INTRODUCTION
The Mediterranean Sea is highly susceptible to biological invasions for several reasons: placement within or near the Atlantic, Erythrean, and Pontic regions, busy maritime traffic, high density of aquaculture farms (Galil & Zenetos, Reference Galil, Zenetos and Leppäkoski2002). Intrinsic reasons for biological invasions of the Mediterranean Sea are linked to its physical features: temperatures are almost tropical in shallow waters in the summer, and temperate in the winter; the recent tendency to global warming is leading to the tropicalization of the basin (Boero, Reference Boero and Briand2002). This may provide greater colonization opportunities for tropical species. Extrinsic reasons for biological invasion are linked to human activities: (a) the opening of the Suez Canal in 1869 allowed entry of Indo-Pacific and Erythrean biota (Boero, Reference Boero and Briand2002; Galil & Zenetos, Reference Galil, Zenetos and Leppäkoski2002); (b) shipping activities transporting species in both fouling and ballast waters (CIESM, 2002); (c) escape of exotic species, used to enhance aquaculture yields, from culturing grounds (CIESM, 2002). In particular, the study of lessepsian immigration from the Red Sea to the Mediterranean Sea through the Suez Canal originated a wealth of information about Indo-Pacific species entering the basin (e.g. Por, Reference Por1978; Spanier & Galil, Reference Spanier and Galil1991). A first list of species that had newly entered the Mediterranean basin via the Suez Canal was compiled by Por (Reference Por1978). Lately, investigations on the Suez Canal revealed an intensification of the Erythrean invasion (Halim & Messih, Reference Halim and Messih1999). Shipping is probably the largest vector for the movement of non-indigenous marine species across the globe (Ruiz et al., Reference Ruiz, Carlton, Grosholtz and Hines1997), and the Mediterranean Sea, with its high-volume shipping routes and degraded habitats, is clearly highly susceptible to ship-transported bioinvasions (CIESM, 2002). Zenetos et al. (Reference Zenetos, Çinar, Pancucci–Papadopoulou, Harmelin, Furnari, Andaloro, Bellou, Streftaris and Zibrowius2005) assembled an annotated list of 963 alien marine species in the Mediterranean Sea. With over 300 species, the Erythrean alien contingent is mainly confined to the eastern part of the basin (Galil & Zenetos, Reference Galil, Zenetos and Leppäkoski2002). At present the entrance of new species is well-monitored and new records of conspicuous species, usually belonging to popular groups such as fish, molluscs, crustacea or to groups that form blooms like dinoflagellates, scyphozoan jellyfish and ctenophores, are continuously published (Boero et al., Reference Boero, Bouillon, Gravili and Piraino2003).
Most Hydrozoa are inconspicuous, both in the hydroid and the medusa stage, and are easily overlooked by non-specialists. The group comprises hundreds of species in the Mediterranean Sea (Bouillon et al., Reference Bouillon, Medel, Pagès, Gili, Boero and Gravili2004); some are well represented both in coastal environments and in ship fouling communities.
In particular, the genus Clytia has a global distribution and includes many species (Govindarajan et al., Reference Govindarajan, Boero and Halanych2006). The life cycle of Clytia includes both polyp and medusa stages. Bouillon et al. (Reference Bouillon, Medel, Pagès, Gili, Boero and Gravili2004) report 12 species of Clytia from the Mediterranean, but for only three of them the life cycle is known (C. hemisphaerica, C. linearis and C. viridicans) (Pagliara et al., Reference Pagliara, Bouillon, Boero and Mills2000; Lindner & Migotto, Reference Lindner and Migotto2002). At present, two species of Mediterranean Clytia are recognized as aliens: C. linearis and C. hummelincki (Boero et al., Reference Boero, Di Camillo and Gravili2005). Clytia linearis was firstly recorded from the basin in the 1950s, but has been overlooked as an alien until recently, whereas C. hummelincki was firstly recorded in 1996 from the coast of Calabria (Ionian coast of Italy) (Boero et al., Reference Boero, Gravili, Denitto, Miglietta and Bouillon1997). The polyp of C. hummelincki is redescribed and developmental stages of the medusa are described herein. The aim of the present study was to estimate the distribution of C. hummelincki in the Mediterranean and to focus on the spread of the species along approximately 150 km of coast by in situ observations. Preliminary observations at several sites along the Salento Peninsula (Ionian coast of Apulia, south-coast Italy) showed that this species thrives only in summer, forming dense carpets at 0.5–2 m depth, on rocks covered by encrusting coralline algae that are intensively grazed by sea urchins (Boero et al., Reference Boero, Di Camillo and Gravili2005).
MATERIALS AND METHODS
Hundreds of hydroid colonies were collected by SCUBA diving along the Ionian coasts of Apulia, Italy (Figure 1A). Rearing experiments were made on fertile colonies of Clytia hummelincki collected in September 2004 along the shallow subtidal coasts of Santa Caterina (40°08.430′N 17°58.828′E), Ionian Sea (Figure 1A). Colonies were reared in glass containers filled with 0.45 µm filtered natural seawater until medusa liberation. Temperature and photoperiod were regulated to match the natural cycle. Artemia nauplii were provided for food every two to three days; water was changed after each feeding session. Colonies were examined daily to detect medusa buds. When buds started to develop, the colonies were fed daily. Colonies kept in the laboratory under controlled temperature conditions (25°C) produced medusae in September. Released medusae (6) were divided into two groups of three individuals each: the first group was reared under the same conditions as the hydroid colony (25°C), while the second was kept at 12°C (preliminary observations showed that gonad development was faster at lower temperatures). All medusae were fed daily and reared until their death. All stages of development were recorded with a video camera connected to either a stereo or a compound microscope.

Fig. 1. (A) Map showing the sampling area in the northern Ionian Sea (Lecce, Apulia, Italy); (B) records of Clytia hummelincki colonies in 2003 survey; (C) records of Clytia hummelincki colonies in 2004 survey.
Surveys of the Salento coast
A first survey along 250 km of the Salento Peninsula, from Torre Specchia to Torre Lapillo (Figure 1A), at 0–5 m depth, was carried out between June and July 2003; a second survey was carried out between June and July 2004, following the sampling-protocol reported in Table 1. Each station was inspected visually by a SCUBA diver along a 100 m horizontal transect, parallel to the coastline, in the depth-range optimal for Clytia hummelincki, to detect the frequency (in terms of presence/absence) of the species. If present at two adjacent stations, the species was considered as being continuously present along the stretch of coast delimited by the two stations. The same procedure was adopted in the case of absence. The distribution of C. hummelincki hydroids was calculated using ArcGis 8.1.
Table 1. Sampling-programme with the indications of presence/absence of the Clytia hummelincki species.

A, absence; P, presence.
RESULTS
Description of Clytia hummelincki (Leloup, Reference Leloup1935)
HYDROID
Colony stolonal, hydrorhiza reticular and anastomosed. Hydrotheca short and wide, ~0.35 mm high and ~0.30 mm wide; hydrothecal rim smooth (Figure 2A). Hydrothecal pedicel long, with several distal annulations (generally three) and four proximal ones; sub-hydrothecal spherule present; diaphragm oblique; hypostome peduncled; hydranth with 22 amphicoronate filiform tentacles. Gonotheca on hydrorhiza, sessile to shortly stalked, truncate, tapering below, 0.8 mm in height and maximum diameter 0.28 mm, with two basal annulations (Figure 2B); only one medusa bud per gonotheca.
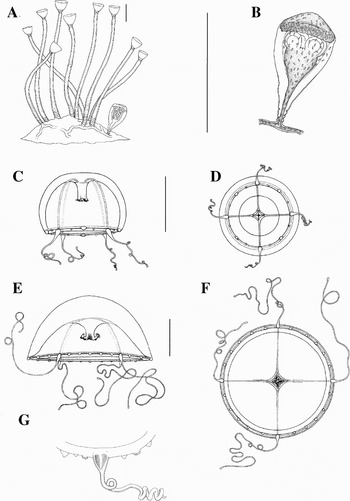
Fig. 2. Clytia hummelincki reared at 25°C: (A) colony with gonotheca; (B) gonotheca with a medusa-bud; (C, D) new born medusa, frontal and oral view; (E, F) 11-day-old medusa, side and oral view; (G) 15-day-old medusa, enlargement of tentacled and atentaculate bulbs, and statocysts. Scale bars: A, 500 µm; B–G, 1 mm.
Cnidome: small microbasic b-mastigophores on both tentacles and hydranth body.
BEHAVIOUR OF HYDROID
Hydranths feed passively: their feeding space coincides with the volume covered by the extended tentacles, and they wait for prey to collide with tentacles. Tentacles with a prey (Artemia nauplii) bend towards the mouth. Up to two prey items can be usually ingested at a time. New ingestions occur about 25 minutes after the preceding one. Every mechanical stimulus is a ‘disturbance’ that causes hydranth retraction into the theca, a typical behaviour of Leptomedusan hydroids (Miglietta et al., Reference Miglietta, Della Tommasa, Denitto, Gravili, Pagliara, Bouillon, Boero and Mills2000).
Medusa reared at 25°C
Three medusae were reared at 25°C for 11 days, but gonad development was not observed.
NEWLY RELEASED (FIGURE 2C,D)
Bell almost spherical, 1.24 mm in diameter, 0.85 mm high; manubrium 0.27 mm long, mouth quadratic, with four lobes; four radial canals, eight bulbs (four large, pyriform, perradial and tentacular ones; four small, rounded and interradial); eight statocysts along circular canal, with one statolyth each; velum ~0.16 mm wide; gonads absent; tentacular tips clavate.
Cnidome: atrichous ishorizas, microbasic mastigophores, on tentacles and manubrium.
Descriptions of further growth stages report on features that were absent or different in previous stages.
FIVE-DAY-OLD MEDUSA
Bell flatter than the previous stage, 1.26 mm high and 3.28 mm in diameter; manubrium 0.41 mm high; 16 bulbs (four large, pyriform, perradial, tentacular; four small, rounded and interradial; eight adradial).
EIGHT-DAY-OLD MEDUSA
Bell almost hemispherical, 3.73 mm in diameter and 1.60 mm high; manubrium 0.42 mm long; mouth quadratic, with large ondulate lobes.
ELEVEN-DAY-OLD MEDUSA (FIGURE 2E,F)
Bell hemispherical, 3.97 mm in diameter and 2.23 mm high. Manubrium 0.48 mm long; mouth quadratic with four large ondulate lobes; 24 bulbs (four tentacular, pyriform, perradial ones; the others small, rounded: four interradial and 16 adradial).
TWELVE-DAY-OLD MEDUSA
Bell hemispherical; 28 bulbs.
FIFTEEN-DAY-OLD MEDUSA (FIGURE 2G)
Bell hemispherical, 4.02 mm in diameter and 2.54 mm high; 38 bulbs, eight tentacles and eight statocysts. No gonads.
Medusa reared at 12°C
Three medusae reared at 12°C developed gonads in 12 days.
FIVE-DAY-OLD MEDUSA (FIGURE 3A)
Bell almost spherical, 1.48 mm in diameter and 1.12 mm high; eight bulbs (four large tentacular, pyriform, perradial ones; four rounded and interradial); eight statocysts along circular canal.

Fig. 3. Clytia hummelincki reared at 12°C: (A) five-day-old immature medusa; (B) male mature medusa (12-day-old). Scale bars: A, 1 mm; B, 2 mm.
EIGHT-DAY-OLD MEDUSA
Bell 1.98 mm in diameter and 1.57 mm high; mouth quadratic; eight bulbs arranged as in five-day-old medusa.
TWELVE-DAY-OLD MEDUSA (FIGURE 3B)
Bell almost spherical, 2.52 mm in diameter and 2.03 mm high; mouth quadratic, with four large ondulate lobes; eight bulbs; eight statocysts along circular canal; four male oval gonads in the upper side of radial canals.
BEHAVIOUR
The medusa catches prey (Artemia nauplii) during swimming, so being a cruising predator (Mills, Reference Mills1981). Captured prey is brought to mouth by tentacle contraction. Clytia hummelincki needs water currents to remain suspended in the water column: this is a problem in rearing because if medusae lie on the bottom they soon die probably due to loss of ability to catch prey. The medusa is very voracious: in a few days specimens ingest up to seven prey with an increasing ingestion of 1–3 prey. Well-fed medusae release captured prey.
REMARKS
The present description does not cover the whole development of the medusa, since gonad maturation and spawning were not observed. The medusae did not grow easily under laboratory conditions and, probably, their natural diet has much different requirements than what is available in an Artemia-based diet. This is, however, the most complete description of the life cycle of this species.
Survey results
FIRST SURVEY (2003)
A total of 217 out of 250 km of the Salento Peninsula coast is rocky, the remainder is characterized by sandy and muddy substrata (Fanelli et al., Reference Fanelli, Piraino, Belmonte, Geraci and Boero1994). Clytia hummelincki colonies were present in half of the surveyed sectors of the Salento Peninsula (125 out of 250 km, 58% of the rocky substrates), from Torre Specchia to Torre Lapillo (Table 1; Figure 1B). The species was particularly abundant in full light, on bare substrates covered by encrusting corallines, being absent on sandy and muddy ones.
Distribution of Clytia hummelincki in the Mediterranean Sea (Figure 4)
The first Mediterranean record of C. hummelincki was from Copanello (Calabria, Ionian Sea), in 1996 (Boero et al., Reference Boero, Gravili, Denitto, Miglietta and Bouillon1997). After its discovery, this species has been widely recorded along the Apulian coast: Tremiti Isles in the Adriatic Sea (S. Fraschetti, unpublished data); Torre del Serpe, Torre Inserraglio and Porto Cesareo in the Apulian Ionian Sea. Colonies of C. hummelincki were recorded from the Croatian coast (C. Di Camillo, unpublished data), Ponza (Naples) (A. Terlizzi, unpublished data), Capo Figari (north-east Sardinia) (P. Guidetti, unpublished data), Portofino (C. Cerrano, unpublished data) and from Majorca Island (P. Schuchert, unpublished data).

Fig. 4. Records of Clytia hummelincki hydroid colonies in the Mediterranean Sea.
World distribution of Clytia hummelincki
The type locality of this species is the West Indies (Leloup, Reference Leloup1935); it has also been recorded from the Gulf of Mexico (Deevey, Reference Deevey and Galtsoff1954), Ghana (Buchanan, Reference Buchanan1957), South Africa (Millard, Reference Millard1966, Reference Millard1975), Papua New Guinea in 1986 (F. Boero & J. Bouillon, unpublished data), Bonaire, the Netherlands Antilles (Bermuda) (Calder, Reference Calder1991), Brazil (Haddad, Reference Haddad1992; Migotto, Reference Migotto1996; Kelmo & Attrill, Reference Kelmo and Attrill2003), North Sulawesi, Indonesia in 2001 (C. Di Camillo, unpublished data), the Galapagos Islands (Calder et al., Reference Calder, Mallinson, Collins and Hickman2003), and the Mediterranean. These records suggest that this species is circumtropical.
Taxonomic remarks
Leloup (Reference Leloup1935) described this species as Laomedea hummelincki due to the features of the hydroid that, having a subhydrothecal spherule, was clearly not a Clytia. Leloup's material, however, was infertile. Millard (Reference Millard1966) described the gonotheca for the first time, reporting it as containing a developing medusa bud. The presence of a medusa in the life cycle is a clear feature of Clytia, the only campanulariid genus with medusae (besides the very peculiar ones of Obelia). Millard (Reference Millard1966) considered the presence of a medusa in the life cycle as having more generic weight than the subhydrothecal spherule and thus transferred the species to Clytia. Cornelius (Reference Cornelius1982) considered the shared feature of a subhydrothecal spherule as a convergence between Clytia and Campanularia–Laomedea. Moreover, the hydrorhiza of Clytia hummelincki is reticular and anastomosed, a typical character of another campanulariid, Orthopyxis. This character, overlooked by Millard (Reference Millard1966, Reference Millard1975) in a colony growing on the surface of Lepas sp. from South Africa, might be a functional specialization of the hydroid stage that occurs in many genera (Boero & Sarà, Reference Boero and Sarà1987). Govindarajan et al. (Reference Govindarajan, Boero and Halanych2006), in a molecular revision of the Campanulariidae, found that C. hummelincki is clearly a member of the clade comprising all the examined Clytia species, having the basalmost position in the clade. From a morphological point of view, the species is clearly referable to Campanularia in the hydroid stage and to Clytia in the medusa stage, constituting a case of ‘inconsistent evolution’ (sensu Boero & Bouillon, Reference Boero, Bouillon and Bouillon1987), resolved by Govindarajan et al. (Reference Govindarajan, Boero and Halanych2006) with a molecular approach.
DISCUSSION
Clytia hummelincki is likely a recent introduction into the Mediterranean Sea (Boero et al., Reference Boero, Gravili, Denitto, Miglietta and Bouillon1997). It is highly improbable, in fact, that such a distinctive species, living in such an easily reachable habitat, passed unobserved in the two centuries of Hydrozoan studies in the Mediterranean. The species is present both in the Atlantic and the Pacific Oceans, and it is difficult, at present, to establish if it entered either from Suez or from Gibraltar (Boero, Reference Boero and Briand2002; Boero et al., Reference Boero, Di Camillo and Gravili2005).
The temperature of the Mediterranean Sea has increased due to global warming. Since the mid-1950s, in fact, an average warming of 0.31°C from the surface to 300 m depth of the world ocean has been recorded (Levitus et al., Reference Levitus, Anotonov, Boyer and Stephens2000; Purcell, Reference Purcell2005), possibly favouring tropical species such as the present one. In general, tropical invasive species gain a distinct advantage over the native Mediterranean fauna (Galil & Zenetos, Reference Galil, Zenetos and Leppäkoski2002).
Most species studied by Galil et al. (Reference Galil, Froglia, Noël and Briand2002) and Golani et al. (Reference Golani, Orsi-Relini, Massuti, Quignard and Briand2002) have been recorded only a few times because their destiny is usually local extinction, but some species are able to replace similar indigenous species.
At present, C. hummelincki can be considered as a successful invader due to its high frequency at 0.5–1 m depth in sea urchin barrens where it forms a ‘belt’ along the Apulian coast. Boero et al. (Reference Boero, Di Camillo and Gravili2005) hypothesized that the rapid expansion of C. hummelincki might be the result of efficient dispersal of the medusa stage mainly obtained by displacement with currents.
Unfortunately, for the moment, its true distribution in the eastern Mediterranean basin is unknown, probably because this species can be easily overlooked by non-specialists. A phylogeographical approach based on DNA sequences might be used to trace the origin of C. hummelincki in the Mediterranean.
The ecological importance of hydrozoan species resides in the feeding habits of their medusae. Purcell (Reference Purcell1989, Reference Purcell1990, Reference Purcell1991a,Reference Purcell and Williamsb, Reference Purcell1997, Reference Purcell2003), Purcell et al. (Reference Purcell, Siferd and Marliave1987, Reference Purcell, Nemazie, Dorsey, Houde and Gamble1994) and Purcell & Arai (Reference Purcell and Arai2001) stressed the role of Aequorea victoria, a hydrozoan species, in the predation on zooplankton of many coastal plankton communities. Jellyfish are potentially important as predators of fish eggs and larvae, as well as being competitors for zooplankton prey with fish larvae and zooplanktivorous fish (Purcell, Reference Purcell2003).
Clytia hummelincki, like other Hydroidomedusae, might play an important ecological role, since it is of the right size to predate on fish eggs and larvae and on their prey too, copepods and larvae, acting as both competitor and predator (Boero et al., Reference Boero, Di Camillo and Gravili2005). The numerous colonies of C. hummelincki probably produce many relatively large medusae. In the Mediterranean Sea, this species might play an important ecological role in the success of fish recruitment by competing with fish by predating on their prey, i.e. zooplanktonic crustacea, possibly feeding also on fish eggs and larvae (Boero et al., Reference Boero, Di Camillo and Gravili2005). The arrival of a new predator not coevolved with the resident species may have a strong impact on local communities, with both competition and predation on species of commercial interest, just as it happened for Mnemiopsis in the Black Sea (CIESM, 2002).
Moreover, the environmental status of the receiving area is a fundamental pre-requisite for the colonization success of alien species. The pre-existing instability of the Posidonia oceanica endemic ecosystem, for example, facilitated the spread of the tropical algae Caulerpa in the north-western basin of the Mediterranean Sea in relation to stress of both natural and anthropogenic origin (Occhipinti-Ambrogi & Savini, Reference Occhipinti-Ambrogi and Savini2003). Therefore, human intervention caused long-term modifications in the Mediterranean Sea environment, preparing a fertile ground for mass bioinvasions of alien species that might alter the original dynamics of the resident communities.
The results of the present study showed that C. hummelincki is particularly frequent on bare substrates, and its propagation, along the Apulian coast, coincides with the frequency of rocky coastal stretches damaged by date mussel fisheries (Fanelli et al., Reference Fanelli, Piraino, Belmonte, Geraci and Boero1994, Reference Fanelli, Giangrande, Miglietta, Morri, Piraino and Rubino1999; Fraschetti et al., Reference Fraschetti, Bianchi, Boero, Buia, Della Tommasa, Denitto, Esposito, Fanelli, Giangrande, Miglietta, Morri, Piraino and Rubino1999), and, therefore, might be caused by the indiscriminate fishing of Lithophaga lithophaga which give rise to barren grounds. Probably the effects of date mussel (Lithophaga lithophaga) collection, and the lack of recolonization due to sea urchin grazing (Fanelli et al., Reference Fanelli, Piraino, Belmonte, Geraci and Boero1994, Reference Fanelli, Giangrande, Miglietta, Morri, Piraino and Rubino1999), heavily damaged hundreds of kilometres of the Salento rocky coasts, transforming them into suitable substrate for C. hummelincki colonies.
In addition, the study of the life cycle of C. hummelincki confirms the relevant role of the temperature on the medusa generation in hydrozoan species (Schierwater & Hadrys, Reference Schierwater and Hadrys1998; Carré & Carré, Reference Carré and Carré2000). Moreover, the stage of maturation reached by C. hummelincki medusae under different temperatures, concurs with the observations reported by Hincks (Reference Hincks1868) for Clytia hemisphaerica. The specimens reared at low temperatures lived shorter lives than those kept at higher temperatures, showing a faster gonad development (Table 2). The same has been observed by Boero & Sarà (Reference Boero and Sarà1987) for Clytia linearis.
Table 2. Features of Clytia hummelincki medusae reared at 25°C and 12°C. Diameters of umbrella are in mm. Unchanged characters are not repeated.

In addition, the patterns of variation of reproduction strategy are affected by temperature in a few invasive hydrozoans (Ma & Purcell, Reference Ma and Purcell2005). The flexible strategy might promote alien invasions and the establishment of populations in new habitats.
The case of the Hydrozoa in general, and of Clytia species in particular, shows that the understanding of ecosystem functioning in terms of species roles is needed for a proper evaluation of biodiversity structure and function. In particular, this species might play an important ecological role in a short time window in a key period of fish population dynamics.
General predictions are not yet available to assess the interactive effects of various environmental and biological factors on invasion success (Carlton & Geller, Reference Carlton and Geller1993; Boero, Reference Boero and Briand2002). Furthermore, Boero (Reference Boero and Briand2002) and Boero & Bonsdorff (Reference Boero and Bonsdorff2007) suggested that the number of variables pertinent to new invaders, and to ecosystem functioning in general, is so great that it is almost impossible to build up predictive models based on insufficient factual information. Moreover, it will be necessary to test for correlations between patterns of invasion and associated environmental data (Ruiz & Carlton, Reference Ruiz and Carlton2003), investigating the changes in ecosystem functioning and in food web structure caused by alien species.
Acknowledgements
This work was supported by MURST (COFIN and FIRB projects) and MATTM Ministries (Italy–Israel Cooperation, R&D Proposal 2007), the Centro Euromediterraneo per il Cambiamento Climatico of Lecce, the European Community (MARBEF NoE, IASON and SESAME projects). Christian Vaglio and Cataldo Pierri helped in the field. Simona Fraschetti, Paolo Guidetti, Peter Schuchert, Antonio Terlizzi, and Egidio Trainito provided information on the distribution of the investigated species.








