INTRODUCTION
All species of turtle, both marine and freshwater, are under varying degrees of predation pressure and require conservation management. Some species are merely threatened, while others are endangered, especially in certain regions of their territory (Hilton-Taylor et al., Reference Hilton-Taylor, Baillie and Stuart2004; Wallace et al., Reference Wallace, DiMatteo, Bolten, Chaloupka, Hutchinson, Abreu-Grobois, Mortimer, Seminoff, Amorocho, Bjorndal, Bourjea, Bowen, Dueñas, Casale, Choudhury, Costa, Dutton, Fallabrino, Finkbeiner, Girard, Girondot, Hamann, Hurley, López-Mendilaharsu, Marcovaldi, Musick, Nel, Pilcher, Troëng, Witherington and Mast2011).
Loggerhead turtles are seasonal visitors to the Indian Ocean coastline of South Africa, including that of KwaZulu-Natal (KZN). For many years the KwaZulu-Natal Sharks Board (KZNSB) has maintained protecting nets offshore of the more popular water-based recreation beaches of the province. This has lead to the accumulation of an enormous database of information on the larger vertebrates that inhabit, or pass through, the region's coastal waters. At the same time the scientists at the KZNSB have engaged in joint research with their peers at other institutions, both internal to, and external to, South Africa.
While the literature relating to turtle biology is extensive, that relating to turtle biochemistry, especially fatty acid biochemistry, is relatively limited. However, the available literature reflects data obtained from several species, tissues and geographical locations. Guitart et al. (Reference Guitart, Silvestre, Guerrero and Mateo1999) published data on the fatty acid composition of various tissues, including adipose and liver, in loggerhead turtles (Caretta caretta); while Guilette et al. (Reference Guilette, Bjorndal, Bolten, Gross, Plamer, Witherington and Matter1991) examined changes in lipid derivatives, also in loggerheads. Huang et al. (Reference Huang, Lin and Chu2005) and Lin & Huang (Reference Lin and Huang2006) reported on the soft-shelled turtle (Pelodiscus sinensis); Ackman et al. (Reference Ackman, Hooper and Frair1971, Reference Ackman, Hooper and Sipos1972) described the lipid profiles of several turtle species, both freshwater and marine, including loggerheads; Joseph et al. (Reference Joseph, Ackman and Seaborn1985), Ackman et al. (Reference Ackman, Takeuchi and Balazs1992) and Seaborn et al. (Reference Seaborn, Moore and Balazs2005) reported on green turtles (Chelonia midas); while Lawniczak & Teece (Reference Lawniczak and Teece2009) assessed changes in the lipid profile during turtle egg development in the common snapping turtle (Chelydra serpentina). Rowe et al. (Reference Rowe, Holy, Ballinger and Stanley-Samuelson1995) also assessed lipid changes during turtle egg development, but in three species, painted turtle (Chrysemys picta), common snapping turtle and Blanding's turtle (Emydoidea blandingii).
During 2008 five loggerhead turtles were part of the by-catch in the nets off South Africa and were brought ashore for dissection. Samples from adipose tissue under the carapace and liver were taken for lipid and fatty acid analysis to ascertain how closely the profiles of Indian Ocean animals resembled those of animals from other regions.
MATERIALS AND METHODS
Unless otherwise stated all reagents were obtained from Merck Pty, Ltd., Randburg, South Africa. The loggerhead liver and adipose samples were obtained from animals found dead in the beach-protecting nets maintained by the KZNSB, Umhlanga, South Africa. Samples were collected from the five turtles in 2008 and were from one adult male, one juvenile female and three adult females. Samples were taken as soon after post-mortem as possible and frozen at −20°C prior to analysis.
Weighed aliquots of the thawed samples were blended and extracted at 4°C overnight using 20 volumes per weight of chloroform:methanol (2:1, v/v) (Folch et al., Reference Folch, Lees and Sloane-Stanley1957). The extracts were purified by washing with 20% of their volume of 0.9% saline at 4°C overnight. The chloroform layer was then removed, dried, and the dry samples made up to 20 ml with chloroform, transferred to glass vials and stored at −20°C.
A 1 ml aliquot of each extract was used to determine total lipid dry weight, and a further aliquot approximating to 20 mg of total lipid was transmethylated using 10% acetyl chloride in methanol to prepare the fatty acid methyl esters (FAME) (Christie, Reference Christie and Christie2003). These were then extracted into hexane, dried under a stream of nitrogen, redissolved in a minimum volume of hexane and the methyl esters separated using a Varian 3400 gas chromatograph run isothermally at 195°C, with a 10% SP2330 on Chromosorb WAW 100/120 6 ft × 1/8 in packed column (Supelco Pty, Ltd, Randburg, South Africa) and with flame ionization (FID) detection. The peaks were quantitated using a Varian 4270 integrator and identified by comparison with authentic FAME standards (Sigma-Aldrich Pty, Ltd, Sandton, South Africa).
Statistical analyses were carried out using a standard package (SPSS). Comparisons between liver and adipose tissue fatty acids were done using the t-test.
RESULTS
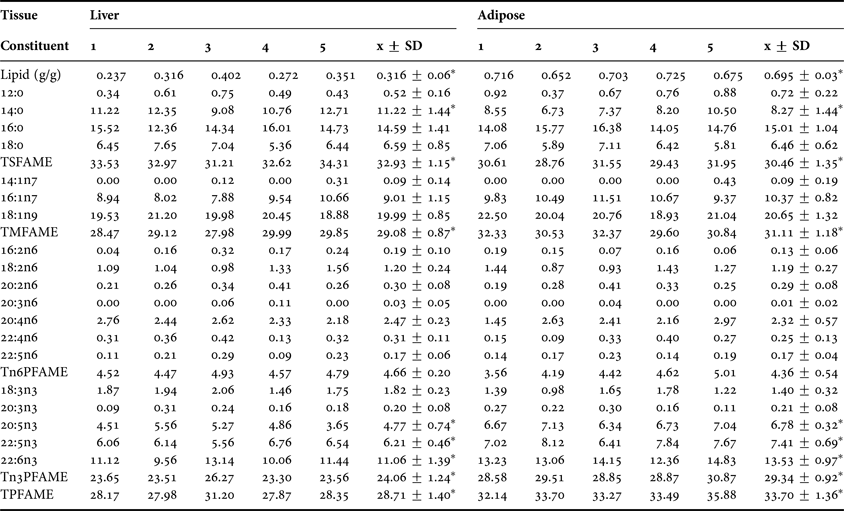
All the individual results of the lipid and fatty acid analyses of liver and adipose are shown in Table 1, along with the mean and standard deviation. The total lipid mass was much greater in adipose tissue than in liver (0.695 g/g vs 0.316 g/g). Within the FAME there were only relatively minor differences in the saturates, with 14:0 (8.27% vs 11.22%) and the total (30.46% vs 32.93%) being significantly reduced in adipose compared to liver. Conversely, within the monounsaturates, the only significant differences were with the total (31.11% vs 29.08%), where adipose was greater than liver. There were no significant differences for any of the n6 polyunsaturates or for their total; however, this was not the case for the n3 polyunsaturates. 18:3n3 (1.40% vs 1.82%) was reduced in adipose, while 20:5n3 (6.78% vs 4.77%), 22:5n3 (7.41% vs 6.21%), 22:6n3 (13.53% vs 11.06%) and total n3 (29.34% vs 24.06%) were all increased. This increase in the total n3 polyunsaturates caused an overall significant increase in the total polyunsaturates (33.70% vs 28.71%) when comparing adipose with liver.
Table 1. The liver and adipose tissue lipid and fatty acid methyl esters profiles of the five loggerhead turtles.

1, adult male; 2, juvenile female; 3–5, adult females; FAME expressed as percentage total; TSFAME, total saturated FAME; TMFAME, total monounsaturated FAME; Tn6PFAME, total n6 polyunsaturated FAME; Tn3PFAME, total n3 polyunsaturated FAME; TPFAME, total polyunsaturated FAME; x ± SD, mean±standard deviation; *, P < 0.05 when comparing liver and adipose.
DISCUSSION
The International Union for the Conservation of Nature and Natural Resources (IUCN—the World Conservation Union) states in the 2004 Red List of Endangered Species that ‘The Testudines (turtles and tortoises) are relatively well covered on the IUCN Red List, with 205 (67%) of the 305 described species evaluated, 128 (42%) of which are listed as threatened’ (Hilton-Taylor et al., Reference Hilton-Taylor, Baillie and Stuart2004). Within the IUCN, the Species Survival Commission Marine Turtle Specialist Group has published a review of the status of marine turtles globally. Of the eleven instances where regional populations of different species were particularly at risk, three related to loggerhead turtles—the north-east Indian Ocean, the north-east Atlantic Ocean and the north-east Pacific Ocean (Wallace et al., Reference Wallace, DiMatteo, Bolten, Chaloupka, Hutchinson, Abreu-Grobois, Mortimer, Seminoff, Amorocho, Bjorndal, Bourjea, Bowen, Dueñas, Casale, Choudhury, Costa, Dutton, Fallabrino, Finkbeiner, Girard, Girondot, Hamann, Hurley, López-Mendilaharsu, Marcovaldi, Musick, Nel, Pilcher, Troëng, Witherington and Mast2011). However, concern for the species extends well beyond these areas, as loggerhead turtles are migratory, so even those animals that spend part of their cycle in the relatively safe waters of South Africa, may well spend time in other areas where they are less protected. Thus, any work which helps to increase our understanding of the natural history of the species may be advantageous in the conservation and management of the species.
In this study we assessed the lipid and fatty acid status of Indian Ocean loggerhead turtles caught in a single period of their cycle. Although one of the five animals was male and one was juvenile, there were no gender or age related differences in either liver or adipose total lipid or fatty acid profiles, although this may reflect the small sample size. The total lipid mass was much greater in adipose tissue than in liver, reflecting the primary lipid storage function of adipose tissue. While there were significant differences between the tissues for some of the saturates, given their largely energy metabolism function, these differences are probably not significant on a holistic level. The differences in the monounsaturates may well also not reflect a metabolically important difference. The overall low levels of n6 polyunsaturates and the high degree of conformity between the adipose tissue and the liver may indicate that these moieties are of limited availability, and hence are conserved. Conversely, the large differences in the n3 polyunsaturates, with significantly greater amounts of most in the adipose tissue, may reflect a greater dietary availability and hence not such a need to conserve them as for the n6.
When comparing our data with those of Guitart et al. (Reference Guitart, Silvestre, Guerrero and Mateo1999) on loggerhead liver and adipose and Ackman et al. (Reference Ackman, Hooper and Frair1971) on loggerhead adipose, there were many more similarities than differences. The only apparently major differences were in the n3 polyunsaturates, where both author groups demonstrated much lower levels of 20:5n3, 22:5n3, 22:6n3 and total n3, which also caused a reduced total polyunsaturated fraction in both tissues. In contrast, soft shelled turtles from China (Huang et al., Reference Huang, Lin and Chu2005; Lin & Huang, Reference Lin and Huang2006) showed markedly different fatty acid profiles for liver, with much greater monounsaturates and lower polyunsaturates. Yet others (Joseph et al., Reference Joseph, Ackman and Seaborn1985; Ackman et al., Reference Ackman, Takeuchi and Balazs1992; Seaborn et al., Reference Seaborn, Moore and Balazs2005) working with green turtles from the central Pacific, showed adipose fatty acid profiles very closely resembling those of our loggerheads. These may reflect true interspecies differences, or they may reflect induced differences, as the Chinese soft shelled turtles were raised in captivity, while the green turtles were wild caught. Lin & Huang (Reference Lin and Huang2006) also showed that captive feeding can change the fatty acid profiles of soft shelled turtles, which findings were mirrored by Peplow et al. (Reference Peplow, Balaban and Leak1990) in captive alligator (Alligator mississipiensis). Vicente-Neto et al. (Reference Vicente-Neto, Bressan, Faria, Oliveira, Vieira, das Gracas Cardoso, de Abreu Gloria and da Gama2010) demonstrated a similar effect in wild vs captive caiman (Caiman yacare, Caiman crocodilus yacare) as did Morpurgo et al. (Reference Morpurgo, Robinzon, Lance and Gelman1993) in wild vs captive Nile crocodile (Crocodylus niloticus). Thus, while reptile fatty acid metabolism is adaptable to the fatty acid composition of the diet, within wild populations of a species there appears to be a predominant conservation of profile. However, in the case of the n3 polyunsaturates in loggerhead turtles there appears to be more variability. Whether this reflects differences between oceanic populations, or differences relating to their greater availability from dietary sources, or both, is not apparent.
ACKNOWLEDGEMENT
The authors would like to express their thanks to the staff of the KZNSB for the provision of the loggerhead turtle adipose and liver samples, without which this work would not have been possible.