INTRODUCTION
Littoral and shallow water tropical meiofauna are well known for their high taxonomic diversity and large number of life forms (Alongi, Reference Alongi1989). Among all meiofauna taxa, nematodes are by far the best studied, and the factors responsible for spatial and temporal variation of their diversity and abundance are fairly well known (e.g. Long & Ross, Reference Long and Ross1999; Ndaro & Ólafsson, Reference Ndaro and Ólafsson1999; Netto et al., Reference Netto, Warwick and Attrill1999; Raes et al., Reference Raes, De Troch, Ndaro, Muthumbi, Guilini and Vanreusel2007). However, studies about the diversity and abundance of other meiofauna taxa are still scant. This applies particularly to the harpacticoid copepods, which attain a relative abundance of up to 82% in coral sand (Gray, Reference Gray1985) and 5% in mangrove silt meiofauna (Rao & Murty, Reference Rao and Murty1988).
Most publications on tropical Harpacticoida deal with systematics, including the description of new taxa and redescription of poorly known species, while the study of community structure has been largely disregarded. This is a consequence of the poor knowledge about tropical fauna in general. The probability of discovering new taxa is very high in the tropics, e.g. Wells (Reference Wells1967) described 43 new species in a study about the harpacticoid fauna of Inhaca Island (Mozambique), Villiers & Bodiou (Reference Villiers and Bodiou1996) recorded 21 undescribed species in samples taken from Fangataufa Atoll (French Polynesia), and Chertoprud et al. (Reference Chertoprud, Gómez and Gheerardyn2009) recognized 30 undescribed species in a small part of Nha Trang Bay (Vietnam). Furthermore, studies about the assemblage structure of tropical benthic harpacticoids are fragmentary and contradictory. For the eastern hemisphere, detailed descriptions are available about the species complexes inhabiting soft sediments in Mozambique, Tanzania, India and Vietnam (Wells, Reference Wells1967; Kondalarao, Reference Kondalarao1984; Arunachalam & Balakrishnan, Reference Arunachalam and Balakrishnan Nair1988; Rao & Murty, Reference Rao and Murty1988; Ansari & Parulekar, Reference Ansari and Parulekar1993; Gheerardyn et al., Reference Gheerardyn, De Troch, Ndaro, Raes, Vincx and Vanreusel2008; Chertoprud et al., Reference Chertoprud, Gómez and Gheerardyn2009). For the western hemisphere, there are descriptions of the harpacticoid assemblages inhabiting coastal sediments of Tuamotu Archipelago, the Bermuda and Virgin Islands, and the western coast of Mexico (Coull, Reference Coull1970; Hartzband & Hummon, Reference Hartzband and Hummon1974; Villiers & Bodiou, Reference Villiers and Bodiou1996; Morales-Serna et al., Reference Morales-Serna, Gómez and Bustos-Hernández2006). So far, a comprehensive analysis of the harpacticoid assemblages from different tropical regions has not been carried out.
The assemblage structure of harpacticoid copepods inhabiting soft sediments of littoral and sublittoral areas of Cat Ba Archipelago (South China Sea) is herein analysed. In addition, a comparative analysis of the shallow-water harpacticoid communities from different tropical regions of the Indian and Pacific Oceans is presented based on published data, and the relationships between biotope type and harpacticoid diversity and abundance, as well as the effect of similar biotopes on the taxocenes of different areas in the tropical region are addressed.
MATERIALS AND METHODS
Original data from the Cat Ba Archipelago
The Cat Ba Archipelago is composed of limestone and located in Ha Long Bay (Vietnam) in the north-western part of the South China Sea. This archipelago lies at about 20 km from the mainland and includes more than 40 islands, the largest one being Cat Ba Island.
Sediment samples were taken at 20 stations from seven locations in the south-eastern part of the archipelago (Figure 1) during April 2008 (in the dry season). Six stations were located in intertidal rock pools, and 14 stations were located in the upper sublittoral zone which was characterized by the presence of mixed coral–quartz sands.

Fig. 1. Locations sampled during spring 2008, at Cat Ba Archipelago (Vietnam). Location (А) combines stations 1–5; (B) stations 6 and 7; (C) stations 8 and 9; (D) stations 10–13; (E) stations 14–16; (F) stations 17–19; (G) station 20.
The studied tidal rock pools were situated not higher than 0.5 m above sea level during high tide. The sea waves swamped the pools regularly, refreshing the water constantly. The depth of the pools varied from 20 to 50 cm, and the walls were covered by encrusting barnacles and young oyster shells. The bottoms of certain pools were covered with sand at the surface of which lie debris. Dense mats of green filamentous algae were observed in certain other pools. Most pools were inhabited by Talitroidea (Amphipoda) and harboured the remains of large invertebrates (mainly crabs).
Triplicate sediment samples were collected from each station in the upper sublittoral zone (during low tide at a depth of 50 cm) and from each tidal rock pool, using a 2 cm-diameter corer (surface area 3.14 cm2). Only the upper 5 cm of sediment were retrieved. In tidal rock pools without sand, samples of debris were collected using the same corer. All samples were fixed with 4% formaldehyde and sieved through a 40 µm mesh.
Granulometric analyses were performed for the sediments collected from all stations (Table 1). Mean grain size, silt content (<63 µm) and content of large-sized fragments (≥2 mm) were calculated.
Table 1. Environmental variables recorded at Cat Ba Archipelago (Vietnam). Mo, modal particle size; Tp, tidal pool.

Identification of harpacticoid copepods
Adult harpacticoids were identified using Lang (Reference Lang1948, Reference Lang1965) and Boxshall & Halsey (Reference Boxshall and Halsey2004). The taxonomic system of European harpacticoids was used for species list compilation (Costello et al., Reference Costello, Emblow and White2001). The taxonomic status of Miraciidae Dana, 1846 follows Willen (Reference Willen2000, Reference Willen2002). Synonymization of species was carried out according to Bodin (Reference Bodin1997). Harpacticoid species and genera were classified into life forms following Chertoprud et al. (Reference Chertoprud, Chertoprud, Kondar, Kornev and Udalov2006), i.e. phytal, planktonic and epibenthic. Also, facultative interstitial (represented by small non-specialized species) and genuine interstitial forms (vermiform and lanceolate species) were recognized.
Literature sources
Information about the harpacticoid copepod fauna from the tropical region was obtained from published literature. The selection of literature sources has been based on three criteria. First of all, the selected paper should contain a detailed list of harpacticoid species as well as a description of the localities where the samples have been collected. Secondly, papers containing data about absolute and relative abundances of species were preferred. Wells (Reference Wells1967) and Gheerardyn et al. (Reference Gheerardyn, De Troch, Ndaro, Raes, Vincx and Vanreusel2008) did not exactly correspond to this criterion, but have been included in the analysis as they both provide a large amount of information about harpacticoids from a poorly studied African region. Wells (Reference Wells1967) has been executed at species level, while Gheerardyn et al. (Reference Gheerardyn, De Troch, Ndaro, Raes, Vincx and Vanreusel2008) include relative abundance data of the dominant genera for the studied area. Thirdly, the investigations should have been based on representative sampling—the total sampling area should be around 200 cm2 or more (including our study at Cat Ba with a total sampling area of 188.4 cm2). All data about abundance and species richness of harpacticoids in the selected publications were standardized to an area of 10 cm2.
The locations of the 11 selected studies about harpacticoids in the tropical region are shown in Figure 2. Associations of coral sands and gravel from the upper sublittoral zone were described along the east coast of Zanzibar (Tanzania) (Gheerardyn et al., Reference Gheerardyn, De Troch, Ndaro, Raes, Vincx and Vanreusel2008), at Fangataufa Atoll (Tuamotu Archipelago, French Polynesia) (Villiers & Bodiou, Reference Villiers and Bodiou1996), along the north-eastern coast of the Bermuda Islands (Coull, Reference Coull1970) and along the southern coast of the US Virgin Islands (Hartzband & Hummon, Reference Hartzband and Hummon1974). At the southern and western coast of Inhaca Island (Mozambique), the species complexes of littoral coral sands and silts were characterized by Wells (Reference Wells1967). Harpacticoid species richness for silty grounds from estuaries has been described for the Mandovi (Ansari & Parulecar, 1993) and Ashtamudi rivers (Arunachalam & Balakrishnan, Reference Arunachalam and Balakrishnan Nair1988) (both in western India). The fauna of silty sediments in mangrove forests has been characterized in detail for the Gautami–Godavari estuarine system (eastern India) (Kondalarao, Reference Kondalarao1984; Rao & Murty, Reference Rao and Murty1988) and for the Urias estuarine system (central Mexico) (Morales-Serna et al., Reference Morales-Serna, Gómez and Bustos-Hernández2006). The harpacticoid fauna of coral sands and mangrove systems from the coast of central Vietnam (Nha Trang Bay) has been described by Chertoprud et al. (Reference Chertoprud, Gómez and Gheerardyn2009). In most of the investigated areas, researchers collected samples during the dry season, with the exception of Fangataufa Atoll and Nha Trang Bay, where samples were collected during the wet season as well. In the estuarine systems of India, Mexico and the Bermuda Islands, samples were collected every month.

Fig. 2. Locations of the selected studies on harpacticoids from the tropical region: (1) Atoll Fangataufa (Tuamotu Archipelago, French Polynesia); (2) Urias river estuarine system (Mexico); (3) Virgin Islands; (4) Bermuda Islands; (5) Zanzibar Island (Tanzania); (6) Inhaca Island (Mozambique); (7) Mandovi river estuarine system (India); (8) Ashtamudi river estuarine system (India); (9) Gautami–Godavari river estuarine system (India); (10) Nha Trang Bay (Vietnam); (11) Cat Ba Archipelago (Vietnam).
Biotope types
Four basic biotope types are recognized for the soft bottom sediments of tropical littoral and shallow systems. The distinction between the different biotope types has been based mainly on aleuropelite content (particle size <0.1 mm) and mean grain size of the sediment particles. Copepod biomass often depends only on the quantitative distribution of the fine aleuropelite sediment fraction (Fleeger & Decho, Reference Fleeger and Decho1987).
WASHED SANDS
Mean grain size ranges from 0.5 to 1.5 mm, with aleuropelite content not exceeding 10%. This biotope type consists of quartz sands with a mixture of coral sand and coral gravel, and usually occurs along exposed coasts and areas not protected by bays. Sediments in these areas are easily suspended by sea currents, thus allowing the deposition of limited amounts of organic matter. Meiofauna usually feeds on bottom microalgae (mainly diatoms) and, to a lesser extent, on ciliates and bacteria, which depend on the presence of organic matter (Alongi, Reference Alongi1989; Azovsky et al., Reference Azovsky, Saburova, Chertoprud and Polikarpov2005).
SILTY SANDS WITH DETRITUS
Mean grain size ranges from 0.3 to 1 mm, with aleuropelite content ranging from 11 to 60%. This biotope type usually occurs in gulfs which are protected from sea currents. Coastal sediments here contain a large amount of organic matter, with small silt content. The hydrogen sulphide layer is located at a sediment depth of 2–4 cm, and food sources for meiofauna are very diverse (Alongi, Reference Alongi1989).
MUDS
Mean grain size ranges from 0.1 to 0.2 mm, with aleuropelite content exceeding 60%. This biotope type is typical for enclosed, narrow gulfs, estuaries, lagoons and sublittoral coastal systems, and often contains large amounts of organic matter. Harpacticoids are very sensitive to oxygen depletion caused by organic matter decomposition, and thus are only able to inhabit a thin well-oxygenated surface layer of the sediment. They are able to penetrate deeper into the sediment only through burrows of macrobenthic organisms (Alongi, Reference Alongi1989).
MANGROVE MUDS
Mean grain size ranges from 0.1 to 0.2 mm, with aleuropelite content exceeding 60%. The present paper deals mainly with muddy habitats which are found in areas dominated by Rhizophora trees. Buttress roots of Rhizophora species produce a vast net of fine roots under the sediment surface. These fine roots prevent mixing and suspension of the sediments, enhancing mud deposition. Usually, Rhizophora trees can be found in enclosed gulfs, estuaries, lagoons, and river deltas.
Statistical analysis
Pairwise similarity of samples was evaluated using the Czekanowski Index (D) (Magurran, Reference Magurran2004): D XY = ∑i=1min(X i, Y i); where X i, Y i are the proportion of individuals belonging to i-th species out of all individuals found from samples X and Y, respectively.
Species richness of different regions was assessed using the Margalef Index (I): I = (W–1)/log2 N; where W is the number of species and N is the number of organisms. In addition, the species/genera ratio for the analysis of local faunistic complexes was used. The relationship between this index and species richness has been previously described for the harpacticoid fauna of the seas of Russia (Chertoprud & Garlitska, 2007).
The taxocene definition and comparison of the taxocene structures
Two methods were used for the classification and separation of taxocene types, namely the modified Braun–Blanquet method and cluster analysis. The method of Braun–Blanquet is widely applied in plant ecology (Mirkin et al., Reference Mirkin, Naumova and Solomeshch2001) and has recently been modified for coastal meiobenthos (Chertoprud et al., Reference Chertoprud, Chertoprud, Kondar, Kornev and Udalov2006). This modified Braun–Blanquet method reaches an eco-faunistic classification of taxocenes on the basis of the deductive–inductive approach. It determines groups of dominant, specific and discriminate species in a series of samples and defines the taxocenes. As a second method, we have used cluster analysis with the average linkage method in the Systat 7.0 software package. As a measure of similarity, we have used the Euclidean distance between the faunas of the different regions: E (x, y) = [b + c]1/2; where b and c represent the number of species (or genera) restricted to species (or genera) group x and y, respectively.
Mean values of the Czekanowski Index have been used as an additional method for the characterization of sample similarity within taxocenes.
Intraregional scale (Cat Ba Archipelago)
The subdivision in taxocenes at the intraregional scale in the Cat Ba Archipelago was based on the relative abundance of species in the community. According to the modified Braun–Blanquet method (Chertoprud et al., Reference Chertoprud, Chertoprud, Kondar, Kornev and Udalov2006), only species with a relative abundance higher than 10% in at least one local sample were considered.
Interregional scale
Harpacticoid species lists from different tropical regions slightly overlap. There are several reasons for this, all of which are related to the poor knowledge about the harpacticoid fauna of the tropics. Certain papers have identified the specimens at the genus level only, or used the operational taxonomic unit, which hampers further comparison between species lists from different sources. Also, species identification in certain other papers is doubtful. Therefore, the similarity between taxocenes calculated on species level is very low (the mean Czekanowski Index (D) is 0.34 ± 0.02), and the reliability of such data is questionable. Conversely, most harpacticoid families show a wide distribution (Chertoprud et al., Reference Chertoprud, Garlitska and Azovsky2010), and the similarity index tends to be 100% (D = 0.96 ± 0.02). This suggests that an optimal taxonomic level for comparison between these taxocenes is at the genus level (D = 0.60 ± 0.07). The specificity of the biotopes and of the regions was assessed at this level.
The subdivision in taxocenes has been made following the modified Braun–Blanquet method (Chertoprud et al., Reference Chertoprud, Chertoprud, Kondar, Kornev and Udalov2006). The relative abundances of genera were used as the initial data to compare taxocene structures at the interregional scale.
RESULTS
Harpacticoid community structure of Cat Ba Archipelago
GENERAL CHARACTERISTICS OF THE FAUNA
Forty-five harpacticoid species belonging to 15 families were collected in the Cat Ba Archipelago. Forty-two species were found in sublittoral sandy sites, and 19 species in the tidal rock pools. The most speciose family was Miraciidae (14 species), followed by Laophontidae and Tisbidae (4 species each), Ectinosomatidae, Dactylopusiidae, and Paramesochridae (3 species each) and Ameiridae, Cletodidae, Harpacticidae, and Leptastacidae (2 species each). The families Canthocamptidae, Canuellidae, Huntemanniidae, Longipediidae, Tachidiidae, and Tetragonicipitidae were represented by only one species each. The species/genera ratio was 1.22.
In the present study, representatives of the genera Harpacticus, Leptastacus, Paraleptastacus, Dactylopusia, Tisbe and Scutellidium were detected for the first time in the South China Sea (Cat Ba Archipelago). Some of these taxa might be particularly abundant in littoral pools.
THE FAUNA OF TIDAL ROCK POOLS
The harpacticoid species list of the tidal rock pools includes 19 species, of which Harpacticus sp. 2, Halectinosoma sp. and Tisbe sp. 2 have not been found in any samples from the upper sublittoral zone. Harpacticus sp. 1, Scutellidium sp., Laophonte sp. 1, Nitocra sp. 1, and Robertgurneya sp. are common inhabitants of tidal rock pools, but are rather rare in neighbouring sediments. Species richness of pools ranged from 2 to 12 species. Mean harpacticoid abundance was 61 ind/10 cm2. This result is similar to that observed for upper sublittoral sandy sediments (74 ind/10 cm2).
All six investigated littoral pools differed from each other in species composition and dominance structure. Mean similarity value of species composition between samples from different pools is 0.15 ± 0.13. Only one species (Harpacticus sp. 1) was found in 5 of the 6 sampled pools. Most species (15) were found in 1, 2 or 3 pools only. Higher values (0.65 ± 0.05) of similarity in assemblage structure of littoral pools with surrounding associations were observed. All species from the tidal rock pools were subdivided into three groups: dominants and subdominants of the surrounding community (6 species), rare members of the surrounding community (10 species), and species which are absent in the surrounding community (immigrants) (3 species). Two of these ‘immigrant' species, Harpacticus sp. 2 and Tisbe sp. 2, are typical phytal representatives, and Halectinosoma sp. is well equipped as a mud-digger species with legs suitable for swimming in the water column. The contributions of each of the three above-mentioned groups of species in rock pools are shown in Figure 3. Species of the first group are the most abundant (81%) in only one pool at Cat Sung Island. Species of the second group dominate in all other pools (58–100%). ‘Immigrant' species occur with rather low abundances in four pools (5–20%).

Fig. 3. Relative abundance (%) of three groups of harpacticoid species (immigrants, dominant and rare species of surrounding communities) in tidal rock pools at Cat Ba Archipelago (Vietnam).
THE TAXOCENES OF SOFT SEDIMENTS FROM THE UPPER SUBLITTORAL ZONE
Three taxocenes (associations) including 42 harpacticoid species were observed in 14 stations located in the upper sublittoral zone (Table 2). Each taxocene was characterized by specific dominant species. The number of rare species ranges from 3 to 27. The mean similarity in species composition between samples within one association is high (Czekanowski Index (D) = 0.81 ± 0.03). Usually, similar biotopes contain one unique type of taxocene. This was true even between similar biotopes from different islands separated by relatively deep straits.
Table 2. Characteristics of the harpacticoid taxocenes in the sublittoral zone at Cat Ba Archipelago (Vietnam). The taxocenes were allocated using the modified Braun–Blanquet method. Relative abundances of species in the different taxocenes are presented.

Taxocene type 1
The Amphiascoides sp.-taxocene (stations 1, 2, 14, 15 and 19) was observed in coarse-grained, washed, and mixed quartz–coral sandy sediments (biotope type A, as described in the Materials and Methods section). This taxocene is typical for the coasts of the Cat Ba, Quai Xanh and Va Sat Islands. The dominant species is the facultative interstitial Amphiascoides sp. (mean relative abundance (m.r.a.) is 54.8%). Subdominant species are represented by the epibenthic Paralaophonte sp. 1 (m.r.a. 8.7%), the true interstitial Paramesochra sp. (m.r.a. 5.4 %) and Tisbe sp. 1 (m.r.a. 6.3%). The mean abundance of adult harpacticoids is 141 ind/10 cm2 and the total number of detected species is 23. The mean similarity of species composition within this taxocene is 0.85 ± 0.04 (Czekanowski Index, D). Only in this taxocene on Cat Ba Archipelago an interstitial harpacticoid fauna is present with high species richness and high relative abundance. This interstitial fauna includes 25% of the total number of species and more than 50% of relative abundance.
Taxocene type 2
Taxocene Stenhelia latioperculata (Itô, 1981)–Paramphiascella sp. (stations 6–11 and 20) was observed in medium-grained, slightly silty sands (biotope type B). It was present in Cat Ba, Cat Sung, Cong Dui and Tai Keo Islands. The dominant species of this taxocene are S. latioperculata (m.r.a. 22.7%) and Paramphiascella sp. (m.r.a. 17.7%). The epibenthic Tisbe sp. 1 (m.r.a. 12.8%) and Miraciidae sp. 1 (m.r.a. 5.7%) as well as the facultative interstitial species, Amphiascoides sp. (m.r.a. 6.4%) and Nitocra sp. 2 (m.r.a. 5%), are less abundant. The mean abundance of adult harpacticoids was rather low (81 ind/10 cm2). The total number of detected species was 32. The mean similarity of species composition between samples within this taxocene is 0.73 ± 0.06.
Taxocene type 3
Taxocene Phyllopodopsyllus sp.–Tisbe sp. 1–Amphiascoides sp. (stations 17 and 18) was observed in medium-grained, slightly silty sands covered by green filamentous algae (this does not correspond to any of the described biotope types in the Materials and Methods section). This taxocene is distinctive for Quai Xanh Island. The epibenthic Phyllopodopsyllus sp. (m.r.a. 20.1%), Tisbe sp. 1 (m.r.a. 18.1%), and the facultative interstitial Amphiascoides sp. (m.r.a. 16.4 %) were observed to be the dominant species. The epibenthic Paralaophonte sp. 1 (m.r.a. 7.1 %) and Microarthridion litospinatus Shen & Tai, Reference Shen and Tai1973 (m.r.a., 10.7 %) as well as the true interstitial Paramesochra sp. (m.r.a. 10.8 %), Apodopsyllus sp. and Paraleptastacus sp. (m.r.a. 3.7% each) were subdominant. The mean abundance of adult harpacticoids was low (69 ind/10 cm2). The total number of detected species was 9. The similarity of species composition between samples within this taxocene was 0.85 ± 0.06.
Comparative analysis of harpacticoid community structure from different tropical regions
SPECIES RICHNESS
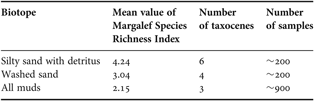
The comparative analysis of species richness in different biotope types of the tropical region revealed an extremely poor harpacticoid fauna in muddy habitats (Figure 4). Maximum harpacticoid diversity was observed in silty sands with detritus. Species richness of washed sands is slightly lower than in silty sands with detritus (Figure 4). The mean number of species in muddy habitats did not exceed 18 species in a small number (about 10) of relatively large samples (50 cm2), as well as in several hundreds of fairly small samples (<10 cm2). Species richness is distinctly higher in sublittoral muddy habitats than in littoral muddy sites. Mean species number in washed sands is 33, whereas a mean of 40.5 species was calculated for silty sands with detritus. Certain genera of the families Miraciidae and Ameiridae were the most frequent taxa, followed by genera of Ectinosomatidae and Canthocamptidae.
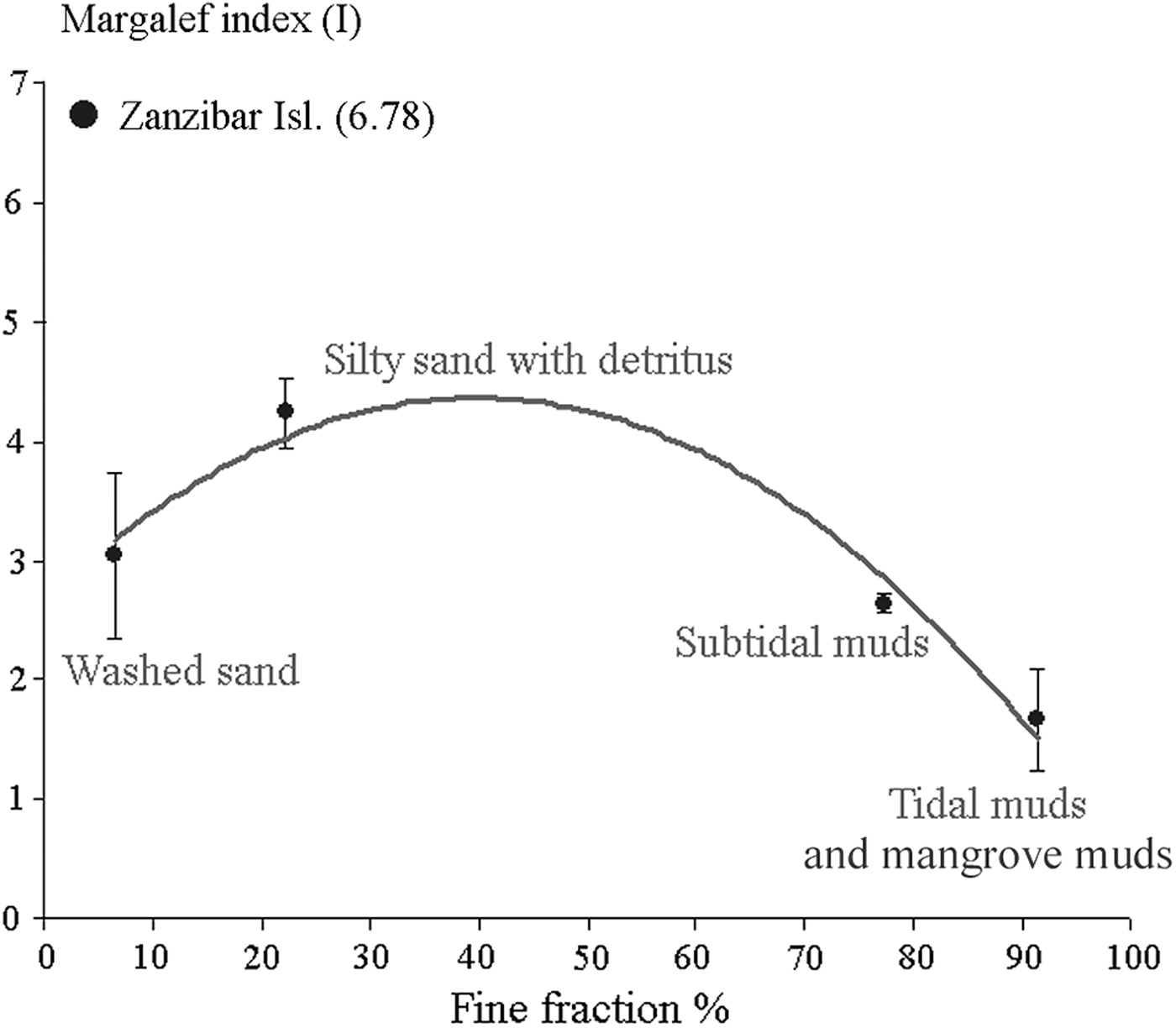
Fig. 4. Relationship between harpacticoid species richness (Margalef Index, I) and aleuropelite (grain size <0.1 mm) weight fraction (%) in different sediment biotope types of the tropical region.
Noteworthy, harpacticoid species diversity of washed coral sands of Zanzibar Island exceeded the values reported in all other selected studies by more than 30% (Figure 4).
SPECIES/GENERA RATIO
The species/genera ratio in the tropical region (excluding the study in Zanzibar) increased with species richness from 1.1 (20 species) to 1.3 (30–40 species), and remained relatively constant around 1.4 when species richness was higher than 50 species (Figure 5). The number of species within one genus usually did not exceed 2.

Fig. 5. Relationship between harpacticoid species/genera ratio (S/G) and species richness in sublittoral and littoral zones from tropical (excluding Zanzibar) and Arctic sites.
Harpacticoid species/genera ratio of washed coral sands at Zanzibar Island was 1.8, which exceeded the values reported in all other selected studies by more than 20%.
ABUNDANCE
Absolute harpacticoid abundance in muddy sediments was lower than in other biotope types at one location (e.g. at Inhaca Island, at Nha Trang Bay, at the Urias river estuarine system). The highest abundance was observed in washed sandy sediments. Harpacticoid abundance in silty sands with detritus was similar to the values recorded for washed sandy sediments, but on average was lower. For example, in Nha Trang Bay harpacticoid abundance in muddy sediments was 17 ± 5 ind/10 cm2, in silty-sands with detritus 83 ± 11.5 ind/10 cm2, in washed sandy sediments 179 ± 37.5 ind/10 cm2.
A similar trend in abundance values for different biotopes was observed across the selected studies in the tropical region (Figure 6). Mean harpacticoid abundance ranged from 12 ± 5 ind/10 cm2 in littoral muds to 132 ± 49.5 ind/10 cm2 in washed sands.

Fig. 6. Variability of adult harpacticoid abundance related to aleuropelite (<0.1 mm) weight fraction in different sediment biotope types of the tropical region.
DIVERSITY AND STRUCTURE OF THE TAXOCENES
The taxocenes from the four main biotope types of the tropics can be subdivided into two groups according to their taxonomic structure, which seems to be characteristic either for sandy or muddy sediments. The mean similarity (Czekanowski Index) between these two groups was low (0.44 ± 0.04), whereas the homogeneity of species structure within the groups was much higher (0.6 ± 0.02). The taxocenes of muddy and sandy sediments have been analysed separately.
The fauna of muds and mangrove muds
Our database compiled from original and literature data about harpacticoid communities from muddy sediments contains 31 genera. Twenty per cent of the genera were found in more than half of the studied loci. Almost all dominant genera are epibenthic. Three types of taxocenes can be distinguished in muddy sediments from the tropics (Figure 7).

Fig. 7. Cluster dendrogram: similarity at genus level of the harpacticoid faunas from muddy sediments and mangroves of the compared areas in the tropical region. *, mangroves in Nha Trang Bay 2004 grow on silty sands with detritus, all other compared mangroves grow on muds.
1. The taxocene Halectinosoma–Diarthrodes. This type of taxocene was observed in the sublittoral region of Nha Trang Bay (Vietnam). Halectinosoma and Diarthrodes were the dominant genera with a total relative abundance of 61%. The Czekanowski Index yielded similarity values of 0.72 ± 0.02 between the sublittoral stations. The distance between the different stations inhabited by this taxocene is about 5 km.
2. The taxocene Stenhelia–Enhydrosoma–Pseudostenhelia–Halectinosoma. This taxocene is common for mangrove forests and estuaries without mangroves from India and Mexico. Stenhelia, Halectinosoma, Enhydrosoma and Pseudostenhelia are the most abundant genera during the dry season, with a total relative abundance of 53%. Also, Kristensenia was observed to be abundant during the dry season in Mexican estuaries. Kristensenia species have been reported only in sites with mangrove vegetation, and this genus is probably restricted to this habitat (Por, Reference Por1983; Gómez & Rocha, Reference Gómez and Rocha2005). The mean similarity between stations within this taxocene is 0.63 ± 0.03. The distances between the different stations inhabited by this taxocene ranges from 800 to 15000 km. Regional differences within this taxocene were noted. Indian and Mexican brackish water estuaries differ in species composition, and most of all during the rainy season. In Mexico, brackish-water species of Cletocamptus, Tisbella and Nannopus were observed, and Onychocamptus, Nannopus, Nitocra and Mesochra were recorded in studies carried out in India.
3. The taxocene Nitocra–Laophontidae. This type of taxocene was observed for Indian estuarine muddy habitats with macrophytes. Nitocra and different genera of Laophontidae (namely Laophonte, Paralaophonte and the brackish-water Onychocamptus) are the dominant taxa. These genera account for a total relative abundance of 67%.
The fauna of washed sands and silty sands with detritus
Sixty-eight harpacticoid genera were reported from sandy sediment in all compared tropical studies, and only 4% were found in more than half of them. Most of the dominant genera are interstitial, and 8 types of taxocenes can be distinguished in sandy sediments (Figure 8). Only two types of taxocenes were found in washed sands, whereas four taxocenes were typical for silty sands with detritus. Two taxocenes were unique for mixed washed and silty sands with detritus.

Fig. 8. Cluster dendrogram: similarity at genus level of the harpacticoid faunas from sandy sediments of the compared areas in the tropical region.
The taxocenes characteristic of washed sands have been reported in the middle sublittoral zone of the Bermuda Islands (taxocene 2) and in the upper sublittoral zone of Zanzibar Island (taxocene 6) (Figure 8). These communities are almost exclusively dominated by interstitial forms of the families Ameiridae (Ameira and Nitocra), Miraciidae (Amphiascus and Typhlamphiascus), Leptastacidae (Leptastacus) (for this family Zanzibar is an exception) and Paramesochridae (Apodopsyllus and Emertonia). The total relative abundance of the dominant genera of taxocenes 2 and 6 are 47% and 65% respectively.
The taxocenes characteristic of silty sands with detritus, are more diverse (Figure 8). Taxocene 1, with a prevalence of the epibenthic genus Phyllopodopsyllus, unites the stations from the littoral zone of Vietnam and the sublittoral zone of the Bermudas. The three other taxocenes (3, 4 and 8) have been reported in littoral and sublittoral zones of Vietnam, the dominant genera being epibenthic (taxocene 8) and interstitial (taxocene 3).
Taxocenes 5 and 7 have been reported from mixed sands (Figure 8). Taxocene 5 unites stations from the Virgin Islands and Vietnam, and epibenthic (Robertgurneya, Stenhelia and Paralaophonte) and interstitial genera (Amphiascus and Ameira) are abundant. Taxocene 7 is more widely distributed in the tropics. This type of community has been reported from Vietnam, the Bermudas and Fangataufa Atoll. Similarity between locations at the genus level is relatively high (0.74 ± 0.05), and epibenthic (Stenhelia, Paramphiascella and Paralaophonte) and interstitial genera (Amphiascoides, Typhlamphiascus, Nitocra and Ameira) are abundant. The ratio of abundance of interstitial to epibenthic dominant genera in washed sands and silty sands with detritus is 3:1 and 1:2, respectively. The relative abundance of interstitial genera is higher in washed sands, while epibenthic genera are dominant in silty sands with detritus.
DISCUSSION
Cat Ba Archipelago
THE FAUNA OF TIDAL ROCK POOLS
The fauna of tidal rock pools is composed of species from surrounding upper sublittoral communities. The similarity in assemblage structure of littoral pools with the surrounding taxocene is high (0.65 ± 0.05). Most immigrant species of tidal rock pools are typical for phytal communities. Most probably, these species are transported with fragments of macrophytes, and account for less than 20% of relative abundance in tidal rock pools. According to our own observations, the Harpacticidae, which is a diverse and abundant family in tidal rock pools, is mostly composed of facultative necrophagous species, which feed on resources that usually accumulate in rock pools. The process of decomposition and softening of dead tissues is very fast in shallow pools due to the relatively high water temperature (≥30°C), thus facilitating consumption by harpacticoids. The first pair of swimming legs of Harpacticus has short hook-like spines, allowing the harpacticoids to hold and to tear dead tissue into pieces. Another species of the same family, Tigriopus brevicornis (Müller, 1776), displays a predatory behaviour on living juveniles of codfish (Gadus morhua) (V.V. Hlebovitch, personal communication). Harpacticoids in the near-bottom water layer cling to the fish and start eating it. The number of attacking specimens of Tigriopus is so large, that they cover the entire fish and give it an orange colour.
The fauna of littoral pools consists mostly of species from surrounding biotopes. The species lists and assemblage structure of pools are very variable and not easy to predict. In addition, organisms which get into the pool have different chances of survival under such new conditions. Thus, a complex consisting of selected taxa gradually forms in these pools. Some of these taxa are well adapted to the surrounding conditions; other taxa are washed into the pool by wave action and because of this are numerous there. According to Jensen (Reference Jensen1979), the second case does not mean that a constant population exists in the pool, because the composition changes permanently, and dead organisms are constantly replaced by newly arriving specimens.
Probably, the rock pools could supply surrounding biotopes with new species (from the rock pools species group ‘immigrants'). This may occur, for example, in the time of a heavy storm, when water from the pools can be splashed into the surrounding biotopes.
THE FAUNA OF THE UPPER SUBLITTORAL ZONE
Three basic types of taxocenes were observed in the upper sublittoral zone in Cat Ba Archipelago, and these were associated with different biotopes (Table 2). Taxocene type 1 inhabits coarse-grained, washed, and mixed quartz–coral sandy sediments (biotope type A), taxocene type 2 was observed in medium-grained, slightly silty sands (biotope type B), and taxocene type 3 occurs in medium-grained, silty sands covered by filamentous algae (specific biotope type). Sediment grain size and the presence of green filamentous algae might determine the structure of these taxocenes. The highest heterogeneity of functional assemblage structure (dominance structure of life forms) occurs in silty sands with detritus. This biotope offers appropriate habitat conditions for many life forms, and which of them will prevail likely has a probabilistic nature, instead of being defined by certain environmental factors. For example, it may be connected to which species were the first to occupy this biotope.
A similar assemblage structure was detected in sublittoral harpacticoid taxocenes of Nha Trang Bay (Vietnam), located further south along the Vietnamese coast. The distance between both locations is more than 1000 km. The dominant species in coarse-grained washed sands of Nha Trang Bay is a different representative of Amphiascoides, while Stenhelia latioperculata and Phyllopodopsyllus are dominant in the medium-grained slightly silty sands of both Cat Ba and Nha Trang Bay (Chertoprud et al., Reference Chertoprud, Gómez and Gheerardyn2009). Soft bottom harpacticoid communities from the northern and central coasts of the South China Sea seem to have a number of the same or related dominant species. The similarity between associations inhabiting coarse-grained sands of Cat Ba and Nha Trang Bay calculated using the Czekanowski Index is 0.62 ± 0.09. This is a high value because the similarity between samples within taxocenes has values from 0.6 tо 0.85.
Comparative analysis of harpacticoid community structure in different tropical regions
SPECIES RICHNESS
The heterogeneous distribution of species richness and diversity of harpacticoid copepods in different biotopes is a well-known phenomenon (e.g. Hicks & Coull, Reference Hicks and Coull1983; Coull, Reference Coull1985; Coull & Dudley, Reference Coull and Dudley1985; Fleeger & Decho, Reference Fleeger and Decho1987). Species diversity of harpacticoids is generally low in muddy sediments, despite the high availability of organic matter in muds, providing a good foraging base for a number of species. Most likely, the low diversity of harpacticoids in tropical littoral muddy sediments is due to higher sediment temperatures during low tide. Further, as the sulphurous layer in muddy sediments occurs near the surface, oxyphilic copepods cannot migrate deeper into the sediment to avoid high temperatures (Alongi, Reference Alongi1989), and undergo more harsh conditions in muddy sediments of tropical littoral and shallow systems. The fact that species richness is higher in silty sands mixed with detritus is probably due to higher habitat heterogeneity and a diverse food availability (organic matter, microphytobenthos, ciliates and small meiobenthos organisms), allowing a larger number of species to occur. Washed sands are usually less diverse, and the species found in these types of sediments are interstitial forms. Also, these species have a limited trophic spectrum when compared to the fauna of silty sands with detritus (Chandler & Fleeger, Reference Chandler and Fleeger1984; Gee & Warwick, Reference Gee and Warwick1984).
The very high harpacticoid diversity and species/genera ratio described from washed coral sands along the eastern coast of Zanzibar (Gheerardyn et al., Reference Gheerardyn, De Troch, Ndaro, Raes, Vincx and Vanreusel2008) might be due to high sediment heterogeneity. Sites with coral gravel and coral fragments supporting a rich fauna are close to the sites with coarse coral sands. Probably, the fauna of these three types of substrates (coral fragments, coral gravel and coral sand) is partially mixed, leading to an increase in species richness and diversity through increased habitat heterogeneity.
SPECIES/GENERA RATIO
The species/genera ratio of the studied tropical areas is consistently lower than in studies from the Arctic zone (White Sea, Barents Sea and Spitsbergen), which were based on a similar number of habitats and locations (Figure 5). The analysed species lists from the Arctic and the tropics were almost similar in number of species. According to our data, the species/genera ratio in the tropics is 1.25 ± 0.15, while in the Arctic it is 1.56 ± 0.08 (Chislenko, Reference Chislenko and Letova1967; Agarova et al., Reference Agarova, Voronova, Galtsova, Yoffe, Letova, Streltcov, Streltcova and Mishustina1976; Letova, Reference Letova and Skarlato1982; Gee & Huys, Reference Gee and Huys1994; Bick & Arlt, Reference Bick and Arlt2005; Chertoprud et al., Reference Chertoprud, Chertoprud, Kondar, Kornev and Udalov2006) (Figure 5). Harpacticoid communities in different Arctic biotopes are usually composed of a similar set of genera, while the taxonomic composition of communities in different tropical biotopes is highly variable and the number of common genera is low. This might be the result of higher variability between biotopes in the tropics than at higher latitudes. These differences might be due to dense packing of ecological niches of tropical macro- and meiobenthic communities, sharpening the competitive relationships (Alongi, Reference Alongi1989). In addition, sediment temperature is higher (≥30°C) in tropical littoral and shallow systems, especially during low tide, leading to oxygen depletion (Kusakin et al., Reference Kusakin, Gulbin and Nguen1998). All this suggests a stronger variation in habitat conditions in tropical muddy and washed sandy sediments with low oxygen content, than in northern latitudes where sediment temperature hardly reaches more than 20°C in summer (Burkovsky, Reference Burkovsky1992).
In addition, an alternative hypothesis to explain the lower species/genera ratio in tropical areas needs to be tested. The higher diversity on genus level in the tropical region compared on the world scale might be due to biogeographical aspects. The Arctic is geographically more unified, and therefore its fauna mainly consists of widely distributed genera. An indirect confirmation of this hypothesis is the fact that the Arctic fauna is not rich and includes many cosmopolitan species and genera (Chertoprud et al., Reference Chertoprud, Garlitska and Azovsky2010).
ABUNDANCE PATTERNS
In the compared tropical areas, total adult harpacticoid abundance is higher in washed sediments with low aleuropelite content than in muddy sediments (Figure 6). Similar values have been reported for the soft sediments of the White Sea (Chertoprud et al., Reference Chertoprud, Chertoprud, Kondar, Kornev and Udalov2006). The relative abundance of interstitial and epibenthic genera in tropical areas varies considerably with sediment type. Interstitial genera dominate in washed sands (relative abundance about 60%). In muddy sediments, interstitial genera do not attain more than 4% of relative abundance, while epibenthic genera are more numerous (not less than 70% relative abundance). The interstitial harpacticoid fauna consists mainly of small forms (total length, ≤ 0.3 mm), while the epibenthic fauna consists of larger animals (total body length about 0.5 mm) (Hicks & Coull, Reference Hicks and Coull1983; Azovsky & Chertoprud, Reference Azovsky and Chertoprud2003). Thus, although abundance of harpacticoids in washed sands is higher, their biomass in these types of sediments is usually lower than in silty sands with detritus.
DIVERSITY AND STRUCTURE OF THE TAXOCENES
Different trends were observed when analysing the taxocene structure of tropical muddy sediments. On the one hand, the dendrogram (Figure 7) shows similar taxocenes in muddy sediments with mangrove forests and in muddy sediments without these forests (taxocene 2). On the other hand, species dominance structures in sites with muddy sediments with mangrove trees from India and Mexico are very different from Vietnamese (Nha Trang Bay 2004) locations (where similar species of mangroves grow on silty sands with detritus) as shown by an extremely low similarity value (D = 0.36 ± 0.01). In sandy sediments close to Rhizophora trees on Thre Island (Nha Trang Bay), a dominant set of species strongly different from that normally found in muddy sediments with mangrove trees was observed by Chertoprud et al. (Reference Chertoprud, Gómez and Gheerardyn2009). According to our data, harpacticoids do not form a specific structure of communities in systems where Rhizophora mangrove forests are present. However, this assertion requires further, more detailed verification. The determining factor during the formation of a given taxocene is probably related to sediment composition and environmental variables, but not to the presence of a particular mangrove species.
From the analysis above, it seems that species diversity is generally lower in muddy than in sandy sediments, where the taxocene is also different (Table 3). The communities from muddy sediments are the most homogeneous throughout the tropical zone, and this fact seems to be supported by the considerable high number of samples taken from this biotope. Harpacticoid communities inhabiting washed sands and silty sands with detritus showed to be more diverse and are also different between areas. In these biotopes, parallel communities prevail on local and global scales. According to Thorson (Reference Thorson1957), parallel communities are composed of different but closely related genera or even species. A possible explanation for the formation of parallel harpacticoid communities on global scales is the geological dispersal by continental drift. It has already been suggested that the assessment of the taxonomic differences between species, and their present geographical distance, will reveal new insights into the interplay between biological and geological mechanisms of speciation and dispersal (Sterrer, Reference Sterrer1973).
Table 3. Integrated characteristics of harpacticoid diversity and composition, and amount of analysed material from original and published data in different biotope types of the tropical region.
The structure of the harpacticoid communities from Mexican and Indian estuarine muddy sediments are highly similar to the communities from White Sea estuaries (D = 0.65 ± 0.07). The main dominant genera in these estuaries are in the tropics—Stenhelia, Halectinosoma, Enhydrosoma and Pseudostenhelia; and in the White Sea—Stenhelia, Halectinosoma, Huntemannia and Tachidius (Chertoprud et al., Reference Chertoprud, Chertoprud, Kondar, Kornev and Udalov2006). The brackish-water genera characteristic for India (Nitocra, Mesochra, Onychocamptus and Nannopus) are, to some extent, similar to those of the White Sea (Nitocra, Mesochra, Onychocamptus, Nannopus and Microarthridion) (Chertoprud et al., Reference Chertoprud, Chertoprud, Kondar, Kornev and Udalov2006). This suggests that the structure of estuarine harpacticoid communities is distributed in different climatic zones and has similar features in the latitudinal range from the tropics up to the Arctic regions.
CONCLUSION
The comparative analysis based on original and published data of the harpacticoid fauna from different tropical areas has led to the following ecological observations. In the compared tropical areas: (1) the highest harpacticoid species richness was observed in silty sands with detritus, and the lowest in muds; (2) the highest harpacticoid abundance was observed in washed sands, and the lowest in muds; (3) mangrove vegetation does not seem to play a determining role in the formation of the harpacticoid taxocene, while the sediment characteristics seem to be more important; (4) the harpacticoid communities inhabiting washed sands and silty sands with detritus showed to be more diverse and are also different between areas; (5) the harpacticoid assemblage structure is most homogeneous for muddy sediments throughout the tropical zone; and (6) the structure of tropical harpacticoid estuarine communities has similar features in the tropics and the Arctic region. Further analyses of the structure of tropical meiobenthic communities will improve our knowledge about the observed patterns.
ACKNOWLEDGEMENTS
This research was supported by Russian Foundation for Basic Research (grant no. 12-04-00284-a). The authors thank their colleague Dr T.A. Britaev from the Maritime Department of the Russian–Vietnamese Tropical Research and Technology Center for providing the materials for this work.













