INTRODUCTION
Throughout the world, except for the Arctic and Antarctica, typically exposed rocky shores, but also more sheltered ones, are occupied by species of the Mytiloidea. The most well known occupants of such shores in the northern hemisphere are species of Mytilus, notably M. galloprovincialis Lamarck, 1918 and Mytilus edulis Linnaeus, 1758 (Seed, Reference Seed1969). In tropical Asia, the occupant of such shores is Septifer virgatus (Wiegmann, 1837) (Ong-Che & Morton, Reference Ong Che and Morton1992; Morton, Reference Morton1995) whereas in the tropical Western Atlantic, for example on the shores of the Cape Verde Islands, the intertidal mytilid is Brachidontes puniceus (Gmelin, 1791) (Morton, Reference Morton2012). In Australia, there occur a variety of species of Xenostrobus, including Xenostrobus securis (Lamarck, 1819), Xenostrobus pulex (Lamarck, 1819), Xenostrobus inconstans (Dunker, 1856) = Volsella inconstans (Dunker, 1856) (Huber, Reference Huber2010) and Xenostrobus hanleyi =Modiolatus hanleyi (Dunker, 1882) (Morton, Reference Morton1999, Reference Morton2004). Xenostrobus pulex has been introduced widely from its Australasian origin into Asia (Morton & Leung, Reference Morton and Leung2015), and the Mediterranean (Gestoso et al., Reference Gestoso, Olabarria and Arenas2012).
More sheltered hard shores are the domain of Brachidontes variabilis (Krauss, 1848) in Asia (Morton, Reference Morton1988). Two species of heteromyarian Mytilidae, that is, Limnoperna fortunei (Dunker, 1857) and Sinomytilus harmandi (Rochebrune, 1881) (Morton & Dinesen, Reference Morton and Dinesen2010), occur in fresh waters throughout South-East Asia, the former being introduced more widely, notably into South America (Darrigran & Pastorino, Reference Darrigran and Pastorino1995; Morton, Reference Morton and Boltovskoy2015a).
Bayne et al. (Reference Bayne, Widdows, Thompson and Bayne1976) reviewed the biology of the Mytilidae as did Gosling (Reference Gosling1992) and within which Yonge (Reference Yonge and Bayne1976) and Morton (Reference Morton and Gosling1992) discussed the evolution of the heteromyarian form in the Mytiloidea and which is, as will be seen, of particular relevance to this study.
This is a study of the poorly known Mediterranean intertidal limestone karst-associated mytiloid Mytilaster minimus (Poli, 1795). The species occurs throughout the Mediterranean (d'Angelo & Garguillo, Reference d'Angelo and Garguillo1978) where there is a suitable intertidal habitat of rocks, particularly the karsted limestone that is characteristic of much of the north-eastern coastline of the sea. Mytilaster minimus also occurs naturally throughout the Adriatic Sea and is recorded from the lagoon of Sacca di Goro on the northern coast of Italy (Mistri et al., Reference Mistri, Fano and Rossi2001) and along the shores of Israel (Fishelson, Reference Fishelson2000) The species is also common in the Bay of Tunis, Tunisia (Zamouri-Langar et al., Reference Zamouri-Langar, Chouba, Ajjabi Chebil, Mrabet and El Abed2011), where it occurs on artificial rocky shores (Antiti et al., Reference Antiti, Daoulatli, Rueda and Salas2013), and in the Aegean Sea (Dogan et al., Reference Dogan, Sezgin, Katagan and Önen2015). It also occurs widely and commonly along extensive lengths of the geodiverse shoreline of Croatia, for example, the Mljet National Park (Šiletic, Reference Šiletić2006).
Mytilaster minimus has also been recorded occasionally from outside the Mediterranean, notably the Canaries Archipelago, as will be described, but all such records are contained within conchological tomes of limited scientific value and it is herein considered that, essentially, the species is a Mediterranean endemic. The sparse scientific Mediterranean literature on M. minimus has, hitherto, mainly focused on the relationship between it and the many marine invaders that have to lesser and greater degrees colonized its habitat. For example, Galil (Reference Galil2007a) estimated that more than 500 alien species have been introduced into the Mediterranean Sea. Galil (Reference Galil2007b) further comprehensively surveyed the taxa introduced along the coast of Israel and identified 296 alien species of which 284 were considered to have arrived from the Red Sea. Mytilaster minimus has also been impacted throughout much of its Mediterranean range by pollution (Riggio et al., Reference Riggio, D'Anna and Sparla1992; Klobučar et al., Reference Klobučar, Štambuk, Hylland and Pavlica2008; Manachini et al., Reference Manachini, Arizza, Rinaldi, Montalto and Sarà2013), and the introduction of those alien species that have displaced it from its natural habitat. One of these bivalves that competes with the native M. minimus is also the similarly mytiloid Brachidontes pharaonis (P. Fischer, 1870). Along the coast of Israel, for example, Rilov et al. (Reference Rilov, Benayahu and Gasith2004) showed that on beach rocks formerly dominated by M. minimus, over a period of 4 years from 1995–1999, B. pharaonis progressively came to dominate the habitat, displacing the native mussel. This invasion of the Sinai, the Suez Canal and the Israeli coast of the Mediterranean by B. pharaonis has been studied by Sariel & Sasson-Frosting (Reference Safriel and Sasson-Frosting1988), Safriel et al. (Reference Safriel, Gilboa and Felsenburg1980) and Sarà et al. (Reference Sarà, Romano, Caruso and Mazzola2000, Reference Sarà, Milanese, Prusina, Sarà, Angel, Glamuzina, Nitzan, Freeman, Rinaldi, Palmeri, Montalto, Martire, Gianguzza, Arizza, Brutto, De Pirro, Helmuth, Murray, De Cantis and Williams2014). Brachidontes pharaonis has also been introduced into Greek waters where M. minimus occurs naturally (Zenetos et al., Reference Zenetos, Vardala-Theodorou and Alexandrakis2005), occupying for example the brackish water Mazoma Lagoon in Amvrakikos Bay on the western coast of the Greek mainland (Nicolaidou, Reference Nicolaidou2007). In relation to this Mediterranean invasion, Sarà & de Pirro (Reference Sarà and de Pirro2011) showed that heartbeat rates in M. minimus and B. pharaonis differed significantly in relation to changes in salinity from brackish (20‰) to hypersaline (75‰). That is, M. minimus was well-adapted to narrow variations in salinity as occur in the surface waters of the Mediterranean but showed signs of stress at salinities greater than 37‰. Conversely, B. pharaonis showed signs of stress only at salinities >45‰ and even exhibited heart rate activity at 75‰. The authors concluded that the Lessepsian immigrant was physiologically better adapted than the native species to invade and be successful in most of the Mediterranean's more transitional aquatic environments. And, thus, better able to outcompete M. minimus.
There is, however, nothing written on the anatomy of M. minimus and little information of substance is available on the species’ biology. Cannicci et al. (Reference Cannicci, Gomei, Dahdouh-Guebas, Rorandelli and Terlizzi2007), for example, showed that the Mediterranean shore crab Pachygrapsus marmoratus (Fabricius, 1787) (Grapsidae) has an omnivorous diet, dominated by algae and, although these authors did not mention M. minimus as an element in this, they did show that the crab fed on small M. galloprovincialis. Giacoletti et al. (Reference Giacoletti, Rinaldi, Mercurio, Mirto and Sarà2016) demonstrated, using feeding trials, that the Mediterranean muricid gastropod Stramonita haemastoma (Linnaeus, 1767), which typically predates and gains most benefit from feeding on the limpet Patella caerulea Linnaeus, 1758, would preferentially attack B. pharaonis as a second choice instead of the native M. galloprovincialis and M. minimus.
Finally, Riggio et al. (Reference Riggio, D'Anna and Sparla1992) described beds of M. minimus in regression in north-western Sicily associated with the colonization of the habitat by M. galloprovincialis and with an increase in pollution, particularly where there was a flow of fresh water from an adjacent stream. Such a location appears to have the same features as the shore and situation at Kaŝtel Stori, Croatia, the study site, where M. minimus occurs naturally.
This, therefore, is a study of the anatomy of M. minimus and has the specific aim of understanding those morphological features that adapt it to life on the pitted karst rocks of the coastline of Croatia. In addition, however, it describes the basic biological features of its relationship on the studied shore with the mytiloid M. galloprovincialis that, as in Sicily (Riggio et al., Reference Riggio, D'Anna and Sparla1992), is colonizing the M. minimus habitat and, it appears, excluding the native occupier.
MATERIALS AND METHODS
The study site for this research on Mytilaster minimus is situated on the northern shoreline of the eastern component of Kaštela Bay, at Kaštel Stari, in Croatia (Figure 1). At this site, there is a seaward projecting outcrop of the dinaric karsted limestone that characterizes the hills and mountains, which dominate this area of Croatia. Associated with this rocky outcrop is the discharge from a freshwater spring and accordingly, salinity readings were made here with a hand-held held multimeter (Mettler Toledo X-matePro MX300) on each visit. At the study site too, water temperatures were recorded to the nearest 0.5°C using the same multimeter. The freshwater spring arises from the Kozjak Mountain and its source waters are characteristically nutrient poor but become contaminated from numerous human activities affecting the local groundwater as it passes downhill to the sea (Barić et al., Reference Barić, Gačić, Grbec, Margeta, Miloš, Onofri, Veldić, Jeftić, Kečkeš and Pernetta1996).

Fig. 1. A map of the study site in Kaštela Bay, Split, Croatia. Also shown is the location of the Mytilus galloprovincialis farm at Marina.
lso identified in Figure 1 is the site of a Mytilus galloprovincialis Monterosato, 1891 farm at Marina, which was established in 2004. Here, in the western component of Kaštela Bay, mussel culturing is on strings suspended in the water column from rafts. Figure 1 also shows that although both components of Kaštela Bay have large outlets to the sea at a gap between the mainland and the island of Otok Čiovo in the west and between this island and the City of Split peninsula in the east, they are, nevertheless, connected by a narrow channel bisecting the city of Trogir. The significance of this, as will be described, is that although M. galloprovincialis is native within the Adriatic, an enhanced supply of larvae from the farm at Marina has been able to penetrate into the eastern component of Kaštela Bay from the western one and, thus, impact M. minimus here. As a consequence, by the end of 2015, M. galloprovincialis had colonized the study rocks where hitherto only M. minimus had been found. It was this observation that stimulated this research and others simultaneously underway, as described in the Introduction to this paper.
Accordingly, and in relation to this study, individuals of M. minimus have been collected from the rocks at Kaštel Stari, monthly since December 2015 and the samples will be used to obtain information on the growth and reproductive cycle of the species in order to obtain a better understanding of its life history trait. For the specific purposes of this study, however, the shell morphometrics, including shell length (from the umbones to the posterior shell margin), shell height (dorso-ventral axis), shell width (left to right across the widest point of the two shell valves) and dorsal valve thickness were obtained for 50 individuals to the nearest 0.1 mm using Vernier callipers. The shell dimensions (length, height, width and dorsal valve thickness) of 50 M. galloprovincialis individuals with shell lengths <16 mm were also obtained, in order to compare the morphometrics of the two, here near sympatric, species.
Similarly, living individuals of M. minimus have been examined with regard to elucidating details of the species’ anatomy and the ciliary currents of the mantle cavity using carmine suspended in seawater. Further, other individuals have been fixed in 4% formaldehyde and, following routine histological procedures, transverse sections of them have been mounted onto slides and stained in Ehrlich's haematoxylin and eosin to facilitate the study of the reproductive cycle (see above) but also to allow a more complete study of the species’ anatomy, as will be described herein.
Reference individuals of M. minimus have been deposited in the collections of the Natural History Museum, London, and have the Registration Number NHMUK 20160400.
RESULTS
Taxonomy
Poppe & Goto (Reference Poppe and Goto1993) record three species of Mytilaster from the shores of the Mediterranean. These include Mytilaster lineatus (Gmelin, 1791), M. marioni (Locard, 1889) and M. solidus Martin in Monterosato, 1872 with M. minimus (Poli, 1795) relegated to a subspecies of this last taxon. Mytilaster minimus is, however, now regarded as a valid species (Gofas et al., Reference Gofas, Le Renard, Bouchet, Costello, Emblow and White2001) and the shell has been described briefly by Hanley (Reference Hanley1843) and by Zenetos et al. (Reference Zenetos, Vardala-Theodorou and Alexandrakis2005). Interestingly, Barbieri et al. (Reference Barbieri, Urgu, Maltagliati, Di Giuseppe, Lardicci and Castelli2011) described a genetic divergence between individuals of M. minimus living in marine and brackish water environments from various locations in Italy. Although this has neither been followed up nor putative names assigned to the ecophenotypes, such a study may one day assist in resolving the nomenclatorial status of the taxa currently assigned to Mytilaster in the Mediterranean.
As will be described and discussed, M. minimus is a member of the Mytilidae and can be characterized by: (i) the chosen habitat of karsted intertidal limestone rocks; (ii) an often highly deformed shell typically <20 mm in length; (iii) ~7 postero-dorsal but no anterior, dissoconch, teeth although secondary teeth develop on the hinge plate subsequently; (iv) a pair of posterior pedal retractor muscles located beneath the similarly paired posterior byssal retractor muscles; and (v) a pair of statocysts hitherto described only for M. galloprovincialis (List, Reference List1902).
Distribution
In addition to the Mediterranean and its daughter seas, (Marina et al., Reference Marina, Urra, Rueda and Salas2012) M. minimus has also been recorded occasionally from outside them on the shores of, for example, Galicia (Spain) by Rolan Mosquera et al. (Reference Rolan Mosquera, Otero Schmitt and Rolan Alvarez1990), Agadir, Melilla, Tang(i)er, Rabat and Casablanca in Morocco (Pallary, Reference Pallary1920), the Basque region of Spain and France (Fischer, Reference Fischer1899) and the Canary Islands by Rodríguez & Sánchez (Reference Rodríguez and Sánchez1997), Hernandez et al. (Reference Hernández, Rolán, Swinnen, Gómez and Pérez2011) and Fernandez-Trujillo & Fernandez (Reference Fernandez-Trujillo and Fernandez1992). These last authors, moreover, consider that the species only occurs outside the Mediterranean in the Canaries (Bacallardo & collaborators, Reference Bacallardo1984) and d'Angelo & Garguillo (Reference d'Angelo and Garguillo1978) record it as so rare in this location as to be of conservation value.
All of the above studies, however, are conchological tomes and there are no scientific studies of populations of M. minimus from any of these localities. Indeed, the senior author of this paper has profiled the shores and flora and fauna of the Atlantic coast of Portugal at Cascais and that of Madeira (Morton et al., Reference Morton, Britton and de Frias-Martins1998, figs 2.3 and 2.5, respectively) and did not record M. minimus from either of these two locations. Further, the senior author has also investigated the Atlantic shores of Morocco and the Canaries and, similarly, not found any individuals of M. minimus. Finally, Morton et al. (Reference Morton, Britton and de Frias-Martins1998) further demonstrated that the larvae of many species of marine organisms leave the Mediterranean with its outflow and some can be carried as far as, for example, to the Açores on the mid-Atlantic Ridge but rarely do such taxa establish themselves as viable long-term populations and this seems to be the case with M. minimus on shores close to the mouth of the Mediterranean. It is significant, therefore, that there are no substantial records nor general studies, nor specific population analyses of M. minimus outside the Mediterranean.
Biology
Figure 2A is a photograph of the rocky outcrop at the study site on the northern shoreline of Kaštela Bay in central Croatia. Associated with this outcrop is a freshwater spring that discharges onto the beach at this point, lowering the salinity of the seawater close to it to 30.3‰ and above the mussel beds at high water to 35‰ compared with open Adriatic seawater salinities of 38.5–39.0‰ (Kršinić, Reference Kršinić2012). At the study site too, water temperatures were recorded from 12 to 24°C over the course of a year, with a mean of 14.7°C.

Fig. 2. Photographs of A, the study site at Kaštela Bay. B and C, Clusters of Mytilaster minimus and Mytilus galloprovincialis on the rocks, respectively, the former zoned higher than the latter.
Figure 2B shows a cluster of M. minimus on the Kaštela Bay rocks. As noted above, M. minimus is generally restricted to the Mediterranean although there are occasional records from the Canary Islands (Fernandez-Trujillo & Fernandez, Reference Fernandez-Trujillo and Fernandez1992), notably from the rocky intertidal of Tenerife (Rodríguez & Sánchez, Reference Rodríguez and Sánchez1997), for example. In the particular situation of Kaštela Bay, however, M. minimus here occupies the upper intertidal occurring as small clumps in concavities and low fissures in the rocks. As will be discussed, here too occur individuals of M. galloprovincialis that have displaced M. minimus from the lower intertidal.
Functional morphology
THE JUVENILE SHELL
The shell of M. minimus has three stages. Prodissoconchs I (PI) and II (PII) are both, presumably like most mytiloids, free-swimming larval stages, but about which there is no information. Prodissoconch II used to be referred to as the plantigrade stage because, as in most mytiloids, it possesses a long thin crawling foot. In many bivalves, this stage is followed by a nepioconch, which may reach a shell length of >1 mm, but nether M. edulis (Ockelmann, Reference Ockelmann1995) nor M. minimus possess this growth stage. The final growth stage, the dissoconch, is formed by the juvenile individual and becomes the permanent shell of the adult. Figure 3A shows an external view of the juvenile shell of M. minimus as seen from the left side. The shell is of the typical mytiloid form, that is, roundly heteromyarian with prodissoconchs I and II (PI, PII) clearly visible and the umbones located close to the end of the valves. Mytilus edulis possesses two (Ockelmann, Reference Ockelmann1995) anterior dissoconch hinge teeth but M. minimus has none. It does however, have up to seven dorsal dissoconch teeth, which are clearly visible through the largely translucent shell. Also visible through the shell is a light brown coloured hinge plate (HP) and the short ligament (L). Of note is that scattered over the lateral surface of both valves are a few byssal setae (BS), which have been planted there by the foot. Byssal setae have been described for other mytiloids such as M. edulis (Board, Reference Board1983), species of Dacrydium (Ockelmann, Reference Ockelmann1983) and Adula (Ockelmann & Dinesen, Reference Ockelmann and Dinesen2009) and Modiolus modiolus (Linnaeus, 1758) (Dinesen & Morton, Reference Dinesen and Morton2014), are diagnostic for the Mytiloidea (Ockelmann, Reference Ockelmann1983) and possibly have a defensive function (Wright & Francis, Reference Wright and Francis1984).

Fig. 3. Mytilaster minimus. (A) An external view of the juvenile, largely transparent, shell as seen from the left side; (B) An internal view of the hinge plate of the left valve of the juvenile shell; (C) A heavily encrusted and eroded adult individual as seen from the right side. (For abbreviations see Box 1.)
Ockelmann (Reference Ockelmann1995) describes and discusses the origins of the hinge teeth of the Mytiloidea. Figure 3B is a more detailed internal view of the anterior region of the dissoconch shell showing the hinge plate (HP) and the dorsal dissoconch teeth (DDT) situated posterior to the short ligament, which comprises a posterior outer ligament layer (POL) and a posterior inner ligament layer (PILL). The dissoconch shell grows progressively and typically rapidly into the juvenile. Byssal attachment typically occurs when a habitat suitable for adult occupation has been chosen and the dissoconch quickly assumes the adult form, which can here in the Adriatic grow to a shell length of ~20 mm. The general colour of the shell is a dark purple brown, this being mostly attributable to the thick periostracum that covers the shell, which presumably has a mineralogy and structure typical of the Mytiloidea (Taylor et al., Reference Taylor, Kennedy and Hall1969) although this has never been studied in M. minimus. As we shall see, however, the shells of M. minimus are both heavily eroded and pitted and covered by epiphytic plants, sponges and bryozoans.
THE ADULT SHELL
A heavily encrusted and eroded adult, dissoconch, individual of M. minimus is illustrated in Figure 3C from the right side. This individual has retained the heteromyarian form typical of the Mytilidae and has produced a stout byssus from a highly mobile foot. Such a shell is however, unusual, as we shall see, but Figure 4 provides illustrations of what can be defined as a generalized shell of M. minimus. Figure 4A shows a shell of M. minimus but only in outline as seen from the right side. It has, again, a typical heteromyarian form but with a somewhat swollen anterior end and a concave ventral surface. When seen from the dorsal aspect (Figure 4B), however, the same shell is wide with its greatest width (a–b) located ~ half way along its anterior-posterior length. There is a distinct ligament (L). The same situation is seen from the ventral aspect (Figure 4C) but here there is a distinct byssal gape (BG). When seen from the posterior (Figure 4D) and anterior (Figure 4E) aspects, the greatly expanded width of the shell is also obvious but the greatest dorso-ventral width (x–y) is situated ~ half way down its dorso-ventral height. The shell is thus generally equivalve, equilateral and medially squat in both its antero-posterior and dorso-ventral aspects.

Fig. 4. Mytilaster minimus. Outline illustrations of the shell as seen from (A) the right valve as seen from the right side; (B) from the dorsal aspect; (C) from the ventral aspect; (D) from the posterior aspect and € from the anterior aspect. (a–b is the greatest shell width ~ half way along the shell length; x–y is the greatest shell width ~ mid-dorso-ventrally). (For abbreviations see Box 1.)
An important feature of the shell of M. minimus however, is that it is thick and solid, adult individuals with a mean shell length of 12.6 mm having a dorsal shell thickness, just posterior to the end of the ligament, of 0.35 mm, whereas M. galloprovincialis individuals of the same shell length had a thickness (measured at the equivalent location) of only 0.15 mm (Table 2).
Box 1. Abbreviations used in the figures
AAM, Anterior adductor muscle (or scar); ABRM, Anterior byssal retractor muscle (or scar); AHT, Anterior hinge teeth; AN, Anus; AU, Auricle; BG, Byssal notch; BGC, Basiphilic gland cells; BGR, Byssal groove; BS, Byssal seta; BU, Buttress; BY, Byssus; BYR, Byssal roots; BYS, Byssal stalk; CA, Ctenidial axis; CRT, Ciliated rejection tract; CSSMG, Conjoined style sac and mid gut; DCAT, Dorsal ctenidial acceptance tract; DD, Digestive diverticulae; DDT Dorsal dissoconch teeth; EGC, Eosinophilic gland cells; ES, Exhalant siphon; ESSP, Siphonal papillae of the exhalant siphon; F, Foot; FP, Fused periostracum; GA, Gonadial aperture; H, Haemocoel; HA, Heart; HG, Hind gut; HP, Hinge plate; IA, Inhalant aperture; IASP, Siphonal papillae of the inhalant aperture; ID, Inner demibranch; ILP, Inner labial palp; IMF, Inner mantle fold; IOL, Inner oral lappet; K, Kidney; L, Ligament; M, Mouth; MA, Mantle; MG, Mid gut; MM, Mantle margin; MMF, Middle mantle fold; O, Ovum; OD, Outer demibranch; OLP, Outer labial palp; OMF, Outer mantle fold; OOL, Outer oral lappett; P, Periostracum; PALID, Point of attachment of the ascending lamella of the inner demibranch to the visceral mass; PAM, Posterior adductor muscle (or scar); PBRM, Posterior byssal retractor muscle (or scar); PBRM(1), Posterior byssal retractor muscle unit 1; PBRM(2–6), Posterior byssal retractor muscle units 2–6; PE, Pericardium; PG, Pericardial gland; PILL, Posterior inner ligament layer; PL, Pallial line; PN, Pallial nerve; POLL, Posterior outer ligament layer; PPRM, Posterior pedal retractor muscle (or scar); PI, Prodissoconch I; PII, Prodissoconch II; PRN(I), Inner component of the pallial retrasctor muscle; PRN(II), Outer component of the pallial retractor muscle; R, Resilifer; RA, Renal aperture; RE, Rectum; R-PA, Reno-pericardial aperture; SL Separated ligament; SP, Siphonal papilla; SS, Siphonal septum; ST, Stomach; STA, Statocyst; STH, Statocyst haemocoel; U, Umbo; V, Ventricle; VM, Visceral mass.
SHELL DEFORMITIES
Examination of a wide range of M. minimus shells (Figure 5) from the rocks at Kaštel Stori, however, exposes the full range of shell variation resulting, probably, from the occupation of the pitted, karst rocks. Outline images of the right shell valves of nine living individuals (Figure 5 A–I) show that although the anterior end is always typically swollen, as seen earlier, in other respects, such as the degree of ventral concavity, dorso-ventral height and the degree of damage notably dorsally (F, G), but also posteriorly (C, E) is sometimes extensive. Figure 5J–N illustrates outline images of the shells of five living M. minimus individuals as seen from the posterior aspect. Once again, there is a high degree of variability from a retained near-equivalve juvenile shell (J) with the greatest width (x–y) being almost horizontal and lower than half way down the dorso-ventral aspect of the shell to four others (K–N) demonstrating variable degrees of flattening and twisting so that x–y is located more dorsally and y (the right valve) is always higher than x (the left valve), so that they are all squat and laterally inequivalve.

Fig. 5. Mytilaster minimus. (A–I) Outline images of the right shell valves of nine living individuals. (J–N) Outline images of five shells of living individuals as seen from the posterior aspect. (x–y is the greatest shell width ~ mid-dorso-ventrally).
The shell of the most deformed individual of M. minimus collected from Kaštel Stori is seen in Figure 6 from the dorsal (A), right lateral (B), posterior (C) and anterior (D) aspects. Also illustrated in transverse section is the outline of a shell of M. galloprovincialis (E) with, in both taxa, once again x–y representing the greatest shell width. From the dorsal aspect (A), not only does growth in width occur initially in this individual of M. minimus but then it occurs more narrowly posteriorly and to thereby grossly disfigure the shell. The same processes also result in the wide separation of the umbones. The shell is also dorso-ventrally flattened (B) thereby enhancing the left and right valve inequality (C), and raising the greatest shell width more dorsally (x–y), but which is best seen from the posterior aspect (D). From the anterior aspect, however (E), the umbones have become widely separate. In contrast, the M. galloprovincialis shell, which is a byssally attached surface rock dweller, not generally occupying pits in the limestone karst, is usually un-deformed and has the outline, when seen from the posterior aspect (E), typical of the species and of its genus.

Fig. 6. Mytilaster minimus. The shell of the most deformed individual collected. As seen from A, the dorsal; B, the right lateral; C, the posterior and D, the anterior aspects. Also illustrated in transverse section is the outline of a shell of Mytilus galloprovincialis. (x–y = greatest shell width).
THE INTERNAL SURFACE OF THE SHELL
The shell of M. minimus is thick and internally darkly coloured (Figure 7) over much of its surface except antero-ventrally. This is caused by the nacre of the shells’ interior being purple above and white below the keel. The opisthodetic, parivincular ligament of M. minimus is internal and relatively long. It comprises two layers, the posterior internal (PILL) and posterior external (POLL) layers, as described for heteromyarian mytiloids by Yonge (Reference Yonge1978), with staining reactions similar to those of Mytilus edulis (Linnaeus, 1758) (Trueman, Reference Trueman1950; Beedham, Reference Beedham1958), indeed of all representatives of the Mytiloidea (Yonge & Campbell, Reference Yonge and Campbell1968). The ligament of M. minimus sits upon a stout, white, resilifer (R) and, as in all mytiloid taxa, the periostracum (P) extends over the ligament, thereby adding another layer. This was termed fused periostracum (Owen et al., Reference Owen, Trueman and Yonge1953) although, since periostracum must cover the entire shell, there is no fusion. The term is nevertheless retained herein simply to emphasize that it is present. Posterior to the ligament, the dorsal margin of the two shell valves are characterized by up to 7 dorsal dissoconch teeth (DDT), which are simple interlocking cones.

Fig. 7. Mytilaster minimus. An interior view of the left shell valve. (For abbreviations see Box 1.)
The muscle scars on the shell of M. minimus (Figure 7) comprise a large posterior adductor muscle (PAM) and, anteriorly to this, a large posterior pair of byssal retractor muscles (PBRM). Located, uniquely, below these is a posterior pedal retractor muscle scar (PPRM). The pallial line (PL) is thin throughout its length except posteriorly underneath the posterior adductor muscle scar where it is thick. This is because of the muscular siphonal septum (SS) located here. The anterior byssal retractor muscle (ABRM) is located just posterior to the umbo and half hidden by the hinge plate. There is a small anterior adductor muscle scar (AAM) situated antero-ventrally.
THE HINGE PLATE
The hinge plate of a juvenile individual of Mytilaster minimus was described earlier (Figure 2B) and that of a young adult (Figure 7) has the same basic form except that three secondary (non-dissoconch) anterior hinge teeth (AHT) have developed, and arise from beneath the umbones (U) and are not added to in number with age and growth. The anterior ventral edge of the left shell valve is formed into small rounded projections that partially hide the adductor muscle scar (AAM).
Figure 8 comprises internal views of the heavily calcified hinge plate areas of an adult individual of M. minimus 13 mm in shell length. Here, the two valves have been drawn with the antero-ventral valve margins depressed so as to be able to see the anterior adductor muscle scar and associated structures more clearly. Viewed in this way, the form of the anterior adductor muscle (AAM) scars are roundly oval and, starting from their anterior faces, the shell valves are calcified to form simple buttresses (BU) that connect with the undersurface of the left and right hinge plates (HP). These add strength to the anterior region of the shell. Figure 8 further shows that secondary, anterior, hinge teeth (AHT) develop on both left and right valves and become heavily calcified with age and increasing size as does the hinge plate (HP) itself beneath and ventral to the umbones (U).

Fig. 8. Mytilaster minimus. Internal views of the heavily calcified hinge plate areas of the A, left and B, right shell valves of a large (shell length = 13 mm) individual. Note that the valves are illustrated when their anterior ends have been depressed to show the buttresses connecting the undersurfaces of the hinge plates to the inner surfaces of the valves at the anterior ends of the anterior adductor muscle scars. (For abbreviations see Box 1.)
MORPHOMETRIC ANALYSES
Morphometric data obtained for the three measured shell axes of M. minimus and M. galloprovincialis are summarized in Table 1 and illustrated in Figure 9. A t-test for independent samples showed that the differences in shell length between the two species were not significant (P > 0.05), which confirms that the selected samples were representative for the comparison of other shell parameters. A t-test for independent samples showed that the differences in shell height and width between the two species were statistically significant (P < 0.05). According to this analysis, therefore, M. minimus individuals are wider and dorso-ventrally shorter than M. galloprovincialis individuals of the same shell height (Figure 10).

Fig. 9. Shell length (A), width (B) and height (C) frequency structure of Mytilaster minimus and Mytilus galloprovincialis obtained from Kaštela Bay, Croatia.

Fig. 10. A comparison of morphometric data between Mytilaster minimus and Mytilus galloprovincialis from Kaštela Bay, Croatia.
Table 1. Morphometric data obtained for Mytilaster minimus and Mytilus galloprovincialis from the rocky shore in Kaštela Bay. Values are presented as means ± SD (minimum-maximum).

The data obtained for the morphometric parameter dorsal valve thickness for both M. minimus and M. galloprovincialis from the rocky shore at Kaštela Bay are presented in Table 2. Figure 11 compares the dorsal valve thickness of M. minimus individuals with those of M. galloprovincialis (N = 15) and with similar shell lengths ranging from 9.2–15.5 and 9.0–15.5 mm, respectively. Dorsal shell length thickness values for the two taxa ranged from 0.26 to 0.45 mm with a mean (±SD) of 0.35 ± 0.06 mm and 0.08 to 0.28 mm with a mean (±SD) of 0.15 ± 0.06 mm, again respectively. A t-test for independent samples showed that the differences between the two species were significant (P > 0.05) and, thus, that for individuals of approximately the same shell length, dorsal valve thickness in M. minimus was more than twice that (233%) of M. galloprovincialis.
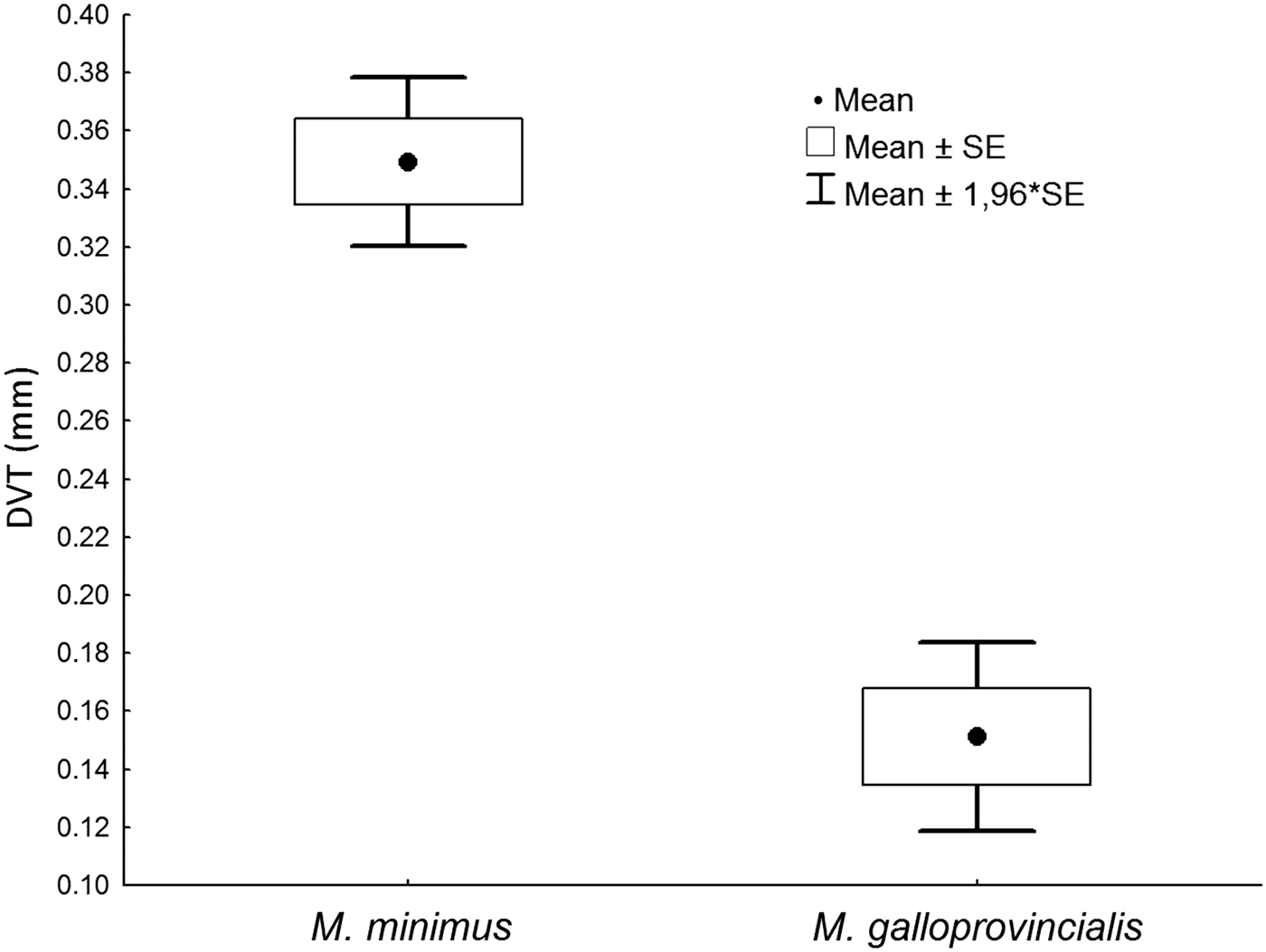
Fig. 11. A comparison of dorsal shell valve thickness (DVT) between Mytilaster minimus and Mytilus galloprovincialis from Kaštela Bay, Croatia.
Table 2. Dorsal valve thickness (DVT) data obtained for Mytilaster minimus and Mytilus galloprovincialis from Kaštela Bay. Values are presented as means ± SD and includes the ranges (minimum and maximum) of shell parameters measured.

THE LIGAMENT COMPARED WITH THAT OF MYTILUS GALLOPROVINCIALIS
Figure 12 comprises illustrations of the internal structures of the ligaments of adult individuals of A, M. minimus and B, M. galloprovincialis. Both are drawn to precisely the same scale. They both have the same structure, except the posterior end of the ligament of M. minimus possesses a row of up to 7 dorsal dysodont teeth (DDT). In comparison, the ligament (L) of M. minimus is twice as wide as that of M. galloprovincialis and is connected to the shell valves by a correspondingly thicker resilifer. Beyond both ligaments posteriorly is fused periostracum (FP). Anteriorly, however, the ligament and periostracum of M. minimus is split (SL). This is natural in this species and results from its epilithic lifestyle inhabiting pits in its occupied rocks (Figure 6), as described earlier. The same split in M. galloprovincialis was caused by the manual separation of the valves – but matching in effect the situation seen in M. minimus.

Fig. 12. Internal views of the ligaments of A, Mytilaster minimus and B, Mytilus galloprovincialis both drawn to precisely the same scale using individuals of the same size. (For abbreviations see Box 1.)
Internal anatomy
THE SIPHONS
Figure 13 is a view of the posterior end of the shell and siphonal apparatus of Mytilaster minimus. The inhalant aperture (IA) is not separated from the pedal/byssal aperture by fusion of the opposite mantle lobes but is separated functionally by their apposition. The exhalant siphon (ES) is formed by fusion between the inner mantle folds only, this being type A (Yonge, Reference Yonge1948, Reference Yonge1957). The expulsion of pseudofaeces from the inhalant aperture is via its dorsal connection with the base of the exhalant siphon. Such a situation is typical of most representatives of the Mytiloidea (White, Reference White1937; Yonge, Reference Yonge1955; Wilson, Reference Wilson1967; Fankboner, Reference Fankboner1971) but atypical of more advanced siphonate bivalves. Both the inhalant aperture and the exhalant siphon bear siphonal papillae (Figure 13). The inhalant papillae (IASP) are formed on the inner surfaces of left and right middle mantle folds and number up to 7 on both sides of the aperture. They are creamy white. The exhalant siphon bears 2 small brown papillae (ESSP) on the dorsal edge of its aperture.

Fig. 13. Mytilaster minimus. The posterior end of the left mantle margin to show the siphonal apparatus, after removal of the right shell valve, mantle lobe and ctenidium. (For abbreviations see Box 1.)
The siphonal septum (Figure 13, SS) connects the ctenidia (ID, OD) to the mantle at the point of fusion of the mantle lobes separating the exhalant siphon from the inhalant aperture. It thus hangs down from the exhalant siphon into the dorsal region of the inhalant aperture and is much larger and more solid than those of both M. modiolus and M. galloprovincialis (Dinesen & Morton, Reference Dinesen and Morton2014, fig. 10). This is reflected in the large scar it creates on both valves where its muscles attach at the pallial line (Figure 7). The septum effectively separates posteriorly the infra-branchial from the supra-branchial chamber and is thought to act as a valve in other mytiloids (Fankboner, Reference Fankboner1971), controlling the volume of water entering the infra-branchial chamber. When the animal is actively filtering, therefore, and the inhalant and apertures are extended into the water column, the septum is held near-vertically regulating the size of the inhalant stream. When the left and right folds of the inhalant aperture are withdrawn, however, the septum folds up left and right.
Each middle mantle fold that forms the inhalant aperture is patterned with a brown stripe. These fuse dorsally towards the exhalant siphon to form a single stripe that encircles it. The siphonal septum is also pigmented brown.
THE MANTLE MARGIN
Figure 14 is a transverse section through the mid-ventral region of the left ventral mantle margin of M. minimus. As noted above, mantle fusions occur dorsally above the exhalant siphon and between the exhalant siphon and inhalant aperture of M. minimus. Mantle fusions, as with the siphons, are of the inner mantle folds only and thus of type A (Yonge, Reference Yonge1957). The mantle margin, as in most bivalves, except for protobranchs and arcoids, comprises three folds, inner, middle and outer (Yonge, Reference Yonge1982). The outer surfaces of the outer folds (OMF) secrete the shell valves. The periostracum (P) is secreted by the epithelia delimiting the periostracal groove, that is, the outer surface of the middle fold (MMF) against the template of the inner surface of the outer fold (OMF). The periostracum is composed of three layers as in all mytiloideans studied hitherto (Beedham, Reference Beedham1958, fig. 1).

Fig. 14. Mytilaster minimus. A transverse section through the mid-ventral region of the mantle margin. (For abbreviations see Box 1.)
The inner fold of the mantle (IMF) is large and at its junction with the general mantle surface there is a ventral ciliated rejectory tract (CRT) that transports unwanted particles posteriorly towards the inhalant aperture for eventual expulsion from the infra-branchial chamber. The swollen inner folds are glandular, internal sub-epithelial cells probably secreting mucus. The central region of this fold is, however, a large haemocoel (H). The pallial retractor muscle, which attaches the mantle to the shell valves at the pallial line on each one, sends fibres into the point of union of the middle and outer folds (PRM(II)), that is, towards the base of the periostracal groove. A second branch of the pallial retractor muscle sends fibres into the inner mantle fold (PRM(I)) where they intermingle with the elements of the haemocoel. There is also a pallial nerve (PN).
Distally, beyond the mantle margin, the general mantle (MA) contains, as in some other mytiloids, such as M. galloprovincialis discussed herein, much of the gonadial tissue of the animal, in this illustrated case the ovaries (O), each follicle of which contains eggs up to about 100 µm in diameter.
The ciliary currents of the left and right mantle lobes of M. minimus are all rejectory and serve to keep the mantle cavity free of either too large or unwanted particles. They are identical to those seen in other mytiloids, for example, Limnoperna fortunei (Dunker, 1857) (Morton, Reference Morton and Boltovskoy2015a) and are thus not illustrated herein.
THE MUSCULATURE
The musculature of M. minimus is seen from the right side in Figure 15. The anterior adductor muscle (AAM) is small and located on the antero-ventral floor of the shell valves. In this respect M. minimus is similar to other mytiloideans (White, Reference White1937; Wilson, Reference Wilson1967). There is also a pair of thin anterior byssal retractor muscles (ABRM) arising from beneath the hinge plate and thus separate from the adductor muscle. In contrast to the anterior adductor, the posterior adductor muscle (PAM) is large, circularly ovate, and anteriorly abuts the large paired adjoining byssal retractor muscles (PBRM). These large muscles reduce the volume of the visceral mass (VM). There is also a pair of small posterior pedal retractor muscles (PPRM) that, uniquely for the Mytiloidea, have their origins beneath the posterior byssal retractors.

Fig. 15. Mytilaster minimus. The musculature as seen from the right side. (For abbreviations see Box 1.)
THE FOOT AND BYSSAL APPARATUS
The foot of M. minimus in relation to the musculature and as seen from the right side is illustrated in Figure 15. It is further illustrated in Figure 16 in more detail. The foot (F) arises from the postero-ventral margin of the visceral mass (VM), which is pigmented dark brown. The foot itself is, however, largely translucent and can be extended enormously. From the swollen base of the foot at its junction with the visceral mass arises a stout byssus (BY). The threads of this arise from a pore, which then develops into a byssal groove (BGR) that extends down the length of the foot. Within the foot can be seen a darkly staining area basally that, as will be shown, corresponds to sub-epithelial eosinophilic glands (EGC) here while throughout the total length of the foot, there are lighter basiphilic glands (BGC).

Fig. 16. Mytilaster minimus. The foot as seen from the right side. (For abbreviations see Box 1.)
A section through the foot and byssal apparatus of M. minimus (Figure 17) shows that the proteinaceous byssus arises from an array of byssal rootlets (BYR), each separated one from another by interspersed, extremely thin (1–2 µm) epithelial membranes. Eventually, these fuse to form a characteristic byssal stalk (BYS) that departs the foot (F) at the byssal groove (BGR). The byssal threads are secreted by an eosinophilic byssal gland (EGC) that comprises cells some 20 µm in length and are arranged around the origins of the byssal rootlets. This was termed the ‘white’ or ‘collagen’ gland in the foot of M. edulis by Brown (Reference Brown1952) and Pujol (Reference Pujol1967). A second basiphilic gland (BGC) is arrayed sub-epithelially within the foot and around the borders of the byssal groove and comprises elongate cells some 25 µm in length. These cells, the ‘purple’ gland of Brown (Reference Brown1952), probably secrete mucus, although Pujol (Reference Pujol1967) thought their secretions might be ‘enzymatic’ and responsible for tanning the collagen produced by the ‘white’ gland.

Fig. 17. Mytilaster minimus. A section through the foot and byssal apparatus. (For abbreviations see Box 1.)
THE CTENIDIA AND LABIAL PALPS AND THEIR CILIARY CURRENTS
The organs of the mantle cavity of M. minimus, as seen from the right side after removal of the right shell valve and most of the mantle lobe covering the right ctenidium, are illustrated in Figure 18. A comparable illustration of those of M. edulis was provided by Kellogg (Reference Kellogg1915, fig. 18). The first detailed study of the bivalve ctenidia was undertaken by Ridewood (Reference Ridewood1903), but elaborated on in a series of classical studies by Daphne Atkins, some of which are referred to herein. The ctenidia of M. minimus, which fulfil the dual roles of respiration and particle capture and transport, comprise two sub-equal demibranchs of which the outer is the longer dorso-ventrally. The upper, dorsal margins of the ascending lamellae of the outer and inner demibranchs are attached to the mantle and the visceral mass, respectively, by ciliary fusions (Atkins, Reference Atkins1937). The ventral margin of the outer demibranch always lies tucked behind the incurving mantle margin with the associated, here thickened, periostracum. Like many other mytiloids also (Fankboner, Reference Fankboner1971) the outer demibranchs of M. minimus are some 3 or 4 filaments shorter at their anterior ends than the inner ones. Fankboner (Reference Fankboner1971) states that ‘a functional advantage for this anatomical reduction is unclear’. For M. minimus, however, the advantage of this arrangement is that it enables the ventral marginal food grooves of both demibranchs to be in contact with the sorting, and hence either acceptance or rejection functions, of the inner surfaces of both inner and outer labial palps thereby greatly increasing the efficiency of particle selection by these structures. The ctenidial-labial palp junction of M. minimus thus falls into Category I elucidated by Stasek (Reference Stasek1963) and is typical of the Mytiloidea in general.

Fig. 18. Mytilaster minimus. The organs of the mantle cavity as seen from the right side after removal of the right shell valve and most of the mantle lobe covering the right ctenidium. (For abbreviations see Box 1.)
The ctenidia of M. minimus are flat, homorhabdic and filibranchiate (eleutherorhabdic). The ascending and descending lamellae of both demibranchs are cross-connected by inter-lamellar junctions, or unions. Similarly, the dorso-ventrally aligned filaments which make up each lamella are connected laterally by inter-filamentary junctions that maintain ctenidial cohesion. The junctions that cross-connect the individual filaments comprise ciliary discs as in other mytiloideans and as such are weak so that the filaments readily separate one from another when damaged. The apices of the ctenidial filaments of all bivalves, save for the carnivorous septibranchs (Morton, Reference Morton2016) comprise a number of ciliary types that collectively fulfil the roles of filtration, particle entrapment and transportation. The currents through the ctenidia, that is, from the infra- to the supra-branchial chambers, are created by lateral cilia that are, thus, largely responsible for the forceful inhalant and exhalant streams into and out of the mantle cavity. The filtering apparatus itself is the responsibility of eulaterofrontal cirri, the fine structure of which have been illustrated and described for M. edulis by Owen (Reference Owen1974). Such a structure is typical of all studied mytiloideans, including M. minimus, and all those bivalves that Atkins (Reference Atkins1938) classified as the Macrociliobranchia, and which were refined histologically by Owen (Reference Owen1978). Other ciliary tracts on each filament head, principally the apical frontal cilia, are concerned with the transport either up or down of those particles flicked on to them by the eulaterofrontal cirri. It is the activities of these cilia that feed particles into the various ctenidial food grooves for onward transmission to and sorting by the labial palps.
The structure and ciliary currents of the ctenidial-labial palp junction of M. minimus are illustrated in Figure 19. The ciliation of the ctenidial surfaces of M. minimus is of type B(I) (Atkins, Reference Atkins1937). That is, acceptance tracts are situated within the ventral marginal food grooves of both demibranchs (ID, OD), in the ctenidial axis (DCAT) and in the junctions of the ascending lamellae of the inner and outer demibranchs with the visceral mass and mantle, respectively. Particles arriving at the anterior end of the ctenidium via (1) the crests of the ventral marginal food grooves of both inner and outer demibranchs; (2) inside the ventral food groove of the outer demibranch; and (3) in all three dorsal food grooves are subjected, before ingestion, to the ciliary selection currents of the labial palps (ILP, OLP).

Fig. 19. Mytilaster minimus. The labial palps and their ciliary currents as seen from the right side. (For abbreviations see Box 1.)
Particles are removed from the anterior ctenidial termini by the un-ridged, posterior edges of the labial palps, the ciliary currents of which subsequently pass the particles onto their ridged sorting region. This function is the attribute of the system of parallel ridges and grooves, which pass selected particles of a suitable nature and size over the crests of the ridges toward the proximal oral groove for ultimate ingestion at the mouth (M). Too large and/or unwanted particles are passed laterally toward the opposite free edge of the palp for rejection. Re-circulatory currents also exist. Details of the labial palp ciliation need not be elaborated upon since they are essentially the same as those described by Fankboner (Reference Fankboner1971) and are typical of mytiloids in general. An important exception to this generalization, however, is that the first ridges closest to the mouth of each palp are enlarged to form swollen lappets (IOL, OOL). These, when separated, allow potential food particles to be accepted for ingestion and to be passed directly into the mouth (M). When they are opposed, however, nothing can enter the mouth. The mouth itself is located between the anterior adductor muscle (AAM) and the paired posterior byssal retractor muscles.
THE CILIARY CURRENTS OF THE VISCERAL MASS
The structure of the visceral mass of M. minimus, as seen from the right side, showing its surface ciliary currents is illustrated in Figure 20. The ciliary currents of the surface of the visceral mass (VM) pass particles downwards dorsally and then postero-ventrally to be concentrated at its postero-ventral pointed tip. From this point, the ventrally directed ciliary tracts of the ascending lamellae of the inner demibranchs presumably remove them. Being too large to enter the ventral marginal food grooves of these demibranchs, such material ultimately passes into the ventral, also posteriorly directed, rejectory tracts of the mantle (not illustrated).

Fig. 20. Mytilaster minimus. The visceral mass as seen from the right side showing its surface ciliary currents and the course of the intestine within it and location of the heart. (For abbreviations see Box 1.)
THE ALIMENTARY CANAL
Figure 20 gives a general picture of the course of the intestine in the visceral mass (VM) of M. minimus, that is, above and between the large paired byssal retractor muscles (PBRM) and below the organs of the pericardium (PE). The course of the intestine in M. minimus is similar to that seen in other mytiloideans (Wilson, Reference Wilson1967). The oesophagus passes upwards from the mouth, which lies between the anterior byssal retractor muscles (ABRM) and the dorsal surface of the anterior adductor muscle (AAM). The oesophagus opens into the stomach (ST), which is located under the antero-dorsal margin of the shell and is surrounded by the dark digestive diverticulae (DD). From the posterior end of the stomach arises the combined style sac and mid-gut (CSSMG), which passes backwards between both blocks of the posterior byssal retractor muscles (PBRM) to a point just anterior to the paired posterior pedal retractor muscles (PPRM). The style sac terminates here, but the mid-gut (MG), now separated from it, loops forwards alone to pass back between the posterior byssal retractors. The mid-gut loops again on the left side of the stomach and turns posteriorly again to loop over the stomach and become the hind gut (HA). This, in turn, penetrates the ventricle of the heart (HA) as the rectum (R), passes between the dorsal surfaces of the posterior pair of byssal retractor muscles, over the posterior adductor muscle (PAM), to terminate in an anus (AN) on the posterior face of this structure facing the exhalant siphon. The histological structures of the style sac and intestine of M. minimus are essentially the same as those described for M. edulis by Giusti (Reference Giusti1971) and for all other mytiloideans studied hitherto.
THE STATOCYSTS
Hitherto, the statocysts of only one species within the Mytiloidea, that is, M. galloprovincialis have been described (List, Reference List1902). In this species each statocyst surrounds an empty capsule, which has a tubular connection to the mantle cavity via a pore on the visceral mass. In the case of the other studied mytiloids, any statocysts, if present, are obscured by the gonads, which fill the visceral mass. This is not the case in M. minimus, however, because, as described earlier, the central region of the visceral mass is an extensive haemocoel. This has allowed identification of a pair of statocysts in the visceral mass of M. minimus (Figure 21). Unlike the situation in M. galloprovincialis, each statocyst (STA) occupies an epithelium-lined cavity some 20 µm in diameter that is partially filled by a granulated core with a single nucleus. By following statocyst structure using serial sections, it is herein confirmed that each one of the pair is not connected to the external surface of the visceral mass by a tube, as in M. galloprovincialis (List, Reference List1902), but is a discrete rounded capsule. Each one is contained within its own component (STH) of the central haemocoel (HA) in the visceral mass.

Fig. 21. Mytilaster minimus. A section through the haemocoel of the visceral mass showing what are possibly a pair of putative statocysts. (For abbreviations see Box 1.)
THE ORGANS OF THE PERICARDIUM
The organs of the pericardium of M. minimus, as seen from the right side, are illustrated in greater detail in Figure 22. The pericardium (PE) is located directly under the short array of dorsal dysodont teeth (DDT) that lie under the dorsal surface of each valve and, thus, the strongest and most protective region of the shell. The course of the rectum (RE) through the medial ventricle (V) of the heart, within the pericardium, between the pair of posterior byssal retractor muscles (PBRM) and over the posterior adductor muscle (PAM) has been described above. The course of the intestine, except for the rectum within the ventricle of the heart, has been ignored in Figure 22. Posterior and ventral to the heart are the paired, pale-brown, kidneys (K). The reno-pericardial apertures (R-PA) of the kidneys are situated on the antero-ventral floor of the pericardium adjacent to the visceral mass containing the digestive diverticulae (DD). The epithelia of the left and right auricles of the heart contain the similarly light brown pericardial gland (AU + PG).

Fig. 22. Mytilaster minimus. The organs of the pericardium as seen from the right side. (For abbreviations see Box 1.)
The renal apertures (RA) open onto the visceral mass to left and right between the ctenidial axis (CA) and the point of union of the ascending lamellae of the inner demibranch (PALID) of the ctenidium with the visceral mass and close to each one of these, the gonadial apertures (GA) also open into the supra-branchial chamber between these two attachment points. That is, excretory products and gametes are discharged into that component of the supra-branchial chamber situated between the ctenidial axis and the inner ctenidial demibranch. Similarly, the anus (AN), hanging freely from the posterior edge of the posterior adductor muscle (PAM) within the same supra-branchial chamber, discharges faeces into the exhalant siphon.
REPRODUCTION
Mytilaster minimus is generally dioecious The large paired gonads are located in mesosomal lobes in, primarily, the postero-dorsal region of the visceral mass and within each mantle lobe as in M. edulis (White, Reference White1937). The gonads do not, however, as in most other mytilids, for example the freshwater L. fortunei (Morton, Reference Morton and Boltovskoy2015a, fig. 5) fill up the central area of the visceral mass as this is occupied by an extensive haemocoel, as described earlier (Figures 17 & 21).
DISCUSSION
The origins of this study and research paper lie in the fact that until 2013, M. galloprovincialis occupied the lower margins of the study site, Kaštela Bay, and much of the coastline of Croatia, but due to a widespread infection in that year by a trematode (Bucephalus sp.) the species largely disappeared from here (SP, personal observations). Importantly, however, M. minimus was unaffected by the trematode. Subsequent to the loss of M. galloprovincialis, there was a corresponding increase in the M. minimus population on the rocky promontory at Kaštel Stori. In 2016, however, the population of M. galloprovincialis recovered from the trematode infection and re-colonized the rocks at Kaštel Stori. Because of the increasing numbers of M. galloprovincialis, M. minimus is now, in 2016, in regression at the site (SP, unpublished data). It was this rapid re-colonization of the hitherto exclusive habitat of M. minimus at Kaštel Stori by M. galloprovincialis that stimulated this research study.
Štambuk et al. (Reference Štambuk, Šrut, Šatović, Tkalec and Klobučar2013) recorded that M. galloprovincialis has a long-lived pelagic larva with a high dispersal potential and that, although such larvae can remain in the pelagic environment for over 3 weeks, the transport of most individuals is restricted to within 10 km of their origin. The >20 km distance between the mussel farm at Marina and Kaštel Stori is not an insurmountable distance and may help account for the rapid re-colonization of the rocks here following recovery from the Bucephalus infection. It was within the context of this scenario and that of the broader problem of the displacement of M. minimus from its habitat by B. pharaonis (and locally and in Sicily by M. galloprovincialis (Riggio et al., Reference Riggio, D'Anna and Sparla1992)) that this study of M. minimus was undertaken. It is, however, interesting that elsewhere, for example north-west Spain, M. galloprovincialis is itself in competition with another invasive mytiloid, Limnoperna (=Xenostrobus (Colgan & da Costa, Reference Colgan and da Costa2013)) securis (Lamarck, 1819) (Comeau & Babarro, Reference Comeau and Babarro2014).
The anatomy of M. minimus has never been described. In general terms, however, this study describes a species, which though adapted to the colonization of karsted limestone rocks, in most respects has a typical mytiloid morphology. It has, however, unlike the epilithic species of Mytilus that colonize the shores of most of the boreal northern Atlantic and Pacific, and almost certainly related to its occupied niche of karsted limestone rock pits, a small, grossly deformed and thickened shell. As a pre-dissoconch juvenile, the shell has the typical heteromyarian form of many mytilids, again for example, species of Mytilus. As it ages, however, and with growth, deformation occurs resulting in a flattened, squat, form with the greatest shell width situated more dorsally, to reduce the dorsal profile of the shell to match the habitat's rugosity. On the similarly limestone shores of the Cape Verde Islands, not even the equally small B. puniceus achieves this degree of deformity (Morton, Reference Morton2012).
This study, however, reveals other unique aspects of the anatomy of M. minimus. Firstly, the gonads are restricted to the dorsal regions of the visceral mass but mostly to the mantle underlying each shell valve. This is not unusual in itself for the Mytiloidea, except that in the studied taxa that make up this superfamily, the gonads also occupy the central region of the visceral mass. In M. minimus, this region is a large haemocoel, which, when considered alongside the fact that each mantle margin also contains a haemocoel, suggests that siphonal extension, and thus filtering activity, is not related so much to adductor muscle/ligament activities, but rather to the pumping of blood from one part of the body to another to create hydrostatic changes within the body.
This visceral mass haemocoel has resulted in, secondly, the discovery of another important morphological feature, namely the visible presence of a pair of statocysts. The variety of bivalve statocyst structure is poorly known and only Morton (Reference Morton1985) has reviewed their structure in the Anomalodesmata revealing a range of complexity with the most sophisticated being demonstrated by the ancient pholadomyid Pholadomya candida G.B. Sowerby I, 1823 (Morton, Reference Morton1980). In comparison with this taxon, the statocysts of M. minimus, and M. galloprovincialis (the latter described by List, Reference List1902) are extremely simple. Whether this is either a primitive phylogenetic feature or a regressed character because of the, in turn, simple byssally-attached lifestyle, is unknown but the absence of tubes connecting each statocyst to the mantle cavity in M. minimus suggests a morphological improvement on those of M. galloprovincialis.
Thirdly, this study demonstrates that the positions of the attachments of the posterior pedal retractor muscles to the shell of M. minimus are unique amongst the Mytiloidea. Morton (Reference Morton2015b) showed that each phylogenetic lineage of the superfamily has a uniquely correlated relationship between the pericardium and the posterior byssal and pedal retractor muscles. In all the studied species of the Mytilidae, therefore, the pericardium is situated anterior to the posterior byssal and pedal retractor muscles – the latter typically small, the former aligned in a row of one or more units. There are six of these for example in M. galloprovincialis. In M. minimus, however, there is one large block of paired posterior byssal retractor muscles and the similarly paired posterior pedal retracts are small and attached to the shell valves beneath these. This character sets M. minimus apart from the other representatives of the Mytilidae.
In conclusion, Figure 23 provides a summary comparison between shell form in A, M. galloprovincialis and C, and D, juvenile and adult individuals of M. minimus, respectively. As a juvenile, M. minimus has the same overall form of M. galloprovincialis when seen from the posterior aspects, but with age its shell becomes squatter, is greatly flattened and, thus, much wider and, as described (Table 1, Figure 10), greatly distorted. Not just this but the shell of M. minimus becomes heavily calcified with age such that the dorsal valve thickness just posterior to the ligament is more than twice the thickness of the equivalent location in M. galloprovincialis for individuals of the same shell length (Table 2, Figure 11). Undoubtedly, such a thickening causes a widening (and thickening) of the ligament too and, thus, that a greater degree of protection is obtained from both shell drilling and surface nibbling predators. Coincidentally, such a thickening protects the most vulnerable organs of the body, namely the pericardium and its contained organs.

Fig. 23. A comparison of shell form and the posterior musculature/pericardial complex in A and B, Mytilus galloprovincialis and C, D and E, Mytilaster minimus. C, is a juvenile individual; D, an adult. x–y = greatest shell width. (For abbreviations see Box 1.)
In addition to this comparison of shell form, moreover, Figure 23B & E compares the posterior musculature/pericardial complex between the two taxa. As described by Morton (Reference Morton2015b), the complex exhibited by M. galloprovincialis is typical of the species of Mytilus (Mytilidae) whereas in M. minimus there has been a loss of five of the six posterior byssal retractor muscle blocks to just one. And the posterior pedal retractor muscles are, as we have seen, now situated beneath the remaining single, but paired, byssal retractor muscle blocks. This places the pericardium and contained heart, not between the anterior-most block of the posterior byssal retractor muscles and the posterior pedal retractor muscles (Figure 23B), but directly up against the former and with the pericardium pushed postero-dorsally upwards (Figure 23E). This difference in anatomy is believed to be related to the anterior-posterior and dorso-ventral compression exhibited by M. minimus in comparison with the more epifaunal M. galloprovincialis so that the space within the dorso-ventrally flattened and width expanded shell of the former species species in relation to length is used most efficiently.
Finally, according to Seed (Reference Seed1969), growth in marine mytilids, for example M. edulis, is particularly seasonal with little or no growth during winter. Mytilaster minimus is, however, significantly smaller (<16 mm) than the shell length values obtained for the other marine mytilid species identified above, indicating an extremely slow growth rate. Whether this is true or not is the subject of ongoing studies in an attempt to understand the growth, age and life history tactic of this unusual mytilid.



























