INTRODUCTION
While many demosponges are known to harbour photosymbionts of various origins (e.g. Schönberg & Loh, Reference Schönberg and Loh2005; Díaz et al., Reference Díaz, Thacker, Rützler, Piantoni, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007; Lemloh et al., Reference Lemloh, Fromont, Brümmer and Usher2009; Thacker & Freeman, Reference Thacker, Freeman, Becerro, Uriz, Maldonado and Turon2012), many calcareous sponges do not appear to be photosynthetic, frequently having pale colours such as yellow or white (e.g. Rossi et al., Reference Rossi, de Moraes Russo, Solé-Cava, Rapp and Klautau2011), implying they may lack this type of symbiont. There is minimal information on any calcarean symbionts, and what is available is usually restricted to reporting their presence (Thacker & Freeman, Reference Thacker, Freeman, Becerro, Uriz, Maldonado and Turon2012), and suggesting the photosymbioses are almost exclusively with cyanobacteria. For example, macrolides of cyanobacterial origin were isolated from New Caledonian specimens of Leucascandra caveolata Borojevic & Klautau, 2000 (D'Ambrosio et al., Reference D'Ambrosio, Tato, Pocsfalvi, Debitus and Pietra1999; Kartika, Reference Kartika2008), although there was uncertainty whether these were present in the living sponge or associated with dead parts of the sampled material. Endocytobiotic, unicellular cyanobacteria were described from Sycon sp. from the Mediterranean (Feldmann, Reference Feldmann1933; Wilkinson, Reference Wilkinson, Schwemmler and Schenck1980; Díaz et al., Reference Díaz, Thacker, Rützler, Piantoni, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007). Burlando et al. (Reference Burlando, Sabatini and Gaino1988, as Clathrina) observed Gram-negative bacteria in Borojevia cerebrum (Haeckel, 1872), and Wilkinson (Reference Wilkinson, Schwemmler and Schenck1980) and Díaz et al. (Reference Díaz, Thacker, Rützler, Piantoni, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007) mentioned endocytobiotic, unicellular cyanobacteria in Clathrina sp. from the Mediterranean, citing the thesis of Duclaux (Reference Duclaux1977). In the southern hemisphere, Díaz et al. (Reference Díaz, Thacker, Rützler, Piantoni, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007) listed a Leucetta sp. from Papua New Guinea as a possible cyanosponge. Pericharax heterorhaphis Poléjaeff, 1883, from the Australian Great Barrier Reef has been reported to contain cyanobacteria distributed in a superficial layer in the sponge, yielding low concentrations of chlorophyll and low rates of carbon fixation (Wilkinson, Reference Wilkinson, Gomez, Birkeland, Buddemeier, Johannes, Marsh and Tsuda1982, Reference Wilkinson1983). This species appears to depend on supplies from the symbionts and has a distribution restricted to shallow water (Wilkinson, Reference Wilkinson1978a, Reference Wilkinsonb, Reference Wilkinson, Schwemmler and Schenck1980; Díaz et al., Reference Díaz, Thacker, Rützler, Piantoni, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007). It can distinguish between symbiont and non-symbiont bacteria digesting only the latter (Wilkinson et al., Reference Wilkinson, Garrone and Vacelet1984). In south-western Australia two calcarean sponges produced fluorescence when scanned with pulse-amplitude modulated fluorometry (Lemloh et al., Reference Lemloh, Fromont, Brümmer and Usher2009), with the symbiont identified as Synechococcus sp.
Apart from the study in south-western Australia (Lemloh et al., Reference Lemloh, Fromont, Brümmer and Usher2009) all of the incidences of light requiring symbionts in Calcarea have been reported from tropical regions, largely in the northern hemisphere. Moreover, all of the symbionts in Calcarea were found to be cyanobacteria, except for Clathrina sp. from Puerto Rico, which was reported to contain diatoms as a response to a bleaching episode (Pleurosigma sp.; Vicente, Reference Vicente1990).
Here we provide an account of Leucetta prolifera (Carter, Reference Carter1878) from south-western Australia, a dark olive-green sponge, a colour which suggests it may be photosynthetic by association with microbial symbionts (Appendix 1). As information about phototrophic Calcarea is very limited to date, in particular about species from Western Australia, we analysed this sponge for pigments, and used molecular taxonomy to identify its putative photosymbionts. We also analysed the membrane fatty acid composition based on the phospholipid derived fatty acids in order to investigate carbon transfer between the symbionts and the sponge.
Leucetta prolifera was first described from Fremantle by Carter (Reference Carter1878) and had not been recorded in the literature until recently (Fromont et al., Reference Fromont, Vanderklift and Klautau2013). It is a common local species in south-western Australia, accounting for 32% of sponge individuals recorded in the Jurien area, ~200 km north of Fremantle (Fromont et al., Reference Fromont, Vanderklift and Klautau2013).
MATERIALS AND METHODS
Sample collection
Three specimens of Leucetta prolifera were collected from Three Mile Reef (31°46′32.16″S 115°40′29.9994″E) in Marmion Marine Park north of Perth at 10 m depth by scuba diving. Sponges were placed in clip seal plastic bags underwater. At the sea surface seawater was poured off, and samples were rinsed with 0.2 µm autoclaved seawater to remove loosely associated microbes. Samples were divided in two, and one subsample was prepared for fatty acid analysis by rinsing with autoclaved and 0.2 µm filtered MilliQ water, then being wrapped in aluminium foil. The other subsample was kept in the original clip seal plastic bag and later used for pulse-amplitude modulated fluorometry, chlorophyll extraction and DNA analysis. Subsamples were kept on ice in the dark. On return to the laboratory (~6 h later) they were frozen at −80°C until further analysis.
Pulse-amplitude modulated fluorometry and chlorophyll extraction
Frozen subsamples of each specimen were screened with pulse-amplitude modulated fluorometry in a maxi iPAM (Walz, Iffeltrich, Germany) at default settings to rapidly confirm whether they were photosynthetic. Pigment extractions were then performed following the protocol of Wellburn (Reference Wellburn1994). During these tasks all material was handled with gloves and all weights were obtained in triplicate at room temperature (20°C), using a desiccator to cool tubes and samples after drying for 24 h at 80°C.
For chlorophyll extraction the samples of sponge tissue were fully defrosted to reach room temperature, then further subsamples of ~ 1 × 1 × 0.5 cm were taken to represent parts with more colour than others that were pale (Appendix 1). Tissue was immediately placed into pre-dried and pre-weighed 15 mL falcon tubes and weighed on analytical scales (Shimadzu AUW 220D, VWR International, Murrarie, Australia; accuracy to 0.00001 g) to obtain sample wet weight (WW). Depending on the size of the subsamples, tissue was then immersed in 2–4 mL of laboratory grade methanol for 2 h in darkness at 20°C, shaking the solution at the beginning and end of the extraction period. After 2 h, turbidity in the extraction solutions was removed by 5 min centrifugation at 300 rpm (Eppendorf 5810R centrifuge, VWR International, Murrarie, Australia). Using micropipettes, aliquots of solutions were taken from the falcon tubes and diluted depending on the strength of the colouration. Transmission properties of the solutions were measured across 450 to 700 nm in triplicate in disposable cuvettes against clean methanol in a UV 1800 Shimadzu spectrophotometer (VWR International, Murrarie, Australia; 1 cm bandwidth). Cuvettes were closed with parafilm to reduce rapid methanol evaporation possibly causing sample bias or risk of inhalation. If necessary, solutions were further diluted with methanol, always taking note of volumes used. After determining that measurements were successful, cuvettes and remaining solutions were decanted for evaporation, without loss of bottom sediment. Open falcon tubes, paired with each respective lid, were left to evaporate, and then dried 24 h at 80°C, closed, allowed to cool and weighed to obtain the dry weight after extraction (DW, dry weight minus pigments in solution). The dry weight is considered to be the more accurate reference than wet weight. This process was repeated with a second set of the remaining material, duplicating the process.
Pigment concentrations for chlorophyll a and b and for carotenoids were quantified according to Wellburn (Reference Wellburn1994) referring to the extraction volumes and dilutions and using the post-freezing wet weights and the post-extraction dry weights as reference. Respective values are considered to be underestimates and conservative, because samples were not directly snap-frozen after sampling. Means of means were calculated, than recalculated omitting all original values that remained significantly lower than 80% of the highest values, thus reducing the bias due to delayed freezing.
Genomic DNA extraction
Approximately 0.5 g of each of the three specimens was extracted for genomic DNA using the ammonium acetate bead beating method as described by Peterson et al. (Reference Peterson, Dahllöf and Nielsen2004). Briefly, 1 mL of extraction buffer (400 mL 6.25 m ammonium acetate; 100 mL Tris with pH 8.0; 40 mL 0.5 m EDTA; 460 mL molecular grade water) was added to a polypropylene tube containing 200 mL of silica beads (Daintree Scientific Products, St. Helens, Australia), 0.015 g PVPP, 300 mL of chloroform:isoamyl alcohol (24:1), and the sample. Bead-beating was performed in an Oscillating Mill MM 200 (Retsch, Haan, Germany), followed by centrifugation (30 min, 15,000 g, 4°C) and collection of the supernatant. This was then precipitated overnight (4°C) with 0.3 m sodium acetate and isopropanol, followed by centrifugation for 30 min (15,000 g, 4°C). Pellets were washed twice with 70% ethanol, dried, and resuspended in 30 µl molecular grade water.
16S gene targeted PE Illumina sequencing
Extracted DNA was quantified and adjusted to 20 ng µL−1. All samples were PCR amplified with universal bacterial primers (27F/519R with barcode on the forward primer) targeting the 16S rRNA gene V1–V3 variable region. PCR primers were used in a 30 cycle PCR using the HotStarTaq Plus Master Mix Kit (Qiagen, USA) under the following conditions: 94°C for 3 min, followed by 28 cycles of 94°C for 30 s, 53°C for 40 s and 72°C for 1 min, after which a final elongation step at 72°C for 5 min was performed. After amplification, PCR products were checked on a 2% agarose gel to determine the success of amplification and the relative intensity of bands. Multiple samples (100 samples) were pooled together in equal proportions based on their molecular weight and DNA concentrations. Pooled samples were purified using calibrated Ampure XP beads. The pooled and purified PCR product was used to prepare DNA libraries by following the Illumina TruSeq DNA library preparation protocol. Sequencing was performed at Molecular Research Laboratories (Shallowater, TX, USA) on a MiSeq following the manufacturer's guidelines. The r1 and r2 files for amplicons >300 bp and <570 bp were joined at Molecular Research Laboratories (Shallowater, TX, USA). Resulting data were subsequently denoised and processed using the MOTHUR MiSeq standard operating procedure http://www.mothur.org (Kozich et al., Reference Kozich, Westcott, Baxter, Highlander and Schloss2013). In summary, sequences <463 bp and sequences containing ambiguous base calls or homopolymer runs above 8 bp were removed. Sequences were aligned with the SILVA 16S rRNA alignment (Pruesse et al., Reference Pruesse, Peplies and Glöckner2007), unique sequences identified and operational taxonomic units (OTUs) (97% similarity) were defined. Chimeric artefacts were also removed using UCHIME (Edgar et al., Reference Edgar, Haas, Clemente, Quince and Knight2011). Any sequences that were classified as mitochondria or eukaryotic as well as any sequences of unknown origin were filtered out of the dataset. Final OTUs were taxonomically classified using BLASTn against a curated database derived from greengenes, RDPII and NCBI (http://www.ncbi.nlm.nih.gov; DeSantis et al., Reference DeSantis, Hugenholtz, Larsen, Rojas, Brodie, Keller, Huber, Dalevi, Hu and Andersen2006; Wang et al., Reference Wang, Garrity, Tiedje and Cole2007). Validity for the identification of photosynthetic microbial taxa was confirmed in Guiry & Guiry (Reference Guiry and Guiry2014). The MiSeq dataset was deposited in the NCBI Sequence Read Archive (SRA) database with the accession number SRR1802581.
Phylogenetic analysis
All sequences were further analysed by comparison with the sponge ARB database (Ludwig et al., Reference Ludwig, Strunk, Westram, Richter, Meier, Yadhukumar, Lai, Steppi, Jobb, Förster, Brettske, Gerber, Ginhart, Gross, Grumann, Hermann, Jost, König, Liss, Lüßmann, May, Nonhoff, Reichel, Strehlow, Stamataki, Stuckman, Vilbig, Lenke, Ludwig, Bode and Schleifer2004; Simister et al., Reference Simister, Deines, Botté, Webster and Taylor2012) which contains over 7500 16S rDNA sequences obtained from sponges. Prior to importing into ARB, sequences were aligned using the SINA sequence aligner (Pruesse et al., Reference Pruesse, Peplies and Glöckner2012). Alignments were manually corrected within the ARB environment, and phylogenetic analyses were done using 880 unambiguously aligned nucleotide positions. Neighbour joining, maximum likelihood and maximum parsimony methods were performed, and bootstrap proportions from 1000 resamplings were calculated using the maximum likelihood/rapid bootstrapping tool within the CIPRES science gateway (http://www.phylo.org/sub_sections/portal/) and viewed using the interactive tree of life (http://itol.embl.de) (Letunic & Bork, Reference Letunic and Bork2006, Reference Letunic and Bork2011).
Analysis of phospholipid derived fatty acids (PLFAs)
PLFAs were extracted from 1.9 g of lyophilized, defrosted sponge tissue by the Bligh–Dyer method (modified; Bligh & Dyer, Reference Bligh and Dyer1959; Lengger et al., Reference Lengger, Hopmans, Sinninghe Damsté and Schouten2012), which, different from methods using only organic solvent, allows extraction of polar lipids by using phosphate buffer and organic solvents in miscible amounts. For this, 15 mL of a mix of methanol/dichloromethane were added to the ground tissue (DCM/phosphate buffer 100 mm, pH 7.4, 2:1:0.8), ultrasonicated for 15 min and centrifuged at 2000 rpm for 3 min. The supernatant was collected, two more extractions were performed and the supernatants united. The solvent ratio was adjusted to methanol/DCM/phosphate buffer (100 mm, pH 7.4) 1:1:0.9 in order to separate the mixture into an aqueous and a DCM phase, with the DCM phase containing the lipids. The DCM phase was collected, and the liquid/liquid extraction on the methanol/phosphate buffer phase was conducted twice more. The combined DCM phases were reduced by rotary evaporation, filtered over cotton wool in DCM/methanol 9:1 and dried under a stream of nitrogen. The phospholipids (PL) were separated from other lipids by gravity column chromatography on 0.8 g of activated (130°C) 60 mesh silica (Guckert et al., Reference Guckert, Antworth, Nichols and White1985; Heinzelmann et al., Reference Heinzelmann, Bale, Hopmans, Sinninghe Damste, Schouten and van der Meer2014). Elution was performed with 4 mL of DCM, 4 mL of acetone and 10 mL of methanol (containing the PL). This fraction was blow-dried under a gentle nitrogen purge and saponified in order to release the fatty acids bound to the glycerol in methanolic KOH (2 N) for 2 h at 80°C under reflux. 2 mL of MilliQ water were added, the pH adjusted to 3, and the PLFA derived fatty acids were extracted from the aqueous phase with hexane, followed by DCM (three times). Fatty acids were methylated for 20 min in 2 mL 30% BF3-methanol at 70°C. The reaction was stopped by adding 1 mL of MilliQ water. The fatty acid methylesters were extracted from the methanol/water phase three times with 2 mL of hexane and diluted to an approximate concentration of 1 mg mL−1 for gas chromatography-mass spectrometry (GC-MS), 100 (δ2H) and 10 mg mL−1 (δ13C) for GC coupled to isotope ratio mass spectrometry (GC-IRMS).
Phospholipid-derived fatty acid methyl esters (PLFAME) were identified by GC-MS on a Hewlett Packard 6890 gas chromatograph (Palo Alto, CA, USA) equipped with a 6890 autosampler and a splitless injector, interfaced to a Hewlett Packard 5973 mass selective detector (Palo Alto, CA, USA). The gas chromatograph was fitted with a 60 m DB5-MS column with 0.25 µm film thickness (J&W Scientific, Folsom, CA, USA). Helium was used as a carrier gas at a constant flow rate of 1.1 ml min−1. One μl sample injections were added and heated to 320°C, with the oven temperature being raised from 40 to 320°C at a rate of 3 °C min−1. The transfer line was maintained at 290°C with mass selector operating with a solvent delay of 3 min in full scan mode for a mass range of 50 to 500 Daltons and held isothermally at 320°C (15 min). Quantification was achieved by GC coupled to flame ionization detection (FID) on a Hewlett Packard 6890 gas chromatograph fitted with FID detection under the same chromatographic conditions as GC-MS (with exception of the He flow which was increased to 1.8 mL min−1). δ13C and δ2H values were determined on a ThermoScientific Trace GC Ultra (Waltham, MA, USA), equipped with a GC IsoLink with a combustion furnace at 1000°C (δ13C) or a high-temperature conversion reactor at 1400°C (δ2H), and the produced CO2/H2 was analysed in a ThermoScientific Delta V Advantage isotope ratio mass spectrometer (Waltham, MA, USA). Chromatographic conditions were identical to GC-MS conditions. The isotopic values were calculated by integrating the mass 44, 45 and 46 ion currents (δ13C) and 1, 2 and 3 ion currents of the peaks (δ2H), and comparison to CO2/H2 reference gas spikes with a known 13C/2H content (Dawson et al., Reference Dawson, Grice, Edwards and Alexander2007). Performance was monitored by regular injections of an n-alkane standard (A. Schimmelmann, University of Indiana, IL, USA), and values were reported in comparison to the references Vienna Mean Standard Ocean Water (V-SMOW) and Vienna Pee Dee Belemnite (V-PDB) and were corrected for the added carbon during methylation, however, δ2H values of the added methyl could not be determined. Standard deviations were between 0.1 and 1.1‰ for δ13C (four analyses) and between 1 and 51‰ for δ2H (in duplicate). Fatty acids are reported with their carbon number, followed by a colon and the number of double bonds. Methyl branched fatty acids are preceded by i for iso and a for anteiso acids, referring to the position of the branching (terminal/second-to-terminal).
PLFA analysis isolates the phospholipids and other intact polar lipids (Heinzelmann et al., Reference Heinzelmann, Bale, Hopmans, Sinninghe Damste, Schouten and van der Meer2014), and provides the lipid profile of the lipids present in active, live cell membranes only. Symbionts and sponge tissue, in this case, were not separated before extraction, thus the lipid profile represents a mixture of these organisms.
RESULTS
Photopigments
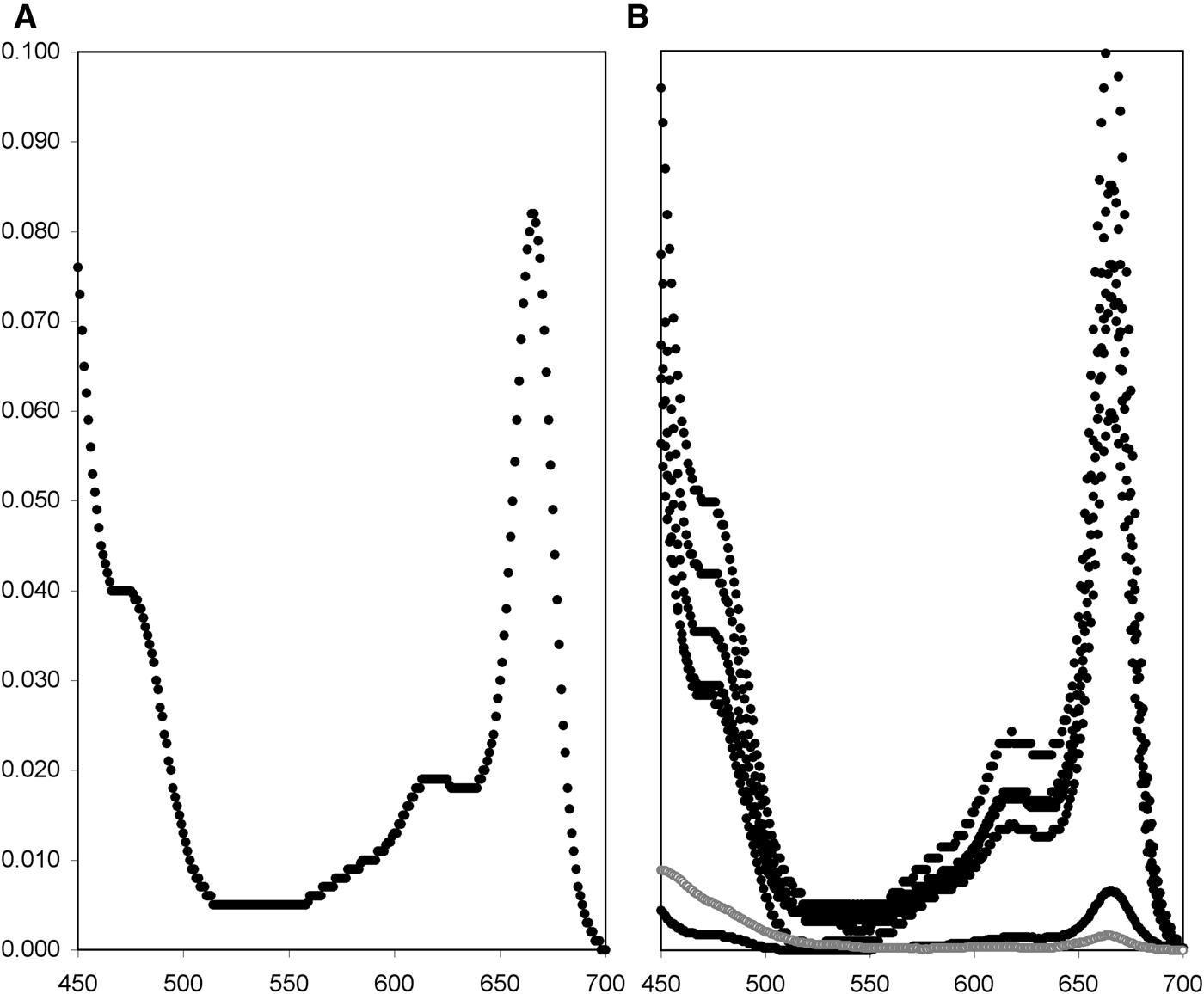
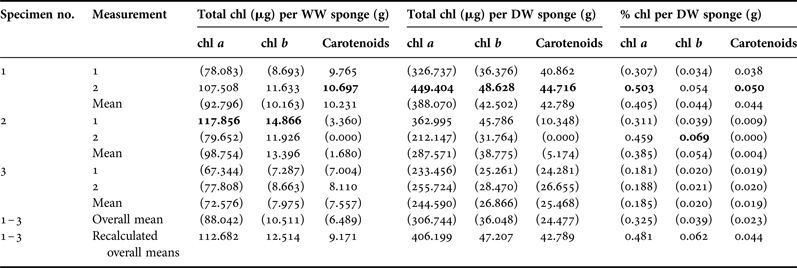
Leucetta prolifera was associated with photosynthetic symbionts. Frozen sponge tissue generated weak fluorescence when checked with pulse-amplitude modulated fluorometry. Methanol extractions and photometry resulted in curves typical for photosynthetic organisms, with clear peaks at 666 nm for chlorophyll a (chl; Figure 1). Pigment concentrations varied widely, as expected when tissue samples are not immediately frozen (Table 1, Figure 1B). After correcting the data by omitting all values significantly below 80% of the highest measures per pigment, we concluded that L. prolifera had average pigment concentrations of 112.7 µg g−1 WW or 406.2 µg g−1 DW for chl a, 12.5 µg g−1 WW or 47.2 µg g−1 DW for chl b, and 9.2 µg g−1 WW or 42.8 µg g−1 DW for carotenoids, or 0.48, 0.06 and 0.04% of the DW, respectively (these values may represent underestimates).

Fig. 1. Transmission spectra for pigments extracted from Western Australian Leucetta spp. (A) Leucetta prolifera spectrum for one of the darkest pieces of tissue. (B) After multiplying all transmission data with the extraction volume and dividing by dry weight, spectra from different measurements become comparable but are not realistic, so the scale on the y-axis was omitted. Black markers are for L. prolifera (two extractions for three specimens), while grey markers represent results from Leucetta sp. from the Onslow area in north-west Australia (Schönberg et al., unpublished data).
Table 1. Leucetta prolifera pigment concentrations for three specimens measured twice.

Means were recalculated omitting values in parentheses that were significantly below 80% of the highest individual pigment concentrations measured (in bold).
Chl, chlorophyll; WW, sponge wet weights after freezing at −80°C; DW, sponge dry weights after extraction.
Olive-green colour in life, a pink aqueous solution from the samples and a light green solution and light pink tissue and sediment at the end of the methanol extraction (Appendix 1) suggested an association with cyanobacteria, and the samples were further subjected to molecular analysis of the symbionts.
Molecular analysis of the microbial community
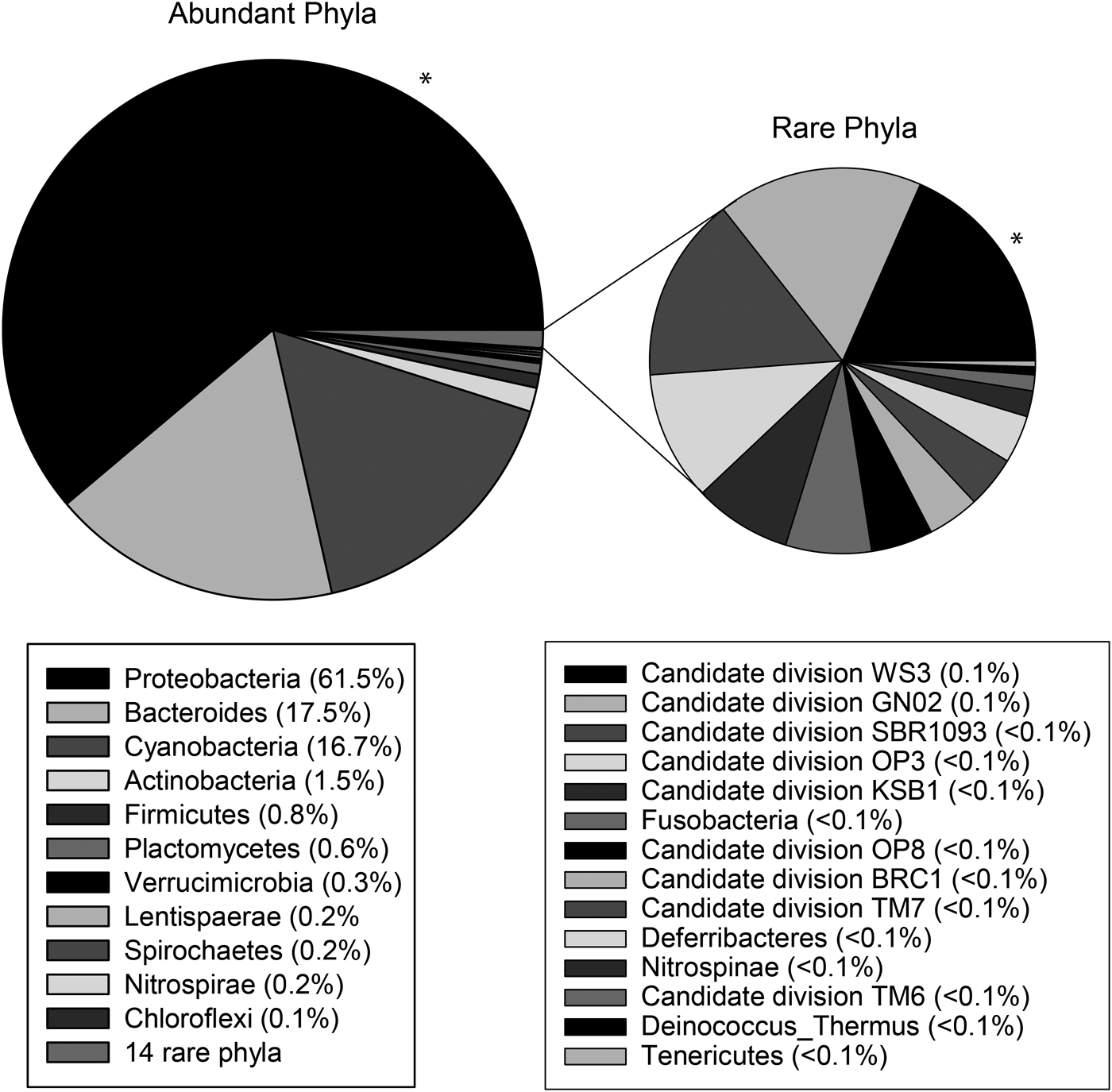
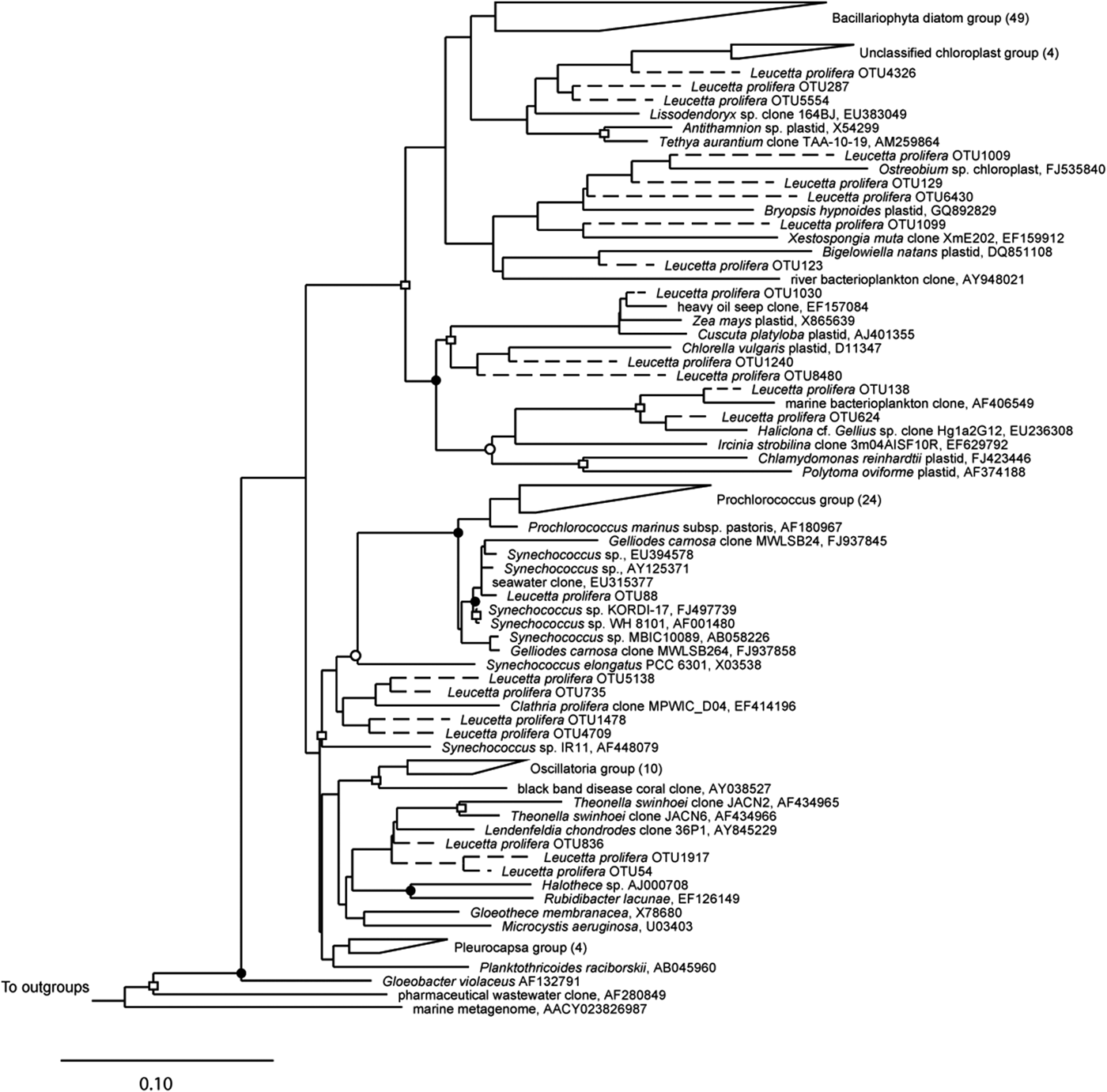
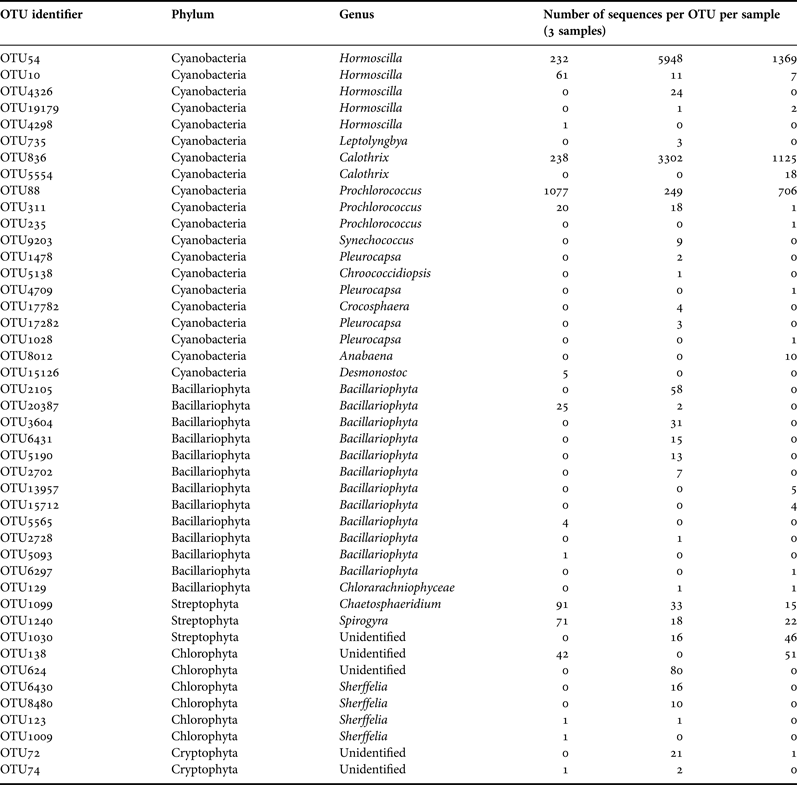
A total of 102,229 high-quality sequences (>300 bp, median length 521 bp) were obtained from the three sponge specimens, with an average of 34,076 ± 7854 sequences per sample. Leucetta prolifera contained representatives from 25 different bacteria phyla, with 61.5% (±7.6%) of sequences belonging to the Proteobacteria, 17.5% (±3.5%) to the Bacteroides, 16.7% (±9.1%) belonging to the Cyanobacteria and 1.5% (±1.5%) to the Actinobacteria. All other phyla contributed with less than 1% to the total sequences (Figure 2). 20 OTUs (97% sequence similarity) fell into the phylum Cyanobacteria, with the two most abundant cyanobacterial OTUs (OTU54: 7549 sequences, 32.7 ± 22.0% of the Cyanobacteria; and OTU836: 4665 sequences, 22.8 ± 11.2% of the Cyanobacteria) most closely related to sequences within the order Oscillatoriales and family Rivulariaceae (Table 2). Phylogenetic analysis placed both of these sequences adjacent to sequences from the cyanobacterium Hormoscilla spongeliae (Gomont) Anagnostidis & Komárek, 1988 (frequently published as Oscillatoria; Figure 3). Cyanobacterial sequences were also acquired from the Chroococcales, Pleurocapsales, Cyanobacteriaceae, and Nostocales. 3114 chloroplast sequences were also obtained. These were placed into 24 OTUs that fell within the Bacillariophyceae (diatoms; 13 OTUs) and the three phyla Chlorophyta (green plants; 6 OTUs), Streptophyta (land plants, 3 OTUs) and Cryptophyta (2 OTUs) (flagellated phytoplankton; Table 2).

Fig. 2. Mean relative abundance (% of all sequences) of bacterial phyla within Leucetta prolifera. Phyla are listed in the legend from most abundant to least abundant and are displayed in the graph in an anticlockwise direction, with most abundant (black, indicated with *) shown in the top right.

Fig. 3. 16S rRNA phylogeny of sequences that fell within the phylum Cyanobacteria and neighbouring chloroplasts. Sequences were aligned with selected sequences from the Cyanobacteria tree from the sponge ARB database (Simister et al., Reference Simister, Deines, Botté, Webster and Taylor2012). The tree shown is a maximum likelihood tree based on long sequences (>1000 bp); shorter sequences were added using the parsimony interactive tool in ARB and are indicated with a dashed line, following Simister et al. (Reference Simister, Deines, Botté, Webster and Taylor2012). Bootstrap values were calculated using the CIPRES science gateway (http://www.phylo.org/subsections/portal/). Symbols indicate bootstrap support as follows: filled circles 60–70, open circles 80–90, open squares >90. Bar signifies 10% divergence. Numbers in parentheses represent number of sequences within wedges.
Table 2. Taxonomic classification of sequences from Leucetta prolifera used in Figure 3. Classifications were assigned using the RDPII, NCBI Taxonomy and greengenes browser (http://www.ncbi.nlm.nih.gov, DeSantis et al., Reference DeSantis, Hugenholtz, Larsen, Rojas, Brodie, Keller, Huber, Dalevi, Hu and Andersen2006; Wang et al., Reference Wang, Garrity, Tiedje and Cole2007).

Phospholipid derived fatty acids and their isotopic values
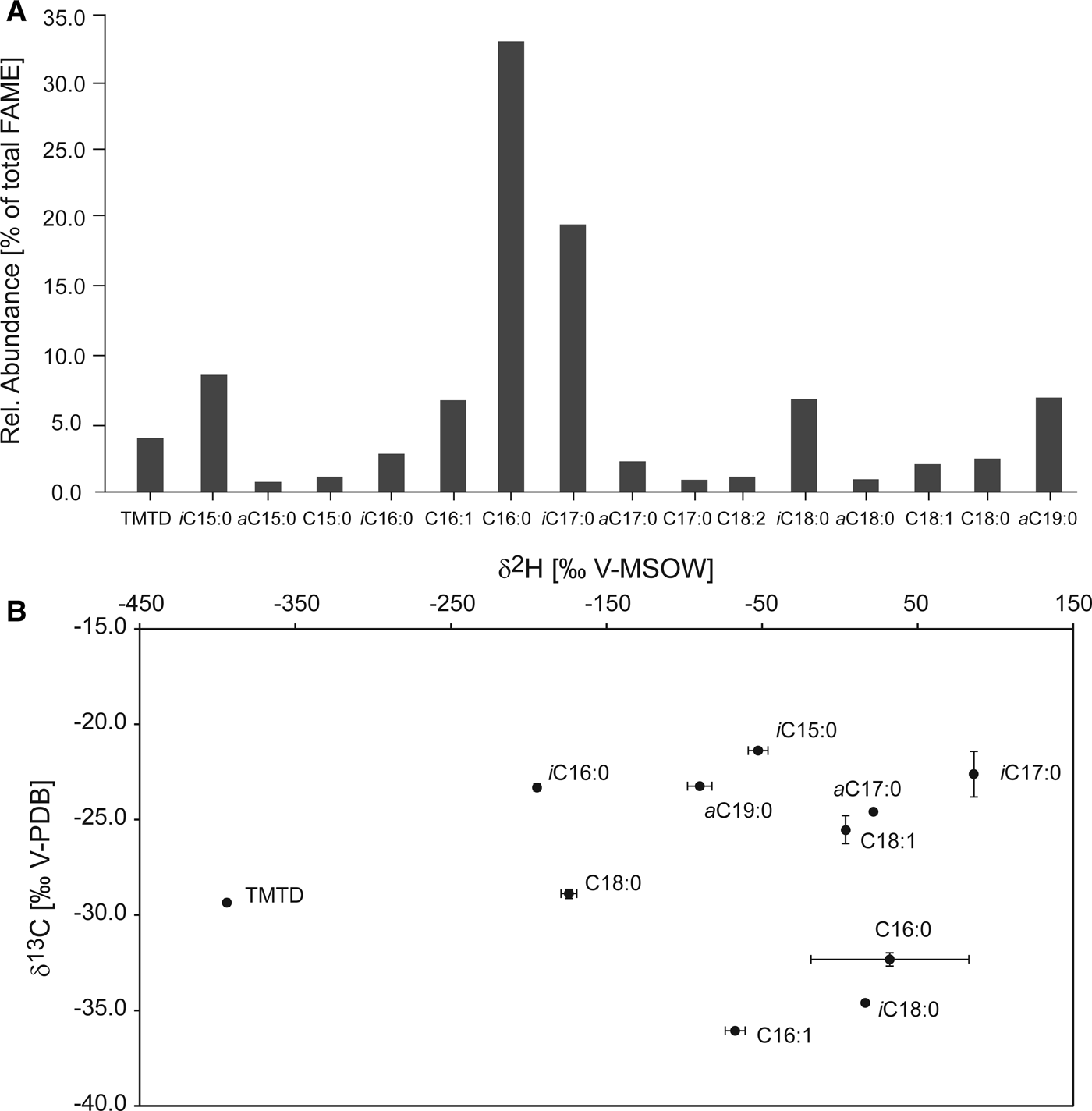
The main fatty acids in the L. prolifera sample making up the PLFA fraction were identified and semi-quantified in their esterified form. The majority consisted of straight chain, saturated hexadecanoic acids (C16:0, 33.1%) and the terminally methylated i-C17:0 (19.7%; Figure 4A). Other PLFA present in considerable amounts were 4,8,12-trimethyltridecanoic acid (TMTD), iC15:0, C16:1 (mix of double bonds at C-6 and C-9,), a-C17:0, and a-C19:0 (all between 2–9%), and traces of other branched and straight chain, unsaturated and saturated fatty acids. Isotope values of the identified, specific fatty acids were determined: δ13C values ranged from −21.5 to −39.4‰ (Figure 4B), with C16:0 and C16:1 being the most strongly depleted fatty acids (−32.3 and −36.1‰, respectively). C18:1 also showed depleted values (−34.6‰), but was eluting close to many other peaks, and its values might have been compromised. Observed δ2H values ranged from strongly depleted (−420‰) to enriched (70‰, Figure 4B), with the most depleted fatty acid being TMTD (−420‰).

Fig. 4. Relative abundances and stable carbon and hydrogen isotopic composition of the PLFA extracted from Leucetta prolifera. (A) Relative abundances in per cent of total of the individual fatty acids analysed as methylesters. (B) δ13C in ‰ V-PDB vs δ2H in ‰ V-MSOW show the distinct isotopic signatures of the individual PLFA. Standard deviations of triplicate measurements are displayed unless smaller than the data points.
DISCUSSION
Identification of the photosymbiont
In the present study we confirmed by pigment, molecular and isotopic analyses that Leucetta prolifera is associated with photosynthetic cyanobacteria. Sequencing results showed that over 16% of all retrieved bacterial sequences belonged to the phylum Cyanobacteria, with the two dominant OTUs most closely related to Hormoscilla spongeliae. Hormoscilla spongeliae has previously been reported from the chondrosid demosponge Chondrilla australiensis Carter, 1873 from south-western Australia, as well as from many sponges in other regions around the globe (e.g. Lemloh et al., Reference Lemloh, Fromont, Brümmer and Usher2009). Interestingly, while the phylogenetic analysis associated both of the two abundant OTUs (OTU54 and OTU836) with H. spongeliae, the greengene comparison placed only the most abundant taxon OTU54 with H. spongeliae, and OTU836 within the genus Calothrix, another cyanobacterium genus. Members of this genus are capable of nitrogen fixation (Eichner et al., Reference Eichner, Rost and Kranz2014) and have previously been reported from other sponges, e.g. in diseased tissue of Carteriospongia foliascens (Pallas, 1766) (Gao et al., Reference Gao, Wang, Lee, Tian, Wong, Bougouffa, Batang, Al-Suwailem, Lafi, Bajic and Qian2014).
It appears that photosymbiosis is more common and symbiont diversity is higher in demosponges than in calcareous sponges (Díaz, Reference Díaz1999; Díaz et al., Reference Díaz, Thacker, Rützler, Piantoni, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007; Thacker & Freeman, Reference Thacker, Freeman, Becerro, Uriz, Maldonado and Turon2012). Apart from Vincente's (Reference Vicente1990) account of diatoms in Clathrina sp., all other records on photosymbionts in Calcarea are of cyanobacteria (see dot points below). Occurrence of diatoms in sponge tissue is common, but may represent parasitism rather than mutualism, or a supplement to the sponge diets during times of stress or limitation (Vicente, Reference Vicente1990; Bavestrello et al., Reference Bavestrello, Arillo, Calcinai, Cattaneo-Vietti, Cerrano, Gaino, Penna and Sarà2000; Cerrano et al., Reference Cerrano, Arillo, Bavestrello, Calcinai, Cattaneo-Vietti, Penna, Sarà and Totti2000, Reference Cerrano, Calcinai, Cucchiari, Di Camillo, Totti and Bavestrello2004; Cárdenas & Rapp, Reference Cárdenas and Rapp2013).
Díaz (Reference Díaz1999) recognized three of 17 families of the Calcarea to contain cyanosponges (Thacker & Freeman, Reference Thacker, Freeman, Becerro, Uriz, Maldonado and Turon2012). Díaz (Reference Díaz1999) was later incorrectly cited that cyanobacteria are found in 17 families of Calcarea (Hentschel et al., Reference Hentschel, Usher and Taylor2006; Usher, Reference Usher2008; Zhu et al., Reference Zhu, Li and Wang2008; Caroppo et al., Reference Caroppo, Albertano, Bruno, Montinari, Rizzi, Vigliotta and Pagliara2012). Presently, with reports of cyanobacterial metabolites in a jenkinid sponge (D'Ambrosio et al., Reference D'Ambrosio, Tato, Pocsfalvi, Debitus and Pietra1999; Kartika, Reference Kartika2008), this number of three families in the Calcarea with cyanosponges can be extended to four for sponges identified at minimum to genus:
• Sycettidae (Sycon sp.; Feldmann, Reference Feldmann1933)
• Clathrinidae (Borojevia cerebrum in Burlando et al., Reference Burlando, Sabatini and Gaino1988, as Clathrina; Clathrina sp. sensu Wilkinson, Reference Wilkinson, Schwemmler and Schenck1980, may be B. cerebrum as well, as also sampled from the Mediterranean)
• Leucettidae (Leucetta sp. sensu Díaz et al., Reference Díaz, Thacker, Rützler, Piantoni, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007; Leucetta prolifera, present publication; possibly Leucetta sp. sensu Schönberg et al., unpublished data, see below – this species differs from L. prolifera; Pericharax heterorhaphis in Wilkinson, Reference Wilkinson1978a, Reference Wilkinsonb, Reference Wilkinson, Schwemmler and Schenck1980, Reference Wilkinson, Gomez, Birkeland, Buddemeier, Johannes, Marsh and Tsuda1982, Reference Wilkinson1983; Wilkinson et al., Reference Wilkinson, Garrone and Vacelet1984)
• Jenkinidae (Leucascandra caveolata; D'Ambrosio et al., Reference D'Ambrosio, Tato, Pocsfalvi, Debitus and Pietra1999; Kartika, Reference Kartika2008)
Photosynthetic activity in L. prolifera
Leucetta prolifera occurs in full light or shaded positions on shallow, exposed rocky temperate reefs. It is common beneath macroalgal canopies, and is incompressible and brittle with a growth form of low vertical ridges with apical oscules (Fromont, personal observation). The presence of photosymbionts in this sponge may aid its ability to coexist with macroalgae, and its very resistant low growth form may protect it from wave surge and macroalgal movement on exposed reefs. Western Australia is renowned for high biomass, high productivity temperate shallow benthic ecosystems, yet is bathed in the relatively low nutrient, southward flowing Leeuwin Current (Fromont et al., Reference Fromont, Vanderklift and Klautau2013). Photosymbiosis in L. prolifera may increase the ability of the sponge to survive in this low nutrient environment, with the clear, warm Leeuwin current waters assisting the growth of the light requiring symbionts. Indeed, Webster & Taylor (Reference Webster and Taylor2012) noted that sponges with symbiotic cyanobacteria may become net primary producers.
Fluorescence generated by the frozen samples was low and not further quantified, but pigment analyses confirmed that L. prolifera contained photopigments. Corrected mean values for chl a concentrations in L. prolifera reached 113 µg per g wet sponge tissue, a value considered intermediate compared with a wide range of other sponges (Erwin & Thacker, Reference Erwin and Thacker2007). The only other study available on pigment extraction from calcarean sponges found much lower values in P. heterorhaphis, i.e. 2.8 µg per g sponge tissue (Wilkinson, Reference Wilkinson, Gomez, Birkeland, Buddemeier, Johannes, Marsh and Tsuda1982).
Coincidentally, another Leucetta species was recently sampled during a thermal event in March 2013 (Lafratta et al., unpublished data; Schönberg et al., unpublished data), in 11.2 m depth near Onslow, north-western Australia. This sponge was almost white in life with a green tinge throughout and was assumed not to be photosynthetic. Yet, when the fresh sample was screened post dive with pulse-amplitude modulated fluorometry as described above, the sponge contour and area were faintly visible as red dots in the image obtained for maximum fluorescence yield. The low signal was initially interpreted as background noise, or reflections off the sponge surface. When extracting a subsample, this sponge still yielded pigment concentrations of 1.61 µg g−1 WW or 6.84 µg g−1 DW for chl a, 0.46 µg g−1 WW or 1.96 µg g−1 DW for chl b, and 1.91 µg g−1 WW or 8.12 µg g−1 DW for carotenoids, or 0.01, <0.01 and 0.01% of the DW respectively, and the respective transmission spectrum had a very low but discernible peak at 666 nm, suggesting remnant presence of chl a (Figure 1). Because the green sheen was uniform, the sponge may have been bleached rather than the results being due to contamination, e.g. through algal material held in choanocyte chambers.
Many symbioses develop as a result of feeding activities, and photosymbiotic calcarean sponges such as Leucetta spp. may originally have obtained their symbionts by filtering them from seawater. Perea-Blázquez et al. (Reference Perea-Blázquez, Price, Davy and Bell2010) found that Leucetta sp. from New Zealand feeds on bacterioplankton, of which the photosynthetic Prochloron and Synechococcus spp. were retained at higher efficiency than heterotrophic bacteria. Lemloh et al. (Reference Lemloh, Fromont, Brümmer and Usher2009) determined that symbionts in the unidentified south-western Australian Calcarea species had 98.8% similarity to a free-living Synechococcus. Wilkinson (Reference Wilkinson, Schwemmler and Schenck1980) demonstrated that sponges can differentiate between non-symbiotic and symbiotic bacteria offered as food, and Hill & Hill (Reference Hill and Hill2012) suggested that where sponges receive nutrients from symbiotic associations, the symbionts have significant control over invading a host, and preventing host digestion.
Possible transfer of photosynthate as determined by stable isotope analysis of PLFAs
The lipid profile generated from L. prolifera was in close agreement with other profiles from Calcarea, and specifically Leucetta species (Schreiber et al., Reference Schreiber, Wörheide and Thiel2006). Different classes of fatty acids can be specific to the organisms in which they occur. The terminally branched methyl fatty acids (iso and anteiso) found in our specimens are unusual in eukaryotes, and are generally considered to be of bacterial provenance. However, they appear to be absent from the often sponge-associated α-proteobacteria (Kaneda, Reference Kaneda1991; Pape, Reference Pape2004; Schreiber et al., Reference Schreiber, Wörheide and Thiel2006; Grice et al., Reference Grice, Lu, Zhou, Stuart-Williams and Farquhar2008), are only present in trace amounts in cyanobacteria (Cohen & Vonshak, Reference Cohen and Vonshak1991) and can thus be considered to be sponge-specific. This was confirmed by Schreiber et al. (Reference Schreiber, Wörheide and Thiel2006), who found iso and anteiso fatty acids in segments of sponge tissue without symbionts (in particular i-C17:0 and a-C19:0 fatty acids). C16:0, C16:1, C18:0 acids, which occurred in large amounts, are rather unspecific. However, cyanobacteria such as the confirmed symbiont in L. prolifera are known to produce these (as well as other very common fatty acids with various double bonds, Cohen & Vonshak, Reference Cohen and Vonshak1991). In addition, some of these major fatty acid constituents could also stem from proteobacteria, a large and diverse bacterial group with members capable of autotrophy, and confirmed to be present in L. prolifera by sequencing (Figure 2). C16:0, C16:1 and C18:0 fatty acids are thus likely to represent a mixture of sponge and symbiont material.
Stable carbon isotopic composition (δ13C) of fatty acids has previously been used to investigate food webs and symbioses as it is linked to biosynthetic pathways and to the carbon metabolism of the producing organism (Schouten et al., Reference Schouten, Klein Breteler, Blokker, Schogt, Rijpstra, Grice, Baas and Sinninghe-Damsté1998). Here, the carbon isotope values of the methyl-branched fatty acids, expected to stem from the sponge, exhibited δ13C values of around −22 to −25‰. The carbon isotope values of the fatty acids which are probably partially derived from the symbionts were more depleted, with C16:0 at −32.3‰ and C16:1 at −36.1‰, as well as C18:0 at −28.9‰. While the δ13C values of the biomass of heterotrophs are in general very similar to that of their carbon source (Grice et al., Reference Grice, Klein Breteler, Schouten, Grossi, De Leeuw and Sinninghe-Damsté1998 and references therein), in a symbiosis where carbohydrates are expected to be transferred, such as here, the fatty acids in the host are isotopically heavier than the ones of the carbon-translocating cyanobacteria (Van der Meer et al., Reference Van der Meer, Schouten, Sinninghe Damste, de Leeuw and Ward2003). This is due to differences in biosynthetic pathways, where carbohydrates are usually isotopically up to 15‰ heavier than fatty acids (Van Dongen et al., Reference Van Dongen, Schouten and Sinninghe Damsté2002). This suggests that, while C16:0 and C16:1 may largely consist of autotrophically produced fatty acids, C18:0 most likely represents a mixture of the same compound produced by the sponge and by the cyanobacteria, and suggests that the sponge receives carbohydrates from its symbionts.
Transfer of organic compounds from the cyanobacteria to L. prolifera could only be partially confirmed by the hydrogen isotopic composition of fatty acids (δ2H). It is highly influenced by the energy source of the producing organism (NADPH pathways), but has previously been shown to exhibit large variability between compounds even within one organism (Zhang et al., Reference Zhang, Gillespie and Sessions2009; Osburn et al., Reference Osburn, Sessions, Pepe-Ranney and Spear2011). In general, photoautotrophs exhibit fatty acids more depleted in hydrogen (−150 to −250‰), while heterotrophic growth produces comparatively 2H-enriched fatty acids (−150 to +200‰). The δ2H values of the sponge-derived fatty acids with methyl branching ranged from +100 to −200‰, while the fatty acids of suspected cyanobacterial, or mixed, origin exhibited very variable δ2H values (C16:0, C16:1 and C18:1, +6, −58 and −175‰, respectively). However, factors influencing the hydrogen isotopic composition of fatty acids in symbiotic relationships are presently inadequately understood and did not allow definite conclusions. In addition, the presence of proteobacteria in the sponge could be responsible for some of the variation found in the isotope values.
A particularly intriguing isotopic composition was that of TMTD. It has been found before in the genus Leucetta and frequently occurs in other sponges of different classes (Calcarea, Demospongiae, Hexactinellida; Schreiber et al., Reference Schreiber, Wörheide and Thiel2006). However, it remained unclear whether its presence reflects the incorporation of chlorophyll degradation products or de novo biosynthesis by the sponge. The carbon and particularly the hydrogen isotopic composition measured here (−29.4/−394‰, respectively) clearly indicate that the source of TMTD in L. prolifera is the phytol of chlorophyll, which has been reported to be extremely depleted in 2H (Chikaraishi et al., Reference Chikaraishi, Tanaka, Tanaka and Ohkouchi2009). Its δ2H value is considerably lower than is typical for isoprenoid synthesis, suggesting that L. prolifera uses a chlorophyll derivative such as phytol from either ingested bacterioplankton or from its cyanobacterial symbiont, again indicating a possible transfer of compounds from the cyanobacteria to L. prolifera. Overall, the carbon and hydrogen isotopic composition of the phospholipid derived fatty acids indicate that L. prolifera uses carbohydrates produced photosynthetically by its cyanobacterial symbiont and that the TMTD present as a membrane lipid in this and other sponges is a chlorophyll degradation product, derived from ingested phytoplankton or the symbiont.
This study of L. prolifera provides new insights into temperate water sponge-symbiont relationships, when most such data are available from the tropics. To date and for calcarean sponges, Wilkinson's classic studies on sponge-microbial associations provide the only data on functional aspects of these photosymbioses (Wilkinson, Reference Wilkinson1978a, Reference Wilkinsonb, Reference Wilkinson, Gomez, Birkeland, Buddemeier, Johannes, Marsh and Tsuda1982; Wilkinson et al., Reference Wilkinson, Garrone and Vacelet1984). This highlights how understudied Calcarea species are in this regard in comparison to demosponges, an imbalance that needs to be addressed for species that are locally as common as L. prolifera.
ACKNOWLEDGEMENTS
We thank Rob Czarnik and Pierre Bouvais for assistance in the field, Geoff Chidlow and Alex Holman for instrumental assistance, and Oliver Gomez for laboratory assistance. Samples were collected under Western Australia Fisheries Exemption number 2384 and Department of Parks and Wildlife licence to take fauna for scientific purposes number SF009715, both to Megan Huggett.
FINANCIAL SUPPORT
Sabine Lengger was funded by an ARC DORA grant provided to Kliti Grice, who also thanks the ARC for funding the purchase of a Thermo Delta V Advantage via an ARC LIEFP grant.
Appendix 1

Fig. A1. Pre-analysis evidence for Leucetta prolifera harbouring cyanobacteria. (A) Olive-green colour of tissue, with pinkish hues. Note that the second piece of the second sponge is paler than the other pieces and obviously experienced pigment loss after sampling. (B) Aqueous solution in the cryovial shows the typical neon-pink colour of fluids that seep out of cyanosponges. (C) Samples after pigment extraction and centrifugation. The solutions are light green, while the tissue is now light pink, as is the sediment.