). The boundary between these provinces varies seasonally between 41°S and 43°S with the northward fluctuations of the cold Malvinas Current in the winter (Balech & Ehrlich, Reference Balech and Ehrlich2008). In this respect, the Patagonian coast provides an opportunity to collect and examine mollusc shell assemblages due to their relative abundance and high preservation potential (Gordillo et al., Reference Gordillo, Bayer, Boretto and Charó2014). Empty shells, mostly bivalves and gastropods, are found along the coast on the active beach and in adjacent deposits in marine terraces (Gordillo & Archuby, Reference Gordillo and Archuby2014).
Data collection and analyses
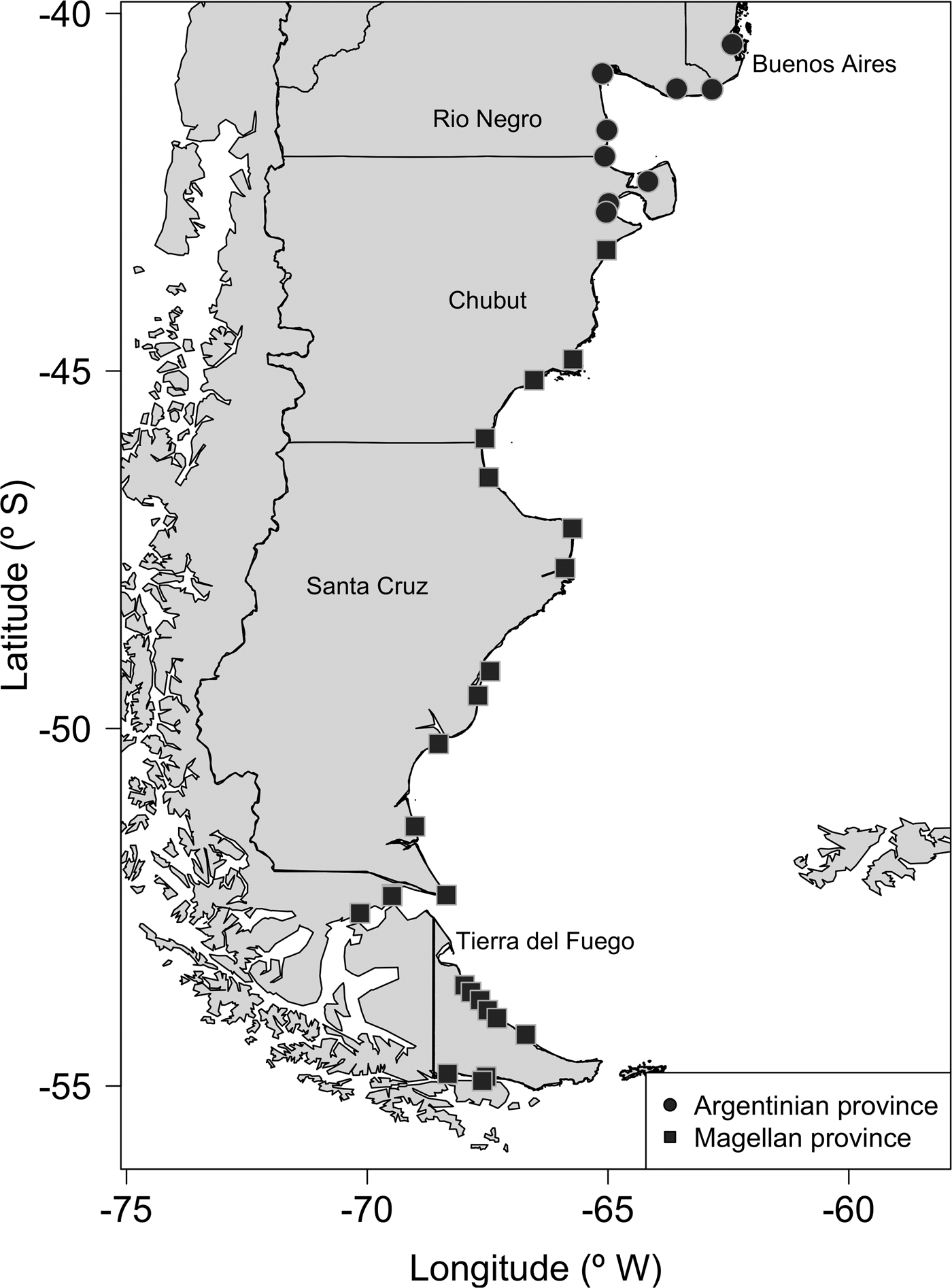
Biological data were obtained from field surveys in 34 beaches (Table 1) along the Argentinean coast between Buenos Aires (Los Pocitos) and Tierra del Fuego (Beagle Channel) (Figure 1), encompassing ~1600 km. At each beach all the dead shells of Trophon geversianus deposited above the high tide sea level were collected. Unlike modern living assemblages, death shell assemblages are time averaged (i.e. they include specimens spanning from a few years to hundreds of years; Behrensmeyer et al., Reference Behrensmeyer, Kidwell and Gastaldo2009), which allows us to include multiple cohorts, thus increasing the robustness of body size estimates. Death shell assemblages potentially contain individuals at various ages including both immature and mature ones. Ideally, size should be compared among individuals at the same age. For that purpose extensive sclerochronological data would be required, which is far beyond the scope of this study.

Fig. 1. Map of the study region, showing the sampled sites.
Table 1. Detail of the 34 study sites (N = 1348). A indicates Argentine province and M corresponds to the Magellanic province. The asterisk indicates the Transition Zone (41°S–43°S) between the Argentine and Magellanic biogeographic provinces.

Taphonomic processes such as fragmentation, dissolution, abrasion and bioerosion can affect shells’ traits. However, most of the shells retain the usual ornamentation suggesting that abrasion and bioerosion were negligible. Other taphonomic attributes were taken into account and minimized when possible, such as fragmentation since only entire (unbroken) shells were used in the analyses. Shell length was measured along an axis passing through the apex to the bottom of the shell with a digital caliper (precision 0.01 mm) and shells were cleaned and weighed using a digital scale (precision 0.01 g). All analyses were performed on log-transformed data given the great spatial variability in the dataset.
For each site climatological information was compiled on five oceanographic variables that could potentially be related to body size: (a) sea surface temperature, (b) concentration of chlorophyll a, (c) concentration of oxygen, (d) concentration of calcite, (e) pH and (f) wave height. For variables a–e, information corresponds to long-term climatological annual means, and was obtained from rasters created by the BioOracle database (Tyberghein et al., Reference Tyberghein, Verbruggen, Pauly, Troupin, Mineur and De Clerck2012, available at http://www.oracle.ugent.be/), with a resolution of 9.2 km. Wave height was included as a proxy of wave exposure along the study area, and data were obtained from the Hexacoral/LOICZ database (http://hercules.kgs.ku.edu/hexacoral/envirodata/hex_modfilt_firststep3dev1.cfm) at 0.5° grid cell resolution.
The relationship between different body size proxies and oceanographic variables was analysed. Two body size proxies were used: (a) mean shell length and (b) relative weight (cubic root of shell weight/shell length). These values were associated to oceanographic variables using a Pearson moment-product correlation. In order to reduce bias introduced by spatial autocorrelation, a frequent problem in ecological data affecting the significance rates of the statistical analyses (Dale & Fortin, Reference Dale and Fortin2009), P-values were corrected for spatial structure using Dutilleul's method. Analyses were carried out using SAM freeware application (Rangel et al., Reference Rangel, Diniz-Filho and Bini2010).
RESULTS
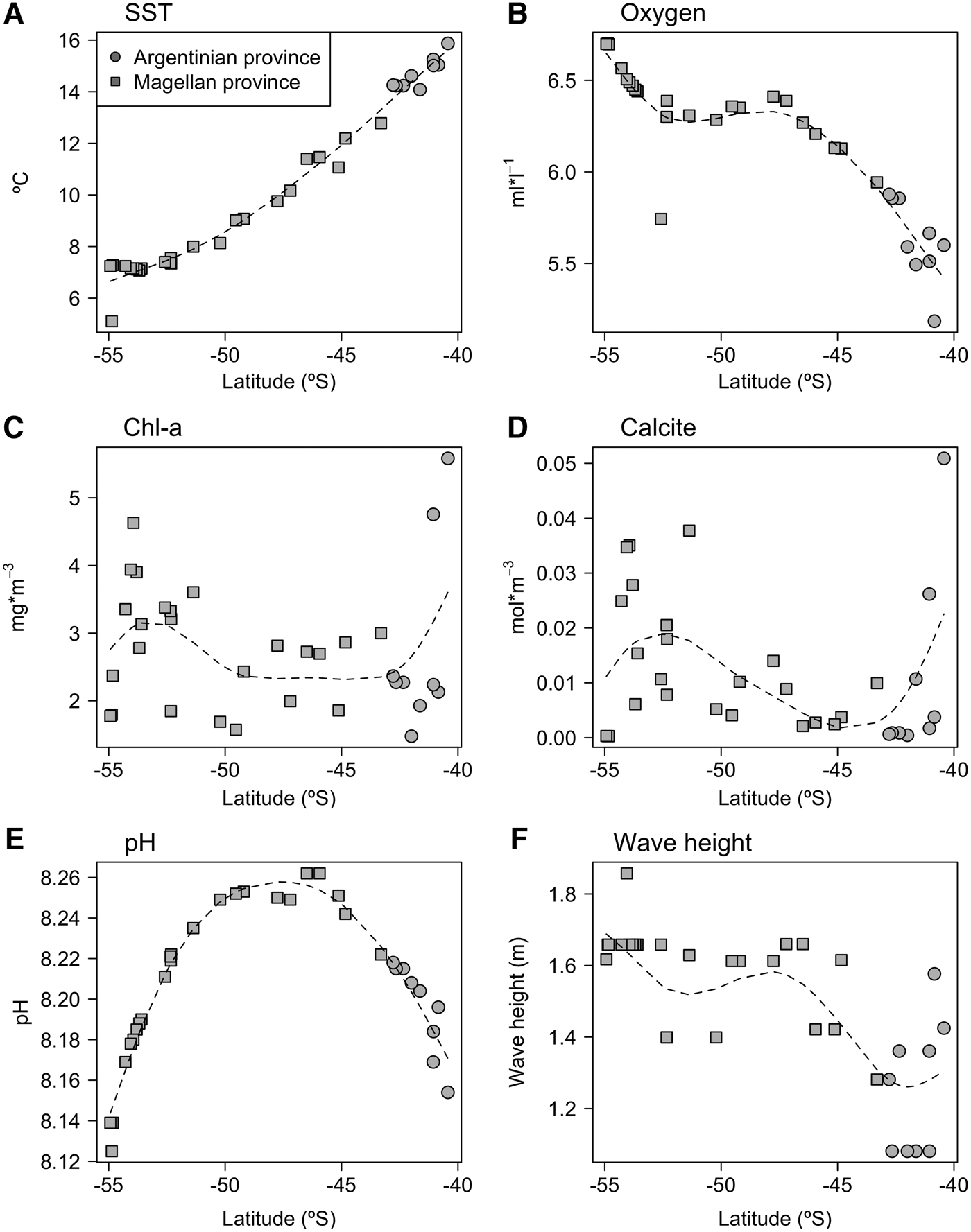
Oceanographic variables show differing latitudinal trends (Figure 2); sea surface temperature varied ~2-fold, decreasing by around 7°C along the latitudinal gradient. The concentration of oxygen increased towards higher latitudes, particularly around the Tierra del Fuego archipelago (52°–54°S). Both chlorophyll a and calcite concentration were much more variable, with lower values observed around mid-latitudes and increasing towards the north and south. In contrast, pH showed a hump-shaped latitudinal pattern with maximum values found around 46°S, declining towards the Buenos Aires and Tierra del Fuego provinces. On the other hand, wave height increased towards higher latitudes.
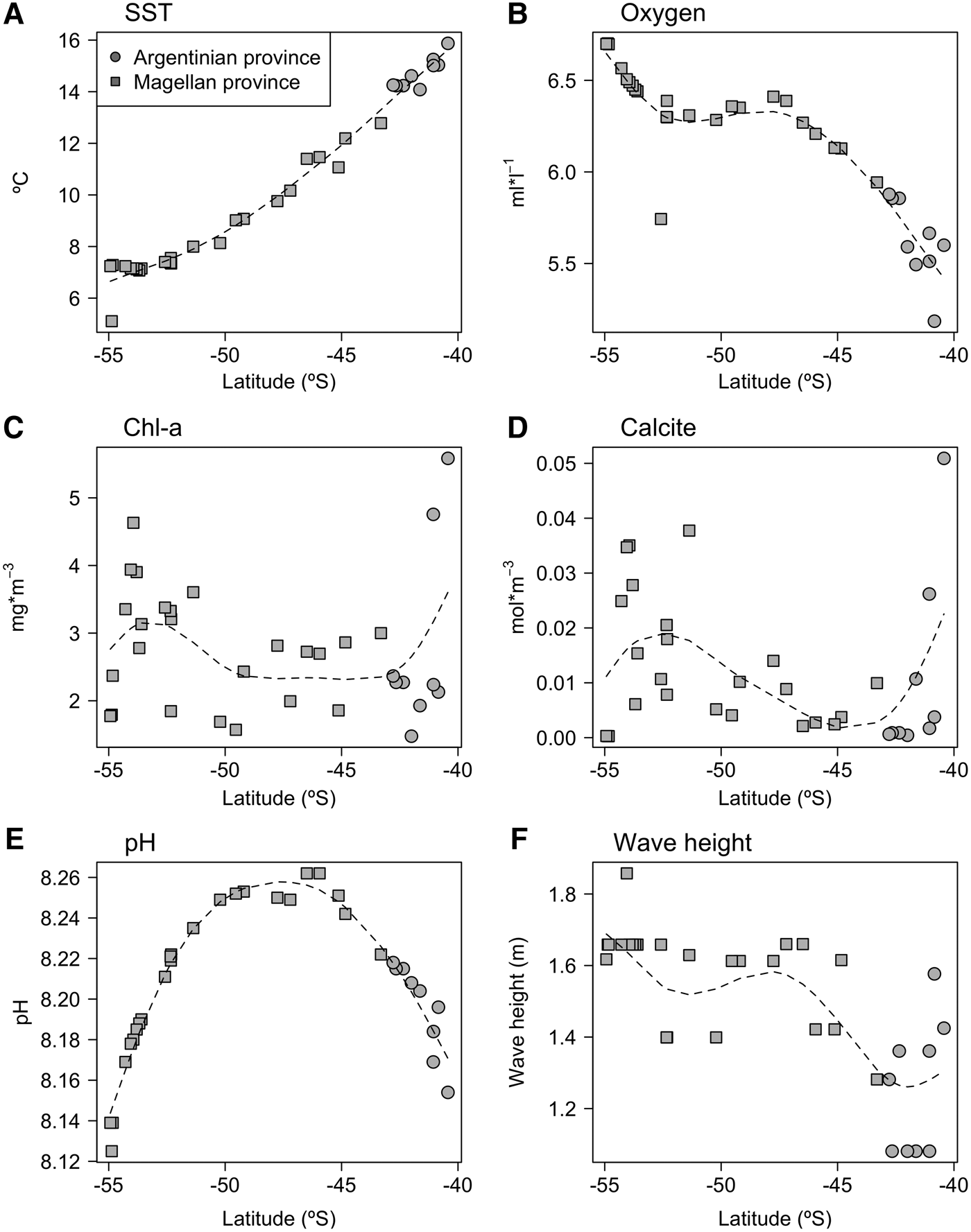
Fig. 2. Latitudinal variation of oceanographic variables. (A) Sea surface temperature; (B) Oxygen concentration; (C) Chlorophyll a concentration; (D) Calcite concentration; (E) pH; (F) Wave height. The dotted line corresponds to a lowess smoothing line.
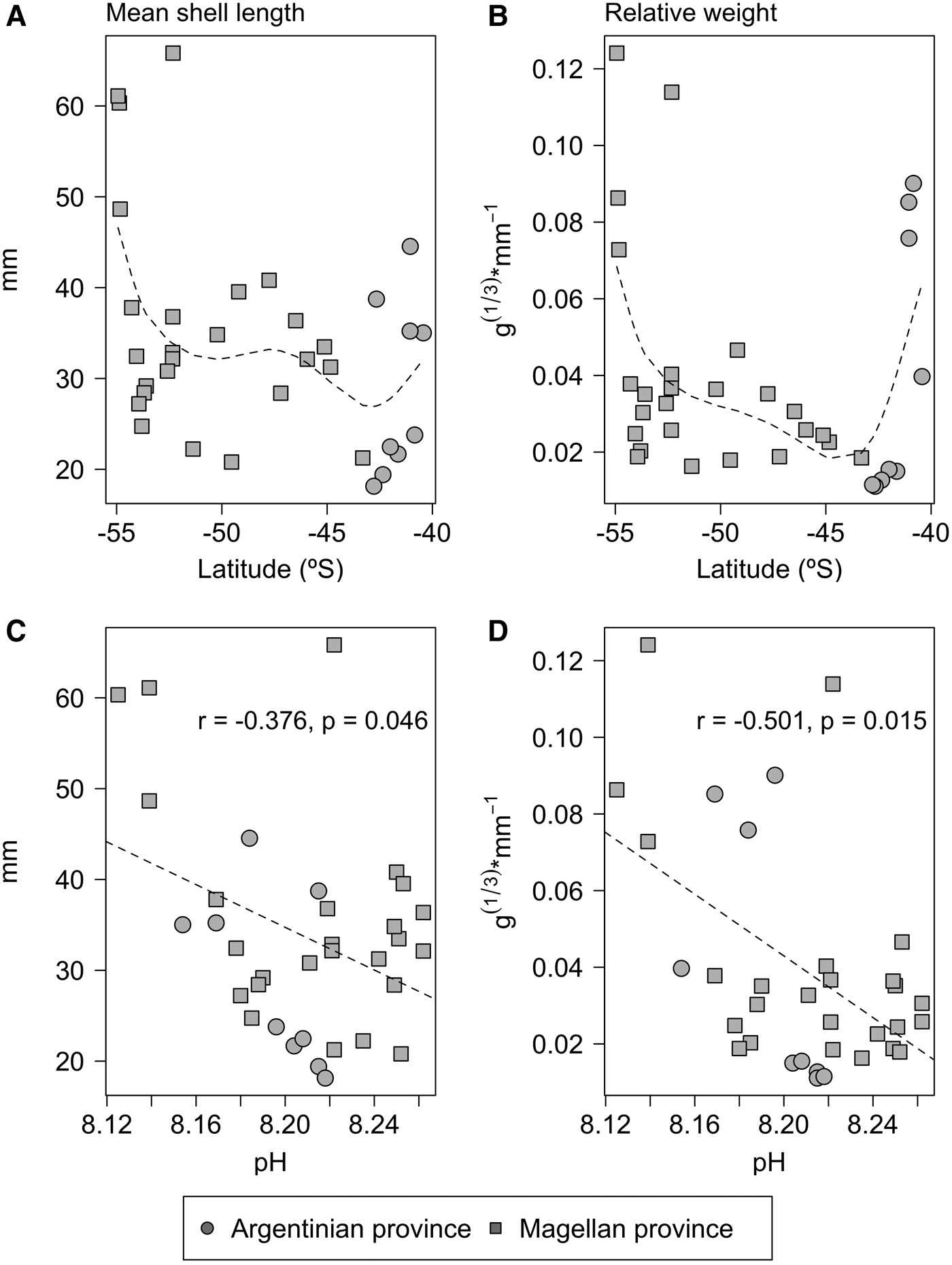
The body size of Trophon geversianus did not show any monotonic latitudinal trends (Figure 3, Table 2), and had a rather complex spatial pattern, with higher values around southern Buenos Aires (41°S), Peninsula de Valdés (47°S) and Tierra del Fuego (54°S) (Figure 3). Despite the lack of latitudinal trends, body size showed strong spatial variation. Mean shell length showed a 3-fold difference between the smallest and largest sites, while for relative weight this difference was 10-fold.

Fig. 3. Latitudinal variation of mean shell length and relative weight and its correlation with pH.
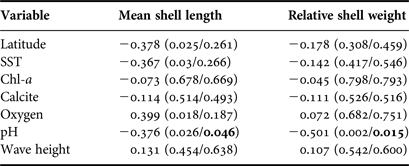
Table 2. Correlation (Spearman's product-moment) between mean shell length and the relative shell weight vs. latitude, sampled shells and oceanographic variables. In parentheses are shown P-values (uncorrected/corrected by Dutilleul's method). Significant values (after correction) are shown in bold.
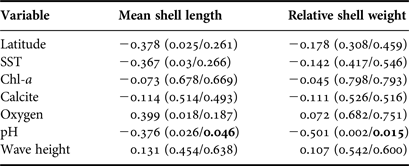
No oceanographic variables, except pH, were correlated to any proxy for body size (Table 2). In the case of pH, this was negatively and significantly correlated to mean shell length (r = −0.38, P = 0.046) and relative shell weight (r = −0.50, P = 0.015) (Table 2 and Figure 3). The negative relationship remained significant even without correcting for spatial autocorrelation in both cases (Table 2). Because the number of shells was very variable among beaches (Table 1), analyses were re-ran using only sites with N > 20 shells, in order to account for possible bias in sampling effort. The trends remained unchanged (data not shown).
DISCUSSION
The analysis of a high number of shells (>1000) from several locations (>30) encompassing 14 degrees of latitude covering two distinct biogeographic regions showed no support for the existence of an increase towards higher latitudes, and no association with sea surface temperature, as expected by Bergmann's rule and the temperature-size rule. In contrast, the spatial variability in body size can be linked to a variation in ocean pH. However, the body size of Trophon geversianus shows high spatial variability throughout the study area. Extremely large individuals were collected at three sites (outliers, see Figure 3A) in high latitudes corresponding to the Magellan Region and Beagle Channel. These areas are unlike the other sites along the Atlantic coast because of the limited exchange of waters with the Atlantic Ocean resulting in different environmental conditions, and its recent geological history strongly influenced by glaciations (Gordillo et al., Reference Gordillo, Coronato and Rabassa2005).
Studies aimed at evaluating the existence of Bergmann's rule in marine molluscs are rather scant (e.g. Frank, Reference Frank1975; Cardoso et al., Reference Cardoso, van der Veer and Kooijman2006; Linse et al., Reference Linse, Barnes and Enderlein2006; Lee & Boulding, Reference Lee and Boulding2010; Watson et al., Reference Watson, Peck, Tyler, Southgate, Tan, Day and Morley2012; Briones et al., Reference Briones, Rivadeneira, Fernández and Guiñez2014). At intraspecific level, there is no consensus about the latitudinal trend followed by the body size of mollusc species, and either positive (Frank, Reference Frank1975; Lee & Boulding, Reference Lee and Boulding2010), negative (Watson et al., Reference Watson, Peck, Tyler, Southgate, Tan, Day and Morley2012) or no correlation (Linse et al., Reference Linse, Barnes and Enderlein2006; Briones et al., Reference Briones, Rivadeneira, Fernández and Guiñez2014) have been reported. A recent analysis carried out at interspecific level showed no support for Bergmann's rule across 59 bivalve families and 4845 species (Berke et al., Reference Berke, Jablonski, Krug, Roy and Tomasovych2013). Similarly, Cardoso et al. (Reference Cardoso, van der Veer and Kooijman2006) found a weak trend for increasing shell length in bivalves at higher latitudes. Given this evidence, and despite the slight poleward increase in mean shell length, our results showed no support of the existence of Bergmann's rule in T. geversianus.
Empirical evidence shows that the relationship between body size and sea temperature is also extremely variable among species (Frank, Reference Frank1975; Linse et al., Reference Linse, Barnes and Enderlein2006; Watson et al., Reference Watson, Peck, Tyler, Southgate, Tan, Day and Morley2012; Briones et al., Reference Briones, Rivadeneira, Fernández and Guiñez2014). Hence, contrary to ecophysiological experiments which show a clear relationship between body size and rearing temperature in ectotherms and thus support the temperature-size rule (Atkinson, Reference Atkinson1994; Irie et al., Reference Irie, Morimoto and Fischer2013), our study shows no significant effect of sea surface temperature on the body size of T. geversianus.
Furthermore, other oceanographic variables may help explain large-scale patterns of invertebrate body size, for example oxygen concentration (Chapelle & Peck, Reference Chapelle and Peck1999) and chlorophyll a (a proxy for food availability; Ho et al., Reference Ho, Pennings and Carefoot2010). These variables showed no correlation with the body size of T. geversianus. Calcium carbonate (CaCO3) solubility is driven by different factors including sea temperature, pressure and pH (Watson et al., Reference Watson, Peck, Tyler, Southgate, Tan, Day and Morley2012). It is expected that increasing acidification will increase CaCO3 solubility, thus making the calcification process more difficult. Indeed, meta-analyses of experimental studies show drastic impacts of acidification on different physiological and ecological processes in molluscs (Kroeker et al., Reference Kroeker, Kordas, Crim, Hendriks, Ramajo, Singh, Duarte and Gattuso2013). The potential impact of acidification on body size per se is less understood. For instance, Watson et al. (Reference Watson, Peck, Tyler, Southgate, Tan, Day and Morley2012) found that the shell mass of five mollusc species increased when living in waters with higher aragonite saturation. On the other hand, in our study both proxies for body size were negatively correlated to pH. We suspect that this apparent contradictory result may arise from the negative relationship between growth rate and the asymptotic body size observed in other molluscs (e.g. Cardoso et al., Reference Cardoso, van der Veer and Kooijman2006). Additional studies addressing the latitudinal variation in growth parameters may shed light on this hypothesis. Alternatively, our results may be influenced by the comparatively high values of pH observed across the south-west Atlantic coast (8.13–8.26) compared with the global ocean (7.95–8.38; Takahashi et al., Reference Takahashi, Sutherland, Chipman, Goddard, Ho, Newberger, Sweeney and Munro2014). In this sense, the functional relationship between body size and pH may be non-linear, and so other responses may be observed in regions with a different pH range.
Additionally, genetic studies will probably improve our understanding of this species given that T. geversianus shows direct development, suggesting that morphological variation is more likely to be caused by genetic differentiation rather than phenotypic plasticity, compared with species with planktonic larvae. Recently, phylogenetic analysis of COI gene fragments showed no consistent differences among individuals sampled in intertidal and subtidal habitats at three sites in Northern Patagonia (Márquez et al., Reference Márquez, Vilela, Lozada and Bigatti2015).
Also, biological interactions may also contribute to shaping latitudinal patterns in the body size of T. geversianus. For instance, the presence of the voracious, invasive crab Carcinus maenas, which lives along Chubut province in the south-west Atlantic coast (Hidalgo et al., Reference Hidalgo, Barón and Orensanz2005), may be inducing phenotypic responses from T. geversianus. Trussell (Reference Trussell2000) found that gastropod shells that have been exposed to predation by C. maenas for a longer time period (~100 years) were heavier, thicker and stronger. Aquarium experiments confirm that C. maenas feed on slow-moving and sessile animals including T. geversianus (Hidalgo et al., Reference Hidalgo, Silliman, Bazterrica and Bertness2007). A taphonomic analysis of shells around the introduced/invasive range of this species (Chubut province, 45°S) (Malvé et al., unpublished data) shows that thicker shells also tend to have a higher repair frequency (probably produced by crabs), thus suggesting a direct link between predation risk and body size.
The inertia of death assemblages to short-term changes in species composition thus turns them into good proxies for long-term or regional-scale studies of benthic assemblages. Our results show that body size has a complex spatial trend of variability that cannot be easily encapsulated by ecogeographic rules (e.g. Bergmann's rule and the temperature-size rule). Molluscan death assemblages can be used to predict climate change effects on marine biodiversity (Warwick & Turk, Reference Warwick and Turk2002), and our study highlights the potential usefulness of biogeographic studies for anticipating the impacts of global change (i.e. ocean warming and acidification) on marine biodiversity. This may be of particular interest in socio-economically important species, such as T. geversianus. Insights into the resolution, fidelity and dynamics of death assemblages’ accumulation now provide a confident basis for using them to evaluate the recent history of modern-day systems representing a valuable resource for understanding our present and future (Kidwell & Tomasovych, Reference Kidwell and Tomasovych2013). Finally, a more complete analysis of the latitudinal variation of life-histories (i.e. growth rates based on sclerochronological analyses), complemented with laboratory experiments and phylogeographic analyses, may provide a much more robust basis for unveiling the patterns and processes which explain the structure and dynamics of T. geversianus along the south-western Atlantic coast.
ACKNOWLEDGEMENTS
We would like to thank two anonymous reviewers for their constructive criticism and useful suggestions that greatly improved the quality of this manuscript. This paper is part of the undergraduate thesis of MEM under the supervision of SG and MMR.
FINANCIAL SUPPORT
The research of SG was funded by PIP-114-201101-00238. The research of MMR was funded by FONDECYT grant no. 1140841.