INTRODUCTION
The members of the family Stromateidae are distributed in North and South America, western Africa and the Indo-Pacific (Froese & Pauly, Reference Froese and Pauly2015). This family contains 17 species belonging to three genera, Pampus, Peprilus and Stromateus. The members of Pampus are commercially important in the areas where they are distributed in the Indo-Pacific region (Last, Reference Last, Carpenter and Niem2001; Liu et al., Reference Liu, Li and Li2002a, Reference Liu, Li and Lib; Parin & Piotrovsky, Reference Parin and Piotrovsky2004).
The genus Pampus was proposed by Bonaparte (Reference Bonaparte1834) and currently comprises seven species distributed worldwide (Liu & Li, Reference Liu and Li1998a, Reference Liu and Lib; Wu et al., Reference Wu, Shao and Lai1999, Reference Wu, Li and Liu2013; Nakabo, Reference Nakabo and Nakabo2002; Liu, Reference Liu and Liu2008; Froese & Pauly, Reference Froese and Pauly2015), Pampus argenteus (Euphrasen, Reference Euphrasen1788a, Reference Euphrasenb), P. chinensis (Euphrasen Reference Euphrasen1788a, Reference Euphrasenb), P. cinereus (Bloch, Reference Bloch1795), P. punctatissimus (Temminck & Schlegel, Reference Temminck and Schlegel1845), P. echinogaster (Basilewsky, Reference Basilewsky1855), P. minor Liu & Li, Reference Liu and Li1998a, Reference Liu and Lib and P. liuorum Liu & Li, Reference Liu and Li2013. An eighth species, P. nozawae (Ishikawa, Reference Ishikawa1904) is considered a synonym of P. cinereus by Liu et al. (Reference Liu, Li and Ning2013b) and of P. argenteus by Eschmeyer (Reference Eschmeyer2015), and it has been included in the present study, while the other synonyms of the species of the genus Pampus were not studied in the present work due to the unavailability of their X-rays.
Due to the presence of morphological similarities between members of this genus, which include general body appearance and colouration, the systematics of the genus Pampus became confusing and several species were misidentified (Cheng, Reference Cheng1962; Haedrich, Reference Haedrich1967; Fowler, Reference Fowler1972; Liu & Li, Reference Liu and Li1998a, Reference Liu and Lib, Reference Liu, Li and Li2002a, Reference Liu, Li and Lib; Dolganov et al., Reference Dolganov, Kharin and Zemnukhov2007). Cui et al. (Reference Cui, Liu, Li and Chu2011) rejected the monophyly of the genus Pampus.
Only a few morphological characters have been used to separate the species of the genus Pampus. Haedrich (Reference Haedrich1967) studied in part the osteology of these species. That study covers briefly the osteology of three species of Pampus, P. argenteus, P. chinensis and P. echinogaster, and an illustration and brief description of the caudal skeleton of P. argenteus is also given. Doiuchi et al. (Reference Doiuchi, Sato and Nakabo2004) in their phylogenetic study of the stromateoid fishes also gave an osteological description for P. punctatissimus only.
Due to the low number of specimens of some species, the present study is considered a preliminary effort to discover distinctive characters that may be available to create a more robust taxonomic system that can facilitate the separation of the different species of the genus Pampus. Therefore, the aims of the present work are (1) to develop formulae for the interdigitation of the pterygiophores of the dorsal and anal fins with the neural and haemal spines of the vertebrae, as well as for the distribution of the dorsal and ventral procurrent caudal-fin rays, and the distribution of the principal caudal-fin rays; (2) to study the vertebral column structure; (3) to describe the morphology of the skeleton of the caudal fin; (4) to evaluate the implementation of these osteological characters to diagnose the species of the genus Pampus; (5) to establish a reference database consisting of additional osteological characters to those already in use in the systematics of Pampus.
MATERIALS AND METHODS
Materials examined
The present study is based on radiographs of specimens (21) of eight species of Pampus. These radiographs, provided by the second author, were obtained from The Fish Data base of Taiwan (Shao, Reference Shao2015) (ASIZP), Natural History Museum, London (BMNH), Institute of Oceanology, Chinese Academy of Sciences (IOCAS), National Science Museum of Tokyo (NSMT), Division of Fisheries Science, Faculty of Agriculture, University of Miyazaki, Japan (MUFS).
Pampus argenteus (Euphrasen Reference Euphrasen1788a, Reference Euphrasenb) (N = 7). 181.5 mm TL, Zhuhai, Guangdong, China, 27 April 2012, ASIZP 20120435; 41 mm SL, Wuchi Taichong, Taiwan, 11 March 2005, ASIZP 0065903; 102 mm SL, Bangkcare market, Bangkok, Thailand, 3 April 1998, MUFS 15140; 90 mm SL, Bangkcare market, Bangkok, Thailand, 3 April 1998, MUFS 15141; 106 mm SL, Bangkcare market, Bangkok, Thailand, 3 April 1998, MUFS 15142; 43 mm SL, Indonesia, 2 January 1984, BMNH 1984.1.2.5; 45 mm SL, Jetino fish market, Indonesia, 23 January 1987, BMNH 1987.1.23.1.
Pampus chinensis (Euphrasen Reference Euphrasen1788a, Reference Euphrasenb) (N = 2). 43 mm SL, No locality data, 6 June 1992, ASIZP 0059767.
Pampus cinereus (Bloch, Reference Bloch1795) (N = 1); 158.0 mm TL, Zhuhai, Guangdong, China, 9 April 2013, ASIZP 2013100.
Pampus echinogaster (Basilewsky, Reference Basilewsky1855) (N = 2). 148 mm SL, Tongkang, Pingtung, Taiwan, 15 August 2004, ASIZP 0064879; 190.0 mm TL, Qingdao, Shandong, China, 9 December 2008, IOCAS 08309.
Pampus liuorum Liu & Li, Reference Liu and Li2013 (N = 1). 185.2 mm TL, Zhuhai, Guangdong, China, 29 April 2012, ASIZP 20120486.
Pampus minor Liu & Li, Reference Liu and Li1998a, Reference Liu and Lib (N = 1). 125.8 mm TL, Humen, Guangdong, China, 14 April 2011, ASIZP 2011040052.
Pampus nozawae Ishikawa, Reference Ishikawa1904 (N = 1). 202 mm, Indonesia; Bali Strait, 1st January 1984, BMNH 1984.1.1.102.
Pampus punctatissimus (Temminck & Schlegel, Reference Temminck and Schlegel1845) (N = 7). 155.5 mm TL, Zhuhai, Guangdong, China, 9 April 2013, IOCAS 2013106; 187 mm SL, 189 mm SL, Kii Peninsula, Wakayama Prefecture, Honshu Island, Japan, 24 January 1998, NSMT-P 54469; 177 mm SL, Kii Peninsula, Wakayama Prefecture, Honshu Island, Japan, 24 January 1998, NSMT-P 54470; 90 mm SL, Bangkcare market, Bangkok, Thailand, 3 April 1998, MUFS 15140; 102 mm SL, Bangkcare market, Bangkok, Thailand, 3 April 1998, MUFS 15141; 106 mm SL, Bangkcare market, Bangkok, Thailand, 3 April 1998, MUFS 15142.
The osteological terminology follows Haedrich (Reference Haedrich1967). The penultimate and antepenultimate vertebrae are referred to as the second preural (PU2) and third preural (PU3) vertebrae respectively (Rosen & Patterson, Reference Rosen and Patterson1969; Rosen, Reference Rosen1973). Bone size of different Pampus species is defined according to the shape of the different bones studied (Table 1).
Table 1. Bone sizes of different Pampus species and their definition

The study of the structure of the vertebral column is based on the method of Naseka (Reference Naseka1996) after modification to fit the vertebral structure of Pampus species, where (T) is total number of vertebrae; (A) is number of abdominal vertebrae; (a1) is number of predorsal vertebrae, i.e. vertebrae anterior to the 1st dorsal-fin pterygophore; (i) is number of intermediate vertebrae, i.e. abdominal vertebrae, parapophyses of which are fused to centra and have no articulation with ribs; (C) is number of caudal vertebrae, including the last complex of preural-ural centra. One other character is also used in the analysis: D-A distance, i.e. number of vertebrae between the 1st dorsal- and the 1st anal- pterygophores. The vertebral structure is represented for each species as a complex (combination) of these character values which is called herein a vertebral formula, and has the following general presentation:
In a general species formula (Table 2, Figure 1B), each character is represented by a mean value. This formula, which includes seven characters, reflects vertebral peculiarities and could be unique for the species.

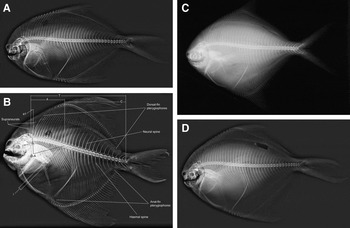
Fig. 1. Radiograph of E, Pampus liuorum; F, Pampus minor; G, Pampus nozawae; H, Pampus punctatissimus.
Table 2. The formula of the structure of the vertebral column of eight Pampus species. T, total number of vertebrae; A, number of abdominal vertebrae; a1, number of predorsal vertebrae; i, number of intermediate vertebrae; C, number of caudal vertebrae, including the last complex of preural-ural centra.

The dorsal- fin formula (Table 3) was adopted, with some modification, from the gobioid formula of Birdsong (Reference Birdsong1975) and Birdsong et al. (Reference Birdsong, Murdy and Pezold1988) and from the carangid formula of Springer & Smith-Vaniz (Reference Springer and Smith-Vaniz2008). The formula was designed to facilitate comparison of the arrangement and relationships of the spinous dorsal-fin pterygiophores and supraneurals with the underlying vertebrae. In this formula, (i), an Arabic numeral with letter (S) on the left of the letter N indicates number of supraneurals preceding the neural spine of the 1st vertebra; (ii), the letter (N), represents the neural spine of the 1st vertebra. If the insertion region is empty then (0) precedes the letter (N); (iii), the digits, separated by hyphens, represent the series of interneural spaces found behind the neural spine of the 1st vertebra (the number indicates the number of pterygiophores present in the spaces); (iv), the superscript number represents the total number of neural spines with corresponding pterygiophores to the end of the dorsal fin (for example, S – N – 1S – 1S, 2P – 18P – 2P – 12P – 28P – 3P – 22P – 3P – 23P – 1P).
Table 3. Number of spines (1), rays (2), supra neurals (3), pterygiophores (4) and the formula for the interdigitation of dorsal-fin pterygiophores with the neural spines (5) of seven Pampus species.

The development of the anal-fin formula (Table 4) is the same as that of dorsal-fin formula. In this formula, (i), the letter (C) denotes for the haemal spine of the 1st caudal vertebra; (ii), the Arabic number on the left of the letter (C) represents the anal-fin pterygiophores preceding the spine of the 1st anal-fin vertebra; (iii), the digits, separated by hyphens, represent the series of inter-haemal spaces found posterior to the haemal spine of the 1st caudal vertebra (the number indicates the number of pterygiophores present in the spaces); (iv), the superscript number represents the total number of haemal spines with corresponding pterygiophores to the end of the anal fin (for example, 7P – C – 22P – 3P – 2P – 32P – 2P – 4P – 3P – 24P).
Table 4. Number of spines (1), rays (2), pterygiophores (3) and the formula for the interdigitation of anal-fin pterygiophores with the haemal spines (4) of seven species of Pampus. C, haemal spine of the 1st caudal vertebra; P, pterygiophore.

The caudal- fin formula was derived following Fricke (Reference Fricke1983), who used it to describe the caudal fin structure of callionymid and draconettid fishes. Some modifications were required to comply with the caudal fin structure of the species of the genus Pampus. The distribution of the branched and unbranched rays of the caudal fin is given. In this formula: (i) the Roman numeral on the left indicates the number of unbranched segmented soft rays in the upper lobe; (ii) the Arabic numeral indicates the number of segmented branched soft rays in the upper lobe; (iii) dashed line between the two sets of numerals is present to separate the counts of the two lobes; (iv) the Arabic numeral indicates the number of segmented branched soft rays in the lower lobe, and (v) the Roman numeral indicates the presence of unbranched soft rays in the lower lobe.
The formulae for the distribution of the dorsal procurrent caudal-fin rays were established for the members of the genus Pampus (Table 5). These formulae were designed to facilitate comparison of the arrangement and relationships of the procurrent caudal-fin rays with the underlying caudal skeleton elements. The formulae list is, in order, (i) the letters (NSPU3 & NSPU2) represent the neural spines of the 3rd and 2nd preural vertebrae; (ii) an Arabic numerical indicates the number of procurrent caudal-fin rays between the neural spines of the 3rd and 2nd preural vertebrae; (iii) the Roman numerals in parentheses represent procurrent caudal-fin rays anterior to the neural spine of the 3rd or the 2nd preural vertebra; (iv) the Arabic numerals in parentheses represent the number of procurrent caudal-fin rays between the neural spines of the 3rd and 2nd preural vertebrae; (v) the Roman numerals in parentheses between (NSPU2) and the 1st epidural (E1) represent the number of procurrent caudal-fin rays opposite (NSPU2) and (E1); (vi) the Arabic numerals in parentheses between (E1) and (E2) represent the number of procurrent caudal-fin rays between (E1) and (E2); (viii) the Roman numerals in parentheses after (E2) represent whether there are procurrent caudal-fin rays anterior to (E2); and (ix) the Arabic numeral in parentheses after (E2) represents the number of procurrent caudal-fin rays falling posterior to (E2). Wherever a ‘0’ is present, it means no procurrent ray is found (for example, (0) NSPU3 (3) NSPU2 (1I) E1 (1I) E 2 (1I)). The formula for the distribution of the ventral procurrent caudal-fin rays is the same as that of the distribution of the dorsal procurrent caudal-fin rays, except it refers to HSPU 1, 2 and 3 denoting the haemal spine of the 1st, 2nd and 3rd preural vertebrae (for example, (1) HSPU3 (1I)(3) HSPU2 (1I) HSPU1 (2I) PH (2I).
Table 5. Formulae for the distribution of the dorsal and the ventral procurrent rays of eight species of Pampus (E, Epural bone; HSPU 1, 2, 3, haemal spines of 1st, 2nd and 3rd preural vertebrae; NSPU1, 2, neural spines of 1st and 2nd preural vertebrae; PH, parhypural; UHB, upper hypural bone).

RESULTS
The osteological characters described for the eight Pampus species are shown in Figures 1 and 2.
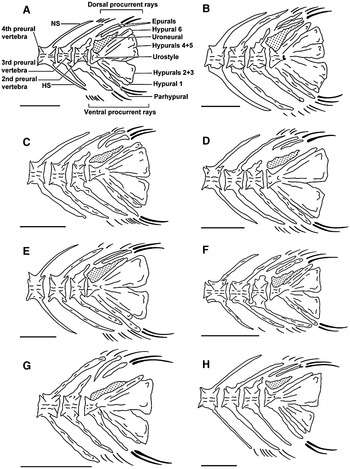
Fig. 2. Caudal skeleton of: A. Pampus argenteus; B, Pampus chinensis; C, Pampus cinereus; D, Pampus echinogaster ; E, Pampus liuorum; F, Pampus minor; G, Pampus nozawae; H, Pampus punctatissimus. Scale bar = 1 mm.
Structure of the vertebral column
The total number of vertebrae of the eight species of the genus Pampus ranges between 30 and 41 (Table 2). The number of abdominal vertebrae is either 15 or 16. Only P. punctatissimus and P. nozawae show 16 vertebrae. The number of predorsal vertebrae varies between two and three. In Pampus argenteus only, there are three predorsal vertebrae and the rest of the species studied have two vertebrae.
The number of intermediate vertebrae varies between one and two. Only P. chinensis and P. liuorum have two intermediate vertebrae. The number of caudal vertebrae range between 15 and 25.
The number of vertebrae present between the 1st pterygiophore of the dorsal fin and the 1st pterygiophore of the anal fin ranges between 12 and 15. In P. minor, there are 12, while there are 13 in both P. punctatissimus and P. argenteus and 15 in P. liuorum. In the remaining species, there are 14 vertebrae.
Pterygiophore interdigitation with neural spines of dorsal fin
The number of spines, rays, supraneurals, and the formula for the interdigitation of dorsal-fin pterygiophores with the neural spines are given in Table 3. In all eight species studied, there is one supraneural anterior to the neural spine of the 1st vertebra.
Pterygiophore interdigitation with haemal spines of the anal fins
The number of spines, rays, and the formula for the interdigitation of anal-fin pterygiophores with the haemal spines are given in Table 4. The 1st anal- pterygiophore of all eight species studied is curved back and its dorsal tip passes over the haemal spines of the 1st, 2nd, 3rd or 4th caudal vertebrae.
Caudal fin formula
The formula for the distribution of branched and unbranched rays in the caudal fin of the eight species studied is as follows: I, 8–5, 0. Hence, the number of principal caudal-fin rays in Pampus is 13 (eight in the upper lobe and five in the lower lobe). The number of unbranched rays in the upper lobe is usually one. Four of the species Pampus studied have multiple branching in the caudal-fin rays, namely, P. argenteus, P. echinogaster, P. liuorum and P. minor.
Distribution of the dorsal and ventral procurrent caudal-fin rays
The formulae of the distribution of the dorsal and ventral procurrent caudal-fin rays are shown in Table 5. Comparative observations have shown that the number of procurrent caudal-fin rays in the upper and lower hypural plates varies between three and six, and seven and 10 respectively.
In the formula of the dorsal procurrent caudal-fin rays, P. liuorum and P. punctatissimus are the only species having one ray anterior the NSPU3. The space between E2 and the upper hypural bone is filled with one ray in P. minor only. In all the eight species studied, there are one or two dorsal-procurrent caudal-fin rays anterior to the 1st epural bone.
For the distribution of the ventral- procurrent caudal-fin rays, there is one species having six rays, three species with eight rays and four species with nine rays. Pampus argenteus is the only species having a procurrent ray anterior to the HSPU3, and all species are shown to have one or two rays anterior to the bones of the ventral side of the skeleton of the caudal fin (Table 5).
Caudal skeleton (Figure 3)
All bones in the caudal fin skeleton are directly or indirectly associated with the last ‘compound centrum’ of the vertebral column (Figure 3). The shape of the neural spine of the PU2 in the eight species of Pampus is shown to be triangular (P. argenteus and P. nozawae), irregular (P. chinensis, P. cinereus and P. liuorum), elongated (P. echinogaster), squarish (P. minor) and wavy (P. punctatissimus). This spine is short and broad in P. argenteus, P. chinensis, P. cinereus and P. minor and long in P. nozawae, while it is narrow in the rest of the species studied and not reaching to the dorsal edge of the body.

Fig. 3. Radiograph of A, Pampus argenteus; B, Pampus chinensis; C, Pampus cinereus; D, Pampus echinogaster. A = number of abdominal vertebrae; a1 = number of predorsal vertebrae, i.e. vertebrae anterior to the 1st dorsal-fin pterygophore; C = number of caudal vertebrae, including the last complex of preural-ural centra; i = number of intermediate vertebrae, i.e. abdominal vertebrae, parapophyses of which are fused to centra and have no articulation with ribs.
The shape of the haemal spine of PU2 in the species studied is broad, except in P. echinogaster, and it reaches to the posterior edge of the lower hypural bones in P. cinereus, P. echinogaster, P. minor and P. punctatissimus, and it reaches the posterior edge of the lower hypural in the remaining four species of Pampus.
In Pampus, the epurals are two blade-like bones located above the urostyle that vary in length. In P. cinereus and P. argenteus, the anterior bone is straight and the posterior is curved. In P. minor, the shape of the posterior epural bone is completely different from the rest of the species studied. It is curved and has a longitudinal depressed area making it look like two bones. In some cases, the anterior is longer than the posterior epural (viz., P. chinensis, P. liuorum and P. nozawae).
In P. chinensis, P. echinogaster, P. liuorum and P. nozawae, the anterior epural is curved and the posterior is straight. In P. punctatissimus, both epurals are elongated and straight. In P. argenteus, P. cinereus and P. nozawae, they reach to the dorsal edge of the body. In the rest of the species, they do not reach the dorsal edge. Both epurals are narrow in P. cinereus, P. echinogaster and P. punctatissimus, while they are broad in P. liuorum. The anterior epural is narrower than the posterior in the rest of the species. The tip of the epural bones can be straight (P. argenteus), rounded (P. cinereus) or pointed (P. echinogaster). The tip of the two epurals can be different in shape, for example with the tip of the anterior bone being pointed, and the tip of the posterior one being straight. This case is found in P. chinensis, P. liuorum and P. nozawae. In all the species of Pampus studied, the two epurals are not fused together.
The uroneural bone is found sitting on the anterior dorsal side of hypural 6 and just behind the tip of the urostyle. It has a broad base and pointed tip and extends over three-quarters of the anterior dorsal surface of hypural 6 in P. argenteus, P. echinogaster and P. nozawae. It is found partially connected to hypural 6 and the urostyle in P. argenteus, P. chinensis and P. nozawae, while it is completely connected to hypural 6 and the urostyle of P. liuorum and P. minor. This bony structure is not connected to any bone of the caudal fin skeleton of P. cinereus, P. echinogaster and P. punctatissimus.
There are six hypurals in species of Pampus. The 2nd and 3rd are fused together to form a lower plate-like bone, and the 4th and 5th are fused to form an upper plate. Except in P. cinereus, the hypurals bones are long. In P. argenteus, the 1st and 6th are narrow, while the remaining four bones are wide. All six hypural bones are broad in P. chinensis, P. echinogaster, P. liuorum and P. minor and narrow in P. cinereus, P. nozawae and P. punctatissimus. The shape of the dorsal side of the 6th hypural bone is curved in P. liuorum and P. punctatissimus, while it is straight in the rest of the species studied. The ventral side of the 4th and 5th plate and the dorsal side of the 2nd and 3rd plate are straight and curved anteriorly at the junction with the urostyle in P. argenteus and P. cinereus and straight with no curve in the rest of the species except P. liuorum, where the ventral side of the 4th and 5th plate and the dorsal side of the 2nd and 3rd plate are both curved and nearly fused completely, leaving only a short distance unfused.
The parhypural is a plate-like bone lying below the urostyle, fused and autogenous to the 1st hypural bone. It is long, straight, not reaching the posterior edge of the hypural plates in P. argenteus, P. cinereus and P. echinogaster, while it is long, curved and not reaching the posterior edge of the hypural plates in P. chinensis and P. punctatissimus. In P. minor, it is very long, straight and extending beyond the posterior edge of the hypural bones. Except for P. argenteus and P. nozawae, this bone is broad.
DISCUSSION
Bonaparte (Reference Bonaparte1834) created the genus Pampus (Stromateidae) which currently contains seven valid species worldwide. Haedrich (Reference Haedrich1967) accepted only three species of Pampus as valid: P. argenteus, P. echinogaster and P. chinensis. Likewise, Last (Reference Last, Carpenter and Niem2001) considered only these three species in his recent review of Stromateidae.
The systematics of fishes of this genus is confusing due to morphological similarities, and such confusion is clear in the following cases. The two pomfret species, P. minor and P. punctatissimus are currently recognized as valid but have previously been synonymized with P. argenteus (Haedrich, Reference Haedrich1967; Lindberg & Krasyukova, Reference Lindberg and Krasyukova1975) because they showed great similarities to this species. The grey pomfret, P. cinereus was originally described by Bloch (Reference Bloch1795) in the genus Stromateus, but later workers have long treated the species as a member of Pampus (Day, Reference Day1876; Deng et al., Reference Deng, Xiong and Zhan1981; Wu, Reference Wu and Chu1985; Liu & Li, Reference Liu and Li1998a, Reference Liu and Lib; Wu et al., Reference Wu, Shao and Lai1999; Nakabo, Reference Nakabo2000, Reference Nakabo and Nakabo2002; Liu et al., Reference Liu, Li and Li2002a, Reference Liu, Li and Lib; Liu, Reference Liu and Liu2008; Cui et al., Reference Cui, Liu, Li and Chu2011; Froese & Pauly, Reference Froese and Pauly2015). Some other authors considered P. cinereus to be a synonym of P. argenteus (Lindberg & Krasyukova, Reference Lindberg and Krasyukova1975; Parin & Piotrovsky, Reference Parin and Piotrovsky2004). Euphrasen (Reference Euphrasen1788a, Reference Euphrasenb), who described P. argenteus for the first time, did not give distinctive morphological characters that can separate it from the other species of Pampus. Since then, the taxonomic status of this species remained unsettled until Liu et al. (Reference Liu, Li, Li and Ning2013a) resolved its identity and separated it from P. echinogaster. Another uncertainty in the taxonomic status of the members of the genus Pampus is the status of the species P. nozawae. This species was originally described by Ishikawa (Reference Ishikawa1904) from Kanagawa, Japan and synonymized with P. argenteus by Lindberg & Krasyukova (Reference Lindberg and Krasyukova1975), and with P. cinereus by Liu et al. (Reference Liu, Li and Ning2013b). The current status of this species is as synonym of P. argenteus (Eschmeyer, Reference Eschmeyer2015). Through the taxonomic history of the members of the genus Pampus, scientists used only morphometric and meristic characters to verify the identity of those fish species (Liu & Li, Reference Liu and Li1998a, Reference Liu and Lib; Liu et al., Reference Liu, Li, Li and Ning2013a, Reference Liu, Li and Ningb). Doiuchi et al. (Reference Doiuchi, Sato and Nakabo2004) accepted the monophyly of Pampus while, more recently, Cui et al. (Reference Cui, Liu, Li and Chu2011) have rejected it.
In the present study some osteological characters are used to add more characters to those previously used to separate the species of Pampus and to settle any misidentification that might occur among its species. Osteological characters still prove to be useful for fish systematics (Day, Reference Day2002; Tyler et al., Reference Tyler, O'Tool and Winterbottom2003). The osteological study presented here illustrates the wide range of useful osteological characters present in the members of the genus Pampus. It is possible to distinguish two groups of osteological characters: (1) exclusive characters that clearly define a species; and (2) characters that are shared by several species.
Structure of the vertebral column
As mentioned above, there are a number of misidentifications among the species of Pampus. The study of the vertebral column in the members of the genus Pampus demonstrates that these species are different in some of their vertebral features. On the whole, the vertebral column of the members of the genus Pampus is characterized by the following features. The total number of vertebrae ranges between 30 and 41. The number of abdominal vertebrae is smaller than that of caudal ones. Predorsal vertebrae are few in number (two or three). The number of intermediate vertebrae varies from one to two. Preanal vertebrae are absent. The 1st anal-fin pterygiophore forms the posterior wall of the abdominal cavity and the anus is therefore placed anterior to the 1st spine of the anal fin beneath the 14th abdominal vertebra.
The results of Liu et al. (Reference Liu, Li and Li2002b) on comparing the different species of Pampus using morphometric and meristic characters are supported here by the formula of the vertebral column features. In particular, the formula obtained for the vertebral column of P. argenteus differs from the remaining six species of Pampus in having a high number of vertebrae, as a result of the high abdominal and caudal vertebral count. Pampus punctatissimus differs from P. cinereus in having slightly more abdominal and caudal vertebrae and fewer posterior vertebrae. The vertebral column characteristics of P. nozawae are different from both P. argenteus and P. cinereus, the two species with which it was synonymized by Liu et al. (Reference Liu, Li, Li and Ning2013a, Reference Liu, Li and Ningb). It differs from P. argenteus in having 37 vertebrae in the vertebral column (41 in P. argenteus), two predorsal vertebrae (three in P. argenteus), and from both P. argenteus and P. cinereus in having 21 caudal vertebrae (25 in P. argenteus and 22 in P. cinereus), and 21 postanal vertebrae (22 in both P. argenteus and P. cinereus).
The study of the vertebral column structure succeeded in separating P. argenteus from the remaining species of Pampus in having three predorsal vertebrae. The remaining seven species of Pampus fall into two groups; one group includes P. punctatissimus and P. nozawae with 16 abdominal vertebrae, and the other group contains P. chinensis, P. cinereus, P. echinogaster, P. liuorum and P. minor with 15 abdominal vertebrae. In the first group, the two species can be separated by the number of caudal vertebrae (18 in P. punctatissimus vs. 21 in P. nozawae). Similarly, species in the second group are separated according to their number of caudal vertebrae (Table 2). The results of the vertebral column structure should be confirmed once more specimens of the eight species become available.
Neural and haemal spines interdigitation with pterygiophore of dorsal and anal fins
As in other teleost fish species (Hollister, Reference Hollister1941; Abe & Takashima, Reference Abe and Takashima1953; Ahlstrom et al., Reference Ahlstrom, Butler and Sumida1976; Birdsong et al., Reference Birdsong, Murdy and Pezold1988; Gill & Edwards, Reference Gill and Edwards2004; Springer & Smith-Vaniz, Reference Springer and Smith-Vaniz2008), the insertion of dorsal- and anal- fin rays are useful to recognizing the eight species of Pampus studied in the present work. The formula of insertion of pterygiophores of both dorsal- and anal- fins appears to be unique for each species of Pampus studied.
For the dorsal fin, the number of supraneurals preceding the dorsal fin is shown to be a good taxonomic tool to identify these species. For example, it is possible to separate P. argenteus from the other species of Pampus by the presence of only two supraneurals in P. argenteus, compared with either three or four in the other species. The other character that distinguishes the Pampus species is whether there is a pterygiophore in the 1st dorsal insertion space anterior to the neural spine (Table 3).
The number of anal pterygiophores preceding the haemal spine of the 1st caudal vertebra is shown to be a feature that separates the species of Pampus into four groups. Pampus liuorum with nine pterygiophores, P. nozawae and P. punctatissimus with eight, P. chinensis, P. cinereus and P. echinogaster with seven and finally P. argenteus and P. minor with six pterygiophores (Table 4).
Caudalfin formula
The number of the branched and unbranched rays of the upper and lower lobe of the caudal fin of the eight species of Pampus studied showed low variability. It is possible to divide these species into three groups based on the number of unbranched rays in the lower lobe of the caudal fin. The first group comprises P. echinogaster, P. minor and P. nozawae missing the unbranched ray from their lower lobe of the caudal fin. The second group includes P. punctatissimus, with only one unbranched ray. The third group embraces P. argenteus, P. chinensis and P. cinereus, with two unbranched rays in their lower lobe of caudal fin. The number of branched and unbranched rays in the upper lobe and the number of branched rays in the lower lobe are shown to be similar in the eight species of Pampus studied. Within the first group, it is possible to separate P. nozawae from P. echinogaster and P. minor for having seven branched rays in the upper lobe of the caudal fin. The caudal-fin ray formula successfully separates P. punctatissimus of the second group from the rest of the six species studied. Within the third group, P. argenteus can be separated from P. chinensis and P. cinereus for having two unbranched rays in the upper lobe of its caudal fin.
In the present study, multi-branching is seen in the caudal-fin rays of one specimen out of four specimens of P. chinensis, out of five specimens of P. cinereus, out of four specimens of P. minor, and out of seven specimens of P. punctatissimus studied in this work. With this low number of multi-branching incidence in the species mentioned above, it is impossible to consider the multi-branching character as a good taxonomic criterion.
Distribution of the dorsal and ventral procurrent caudal-fin rays
The procurrent caudal-fin rays are unsegmented and spine-like. In blennioid fishes, the posteriormost dorsal procurrent ray is counted as a principal caudal-fin ray (Springer & Gomon, Reference Springer and Gomon1975). However, it is counted as a procurrent ray in the present study because it resembles the anterior procurrent caudal-fin rays (short, segmented, spine-like with a smooth rather than knob-like base), rather than the long and segmented rays on the upper and lower hypural plates.
To facilitate the presentation of the distribution of the dorsal and ventral procurrent caudal-fin rays, two formulae were developed. Both formulae show the specific differences between the eight species of Pampus studied. For the formula for dorsal procurrent caudal-fin rays, there is variation in the number of procurrent caudal-fin rays inserted between the NSPU3 and NSPU2, while there are no insertions between the NSPU1, E1 and E2. Instead the dorsal procurrent caudal-fin rays fall anterior to these elements.
The distribution of the ventral procurrent caudal-fin rays in the eight species of Pampus studied is characterized in having the space between HSPU3 and HSPU2 either empty or filled with one or three procurrent caudal-fin rays, and the spaces between the HSPU2, HSPU1 and PH are either filled with procurrent caudal-fin rays or are all anterior to the procurrent caudal-fin rays.
Doiuchi et al. (Reference Doiuchi, Sato and Nakabo2004) discussed the feasibility of using the shape of the ventral procurrent caudal-fin rays in phylogenetic studies, basing their assumption on Johnson (Reference Johnson1975). Therefore, the distribution of these rays and the dorsal procurrent caudal-fin rays given in the present study will benefit any study on the relationship of the members of the genus Pampus in the future.
Members of the genus Pampus have a stromateoid-type caudal fin skeleton (Haedrich, Reference Haedrich1967). The skeleton includes the second preural centrum (PU2); six hypurals with fusion of 2 + 3 and 3 + 4, which in turn are fused to the urostyle complex; an autogenous parhypural bone located ventral to hypural plate 2 + 3; two epurals; and one pair of uroneural. Within this general structure of the caudal-fin skeleton, the shape of different elements of the caudal skeleton can be used to separate the eight species of Pampus studied.
In the present study, several characteristics of the caudal skeleton of the eight Pampus species are considered advanced in terms of the reduction of the number of the osteological elements. Such characters are: the reduction of the number of epural bones from three to two; the fusion of the hypural plates 2 and 3, and 4 and 5; and the fusion of the pair of uroneural bones. Therefore, Haedrich (Reference Haedrich1967) considered the members of Pampus the most highly derived genus of the family Stromateidae. Further fusion of the hypural plates, 2 + 3 and 4 + 5 is observed in P. liuorum, where the gap between these two plates is smaller than any gap between the two plates in the remaining species of Pampus studied.
The shape, size and width of the different caudal skeleton elements are useful as taxonomic criteria to separate the species of Pampus studied. The distinctive shape of the posterior epural bone in P. minor, which has a longitudinal depression; the different shape, size and width of the parhypural bone in P. nozawae, P. punctatissimus, P. argenteus and P. chinensis; and the shape of the neural spine of PU2 are all considered good taxonomic criteria to recognize these species.
Horn (Reference Horn, Moser, Richards, Cohen, Fahay, Kendall and Richardson1984) counted the parhypural as a hypural bone in the stromateoid genera. In the present study, the parhypural was considered separately from the hypurals (Fujita, Reference Fujita1990).
The uroneural bone shape and its position in the skeleton of the caudal fin of the species of Pampus are not sufficiently distinct to differentiate between individual species, but these characters can be used to separate species into groups. From the preliminary results, it is possible to separate the eight species of Pampus into three groups based on whether the uroneural bones are connected or not connected to hypural 6 and the urostyle.
The description of the morphology of the caudal fin skeleton of P. argenteus and P. punctatissimus given by Haedrich (Reference Haedrich1967) and Doiuchi et al. (Reference Doiuchi, Sato and Nakabo2004) resembles that obtained for the two species in the present study. No comparison was made for the caudal-fin skeleton of the remaining six Pampus species due to the lack of osteological descriptions for these species.
The osteological results obtained from the present study indicate that P. nozawae is morphologically characterized by the vertebral column structure, interdigitation of the dorsal- and anal-fin pterygiophores with the neural and haemal spines of the vertebrae, distribution of the dorsal- and ventral-procurrent caudal-fin rays, and the structure of the skeleton of the caudal fin. The evidence presented here strongly indicates that P. nozawae is a valid species of the genus Pampus. Molecular evidence is needed to support the differences in the osteological characters obtained in this study for P. nozawae in order to designate a neotype for this species as the holotype of P. nozawae is missing (Eschmeyer, Reference Eschmeyer2015).
KEY TO SPECIES OF PAMPUS BASED ON THE FIVE OSTEOLOGICAL CHARACTERS STUDIED IN THE PRESENT WORK:
-
1a. Presence of 8 anal-fin pterygiophores anterior to the haemal spine of the 1st caudal vertebra ……… (2)
-
b. Presence of fewer than 8 anal-fin pterygiophores anterior to the haemal spine of the 1st caudal vertebra ……… (4)
-
2a. Presence of 14 consecutive vertebral insertions with 2 pterygiophores immediately posterior to the haemal spine of the 1st caudal vertebra; presence of 21 caudal vertebrae; presence of 2 consecutive dorsal vertebral insertion with one dorsal-fin pterygiophore in the 5th insertion immediately posterior to the neural spine of the 1st vertebra; presence of 2 dorsal procurrent caudal fin ray in the front of NSPU3; presence of 2 ventral procurrent caudal-fin rays between HSPU3 & HSPU2; parhypural bone straight ……… P. nozawae
-
b. Presence of less than 14 consecutive vertebral insertions with 2 pterygiophores immediately posterior to the haemal spine of the 1st caudal vertebra; presence of more than 21 caudal vertebrae; presence of more than 2 consecutive dorsal vertebral insertions with one dorsal-fin pterygiophore in the 5th insertion posterior to the neural spine of the 1st vertebra; presence of less than 2 dorsal procurrent caudal fin rays in the front of NSPU3; parhypural bone not straight ……… (3)
-
3a. Presence of 5 consecutive vertebral insertions with 2 pterygiophores immediately posterior to the haemal spine of the 1st caudal vertebra; presence of 22 caudal vertebrae; presence of 7 consecutive vertebral insertions starting from the 4th vertebral insertion posterior to the neural spine of the 1st vertebra; presence of one dorsal procurrent caudal fin ray in front of the 1st epural bone; presence of 3 ventral procurrent caudal-fin rays between HSPU3 & HSPU2; parhypural bone curved through all its length P. liuorum
-
b. Presence of one vertebral insertion with 3 pterygiophores immediately posterior to the haemal spine of the 1st caudal vertebra; presence of 18 caudal vertebrae; presence of 7 consecutive dorsal insertions of one dorsal-fin pterygiophore in the 3rd vertebral insertion immediately posterior to the neural spine of the 1st vertebra; presence of 2 dorsal procurrent caudal-fin rays in the front of the 2nd epural bone; presence of one ventral procurrent caudal-fin ray in the front of the HSPU3; parhypural bone curved at posterior tip ……… P. punctatissimus
-
4a. Presence of 7 anal-fin pterygiophores anterior to the haemal spine of the 1st caudal vertebra; presence of 18 caudal vertebrae; presence of 3 consecutive vertebra insertions with 3 dorsal fin pterygiophores starting from the 4th vertebral insertion posterior to the neural spine of the 1st vertebra; presence of 2 dorsal procurrent caudal-fin rays in front of the 1st epural bone; presence of 3 ventral procurrent caudal fin ray between HSPU3 & HSPU2; anterior epural bone long ……… P. chinensis
-
b. Presence of 6 anal-fin pterygiophores anterior to the haemal spine of the 1st caudal vertebra; presence of 18 caudal vertebrae ……… (5)
-
5a. Presence of 4 consecutive vertebral insertion with 3 anal-fin pterygiophores starting from the 2 ns vertebral insertion posterior to the neural spine of the 1st caudal vertebra; presence of 15 caudal vertebrae; presence of 7 consecutive dorsal insertions of one dorsal-fin pterygiophore in the 4th insertion immediately posterior to the haemal spine of the 1st vertebra; presence of one dorsal procurrent caudal fin ray in front of the 2nd epural bone and one dorsal procurrent caudal fin ray between 2nd epural bone and urohyal bone; presence of 2 ventral procurrent caudal-fin rays between HSPU2 & HSPU1; 2nd epural bone with a longitudinal depression P. minor
-
b. Presence of one vertebral insertion with one anal-fin pterygiophore posterior to the haemal spine of the 1st caudal vertebra ……… (6)
-
6a. Presence of only one vertebral insertion with 2 pterygiophores in the 8th vertebral insertion posterior to the haemal spine of the 1st caudal vertebra; presence of 3 predorsal vertebrae; presence of 2 dorsal vertebral insertions with 2 dorsal-fin pterygiophores posterior to 11th vertebral consecutive insertions of one pterygiophore; presence of 3 dorsal procurrent caudal-fin rays between NSPU3 & NSPU2; presence of one ventral procurrent caudal-fin ray anterior to the HSPU3; parhypural bone narrow ……… P. argenteus
-
b. Presence of more than one vertebral insertion with 2 anal-fin pterygiophores starting from 8th vertebral insertion ……… (7)
-
7a. Presence of 2 consecutive vertebral insertions with 2 anal-fin pterygiophores starting from the 8th vertebral insertion posterior to the haemal spine of the 1st caudal vertebra; presence of 22 caudal vertebrae; presence of 2 dorsal-fin pterygiophores and one supraneural spine in the 2nd vertebral insertion posterior to the neural spine of the 1st vertebra; presence of one ventral procurrent caudal fin ray in front of HSPU3; irregular shape of the neural spine of the 2nd preural vertebra; parhypural bone broad ……… P. cinereus
-
b. Presence of 3 consecutive vertebral insertions with 2 anal-fin pterygiophores immediately posterior to the haemal spine of the 1st caudal vertebra; presence of 18 caudal vertebrae; presence of 24 caudal vertebrae; presence of 11 consecutive vertebral insertions with one dorsal-fin pterygiophore posterior to the 4th vertebral insertion; presence of 2 dorsal procurrent caudal-fin rays between NSPU3 & NSPU2; presence of 3 ventral procurrent caudal-fin rays between HSPU3 & HSPU2; elongated shape of neural spine of the 2nd preural vertebra; anterior epural bone short. ……… P. echinogaster
ACKNOWLEDGEMENTS
Our sincere thanks are due to K.T. Shao, fish database of Taiwan, Oliver Crimmen and Patrick Campbell, Natural History Museum, London, Keiichi Matsuura, National Science Museum of Tokyo, Yuk Iwatasuki, Department of Marine Biology and Environmental Sciences, Faculty of Agriculture, University of Miyazaki and Institute of Oceanology, Chinese Academy of Sciences, China for giving us the permission to use the radiographic images of Pampus specimens found in their collection. Many thanks to Joacin Naslund from Goteborg, Sweden for his technical assistance.
FINANCIAL SUPPORT
This research was financially supported by the National Natural Science Foundation of China (Grant Nos 31172053, 41276166).