INTRODUCTION
The family of Grapsidae is the richest decapod family among the intertidal species. Pachygrapsus marmoratus is a species of crab, sometimes called the marbled rock crab or marbled crab, living in the intertidal belt of rocky shores throughout the Mediterranean Sea, Black Sea and north-western Atlantic from Brittany to Morocco, including the Canary Islands, Normandy, the Azores and Madeira (Ingle, Reference Ingle1980; Cannicci et al., Reference Cannicci, Paula and Vannini1999; Dauvin, Reference Dauvin2009, Reference Dauvin2012). It is widespread in southern Europe (Cannicci et al., Reference Cannicci, Gomei, Boddi and Vannini2002), from the Black Sea to the Moroccan coast, and along the Atlantic coasts of Portugal, Spain and France (Ingle & Clark, Reference Ingle and Clark2008). It has recently been observed as far north as Southampton in the English Channel (Ingle & Clark, Reference Ingle and Clark2008). This expansion of range may be due to the warming of the surface waters (Dauvin, Reference Dauvin2009).
Pachygrapsus marmoratus exhibits a semi-terrestrial lifestyle. It is a flexible, omnivorous species that actively searches for food, relying on the intertidal community throughout its post-larval life (Cannicci et al., Reference Cannicci, Gomei, Boddi and Vannini2002). Hard-shelled organisms such as limpets, barnacles and mussels, as well as algae, constitute its food items (Cannicci et al., Reference Cannicci, Gomei, Boddi and Vannini2002, Reference Cannicci, Gomei, Dahdouh-Guebas, Rorandelli and Terlizzi2007; Silva et al., Reference Silva, Boaventura, Flores, Ré and Hawkins2004). It spends most of its time active in this zone (below and above water) (Cannicci et al., Reference Cannicci, Paula and Vannini1999). This species can be separated from other Pachygrapsus members by a four-segmented antennula exopod, a uropod protopod with two setae and a uropod exopod with 17 setae (Pessani et al., Reference Pessani, Tirelli and Flagella2004). Pachygrapsus marmoratus have an ability to move quickly, and hence they can escape to the hollows, which makes them difficult to catch (Aydın et al., Reference Aydın, Karadurmuş and Mutlu2013a; personal observation).
Stomach contents studies reveal that crabs and their larvae are important food sources for fish, crabs and other marine organisms (Şen, Reference Şen2007; Aydın et al., Reference Aydın, Karadurmuş and Mutlu2013b). Pachygrapsus marmoratus plays an important role on the Black Sea coast ecosystems as a component of the diet of fish such as bream, scorpion fish, meager and bass. Georgiev (Reference Georgiev2006) and Şen (Reference Şen2007) also found that P. marmoratus are among the dietary items of octopus and otters.
Although P. marmoratus is the most dominant intertidal brachyuran on the rocky Black Sea shore of Turkey, and its habitat includes the whole intertidal zone (Aydın et al., Reference Aydın, Karadurmuş and Mutlu2013a), there is still a paucity of information about it in the region (Selimoğlu, Reference Selimoğlu1997). In addition, a detailed study of the reproductive biology of the species in this region would be highly relevant. Therefore, the specific objective of this study was to determine the size composition, biological characteristics and reproductive biology of P. marmoratus on the Black Sea coasts of Ordu, Turkey.
MATERIALS AND METHODS
Study area and sampling
The study was carried out at 41°08′44″–40°57′28″N 37°10′29″–38°06′57″E (the Black Sea, Turkey; Figure 1). Crabs were collected by hand monthly between November 2012 and October 2013. The samples were collected within a period of 3–6 h by the same persons between the 10th day and 20th day of the month. Small individuals were easier to catch as they were generally found among the mussel populations. However, the large ones were very active and tended to escape to crevices, compelling us to collect them before escaping. It is equally noteworthy that crabs appeared to select the crevices during the winter months. The samples were kept in ice cold water until measurement.

Fig. 1. Map of the study area: (A) position of the Black Sea; (B) map of the sampling location (Turkey).
Measurements
Sex distinction was made based on typical characteristics of the abdomen. In most males the abdomen is narrower and triangular, while in females it is broader and rounded (Wenner, Reference Wenner1989; Guerao & Rotlland, Reference Guerao and Rotllant2009). Monthly collected samples were separated into sexes and counted, and the sex-ratio was calculated (M:F) according to size-classes of carapace width (CW).
The carapace length (CL) was measured with a 0.01 cm precision Vernier caliper from the edge of the frontal region near the eye to the base of the carapace backwall, while CW with spines was taken from the tip of the left dorsal spine to the tip of the right dorsal spine. The CL and CW of the male, female and ovigerous females were measured to the nearest 0.01 cm and crab fresh weight (W) was determined using a balance with an accuracy of 0.01 g.
In the determination of growth rates of CW and W, the following formulae were used:
where n is length-class (Ricker, Reference Ricker1975).
The CW–W relationship for both sexes was calculated following the method of Ricker (Reference Ricker1973, Reference Ricker1975):
where W is crab fresh weight (g), L is carapace width (cm), a is the intercept of the regression curve (a constant) and b is the regression coefficient (an exponent).
The condition factor (K) for both sexes was calculated using the method of Bagenal & Tecsh (Reference Bagenal, Tesch and Bagenal1978):
where K is condition factor, W is fresh weight of crabs (g) and L = CW (cm).
Reproduction, fecundity and egg diameter
For fecundity estimation, 91 ovigerous P. marmoratus were examined. The gonadosomatic index (GSI) of the female crabs was calculated with the following formula (Bagenal, Reference Bagenal1978):
In order to obtain the data related to reproductive biology, the pleopod eggs were removed from females and the total weight of eggs were measured using a balance with a sensitivity of 0.0001 g. Then, sub-samples were taken randomly from different areas and weighed, and eggs were counted over a glass slide after dripping 30% glycerin over the samples to separate the eggs. Fecundity was estimated by the gravimetric method. Moreover, subsamples of 50 eyed eggs from each ovigerous female were placed on a moistened filter paper in a Petri dish and then egg diameters were measured with a dissecting microscope equipped with a calibrated ocular micrometer. The total number of eggs of each female was expressed (Bagenal, Reference Bagenal1978; Kwei, Reference Kwei1978; Jones et al., Reference Jones, Mc Conaugha, Geer and Prager1990; Prager et al., Reference Prager, Mc Conaugha, Jones and Geer1990) as:
where F represents the number of eggs, X stands for sub-sample weight (g), W 0 denotes the weight of ovary and n represents the number of eggs in the sample. The relationship between F and CW was analysed using the equation:
where, b is the slope of curve, a is a constant, CW is carapace width (cm) and F is fecundity.
Statistical analysis
Pearson's χ2 analysis was performed to test if sex-ratios of monthly collected samples deviate from 1:1. Normality of the data was checked using the Shapiro–Wilk test. Comparisons of female and males in terms of monthly normally and non-normally distributed variables were performed using the independent t and Mann–Whitney U-tests, respectively. In the former analysis, homogeneity of variance was tested using Levene's test. Since distributions of CL, CW, W, egg number and diameter were non-parametric according to the Shapiro–Wilk test, relationships between them were tested using Spearman correlation analysis (Tunca et al., Reference Tunca, Ucuncu, Ozkan, Ulger and Tekinay2013). Population distribution (%), growth rate (%) and egg number against size-classes were modelled using the cubic model, whereas the linear model was chosen to explain the relationship of CW with egg diameter and egg number based on R 2 value (Üçüncü et al., Reference Üçüncü, Tunca, Fikirdeşici and Altındağ2013).
RESULTS AND DISCUSSION
Sex-ratio and size distribution
A total of 1503 specimens were measured, ranging from 0.9 to 4.3 cm in CL over the study period. Out of 1503 specimens sexed, 753 (50.09%) were males and 750 (49.90%) females. Overall, males outnumbered females, but the sex-ratio (females:males) was not significantly different from the equal sex-ratio (χ2 = 0.01, d.f. = 1, P > 0.05). This finding is inconsistent with that (1:1.27) of another study conducted in the Black Sea by Selimoğlu (Reference Selimoğlu1997). On the other hand, somewhat similar sex-ratios of 1:0.9 and 1:1 were reported from Portuguese waters by Flores & Paula (Reference Flores and Paula2002a, Reference Flores and Paulab). The sex-ratios according to CW classes are presented in Table 1.
Table 1. Sex-ratios of male and female Pachygrapsus marmoratus according to size-classes.

NS, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The population showed a different pattern below and above 3.5 cm size. The number of male individuals in the 1–1.5 cm class was more than females, whereas they were almost equal in the 1.5–1.9 cm class. The females outnumbered males in the 2–2.49 cm class and this trend was kept up in the 3.5 cm size. However, from this point (3.5 cm) to the maximum size, the number of males became dominant in the population. These results are consistent with the existing literature. Accordingly, size-ranges of male and female marbled crabs caught from Normandy coasts were reported as 0.9–4.8 and 1.7–3.6 cm (Dauvin, Reference Dauvin2009). Flores & Paula (Reference Flores and Paula2002a) also reported comperable size-ranges of male and femal marbled crab from two different localities in Portugal.
The distribution of crabs in the population suggested that large sized individuals were more likely to be male rather than female (Figure 2). Greater numbers of males and females were seen in the 3.5–3.99 cm and 2.5–2.99 cm size-classes, respectively. Maximum size of males was greater than females. Monthly size distributions by sex are presented in Figure 3.

Fig. 2. Cubic regression modellings for male and female crab population distributions according to size-classes.

Fig. 3. Size–frequency distributions of marbled crab by sexes according to sampling dates.
Monthly distribution of the P. marmoratus population according to sex is given in Figure 4. Ovigerous females were observed during only three months, implying that spawning takes place for a quite short period in P. marmoratus.

Fig. 4. Monthly population distributions of Pachygrapsus marmoratus according to sexes.
The mean values of CL, CW and W for marbled crab were determined as 2.55 ± 0.02 cm, 2.90 ± 0.02 cm and 14.21 ± 0.27 g for all individuals, respectively (Table 2). There were significant differences among male, female and ovigerous P. marmoratus in terms of monthly morphometric measures (P < 0.05).
Table 2. Monthly variations in biometric measurements (CW and CL in cm, W in gm) of male (753), female (659) and ovigerous female (91) Pachygrapsus marmoratus collected from the study area.

Monthly growth variables between November and May, excluding December, were significantly higher for males than females, but this difference disappeared after May. A possible reason for this divergence between sexes could be higher energetic costs of spawning in females as compared with males.
In the present study, minimum, maximum and mean CW values for all samples were 1.1, 4.8 and 2.9 cm, respectively. In another study in the Black Sea, mean CW of marbled crabs was reported as 3.57 cm by Selimoğlu (Reference Selimoğlu1997). This big difference between the studies could be due to the fact that this researcher studied with a sample that was smaller in number with smaller size-classes compared to our investigation. The CW distribution of specimens collected over the study period showed that 77.38% of P. marmoratus were in a range from 2 to 3.99 cm (Figure 5).
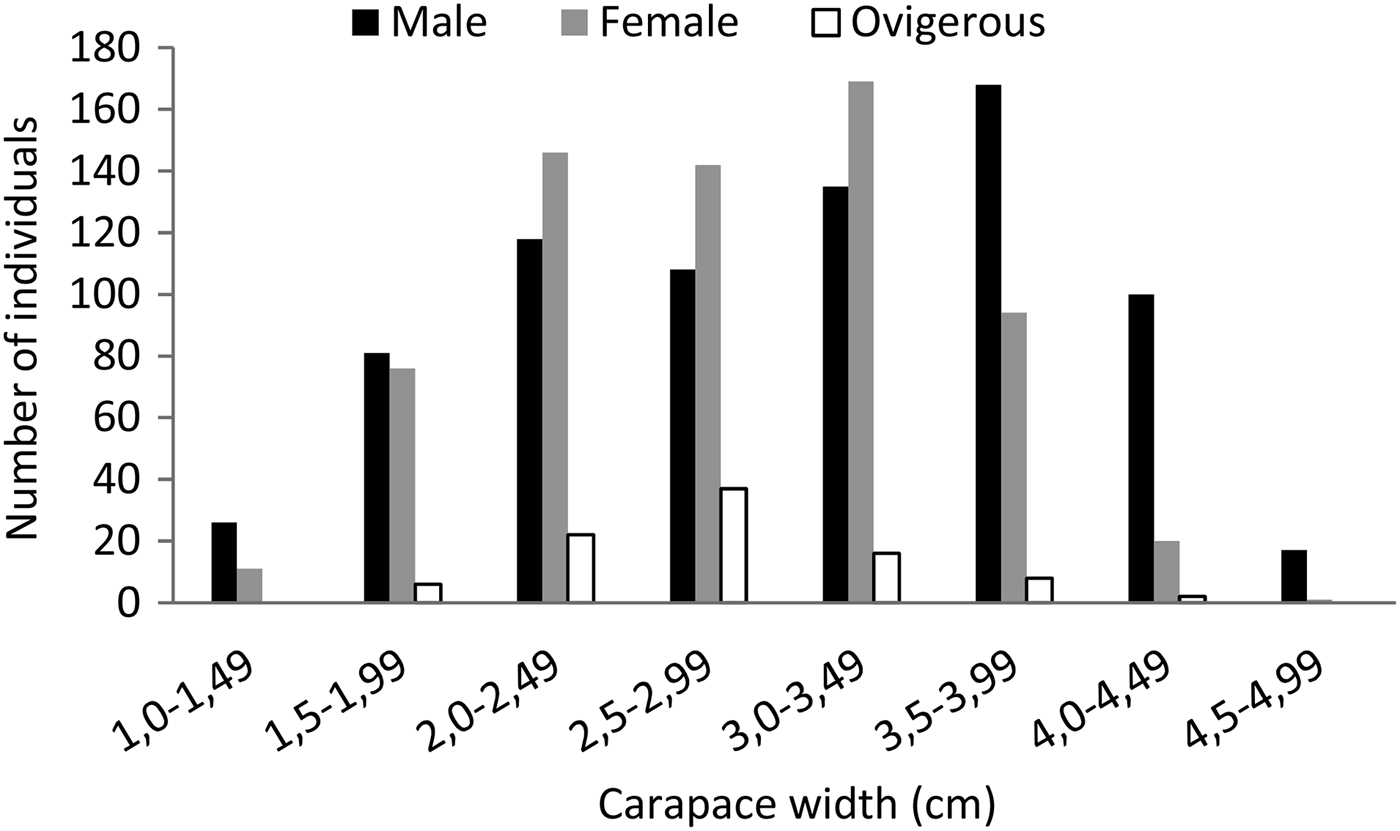
Fig. 5. Carapace width frequency distribution of male (black), female (grey) and ovigerous female (white) Pachygrapsus marmoratus.
In the present study, highest CW and W increments were recorded as 40.16% and 160.40%, respectively, in male crabs (Tables 3 and 4). Expectedly, as far as growth rate by sex is concerned, males showed higher values than females.
Table 3. Mean (±standard error) CL (cm), CW (cm), W (g) and size increment rates for male Pachygrapsus marmoratus from the study area.

Table 4. Mean (±standard error) CL (cm), CW (cm), W(g) and size increment rates for female Pachygrapsus marmoratus from the study area.

The regression models of CW and W growth increments in both sexes are shown in Figure 6. The most remarkable difference between the sexes in terms of the models is that % growth in males started from higher levels and lasted for a longer period compared with females. Moreover, the stage of growth stagnancy in males appeared to occur at higher weights than females, allowing male individuals to grow at a higher rate. These findings also support the population distribution models of males and females. The higher growth range of males could result from the fact that females reach sexual maturity earlier. Indeed, sizes on reaching sexual maturity in female and male marbled crabs were found to be 9.9 and 10.6 mm, respectively (Flores & Paula, Reference Flores and Paula2002b). Another reason for higher growth of males could be differences between the sexes in diet preference, when the findings of Cannicci et al. (Reference Cannicci, Gomei, Boddi and Vannini2002) and Silva et al. (Reference Silva, Boaventura, Flores, Ré and Hawkins2004) are considered. Last but not least, intense competition and fighting among the males could also lead to a pressure for growth.

Fig. 6. Relationships between growth rates (% CW and W) and size-classes in male and female marbled crab.
The parameters of the regressions used in analysis of growth between CL and W, CW and W and CL and CW are given in Table 5. The b values of CW and W of male, female and ovigerous were 3.10, 3.06 and 2.95, respectively. Deviations of b values from 3 were significant for both sexes (χ2 test; P < 0.05). Selimoğlu (Reference Selimoğlu1997) found the relationship between CW and W to be W = 0.0008 CW 2.83 for crabs caught from the Black Sea.
Table 5. Regression parameters of the relationship between W and CW, W and CL and CL and CW.

The mean condition index values were calculated as 49.03 for males and 45.79 for females. The highest monthly condition index value (56.42) was recorded in March for males and (42.09) in May for females (Figure 7). The mean condition indices of males and females were significantly different (χ2 test; P < 0.05).

Fig. 7. Monthly variations in the condition index of male and female Pachygrapsus marmoratus from the Black Sea (*and ○ indicate the values that are placed in out of the distributions).
Reproduction, fecundity and egg diameter
Ovigerous females were observed only from May to July 2013, with the highest proportion in June (0.21%). Spawning began to take place in May when surface water temperature reached 18°C. In agreement with this, a study in Portugal reported that individuals with eggs were found in May and June, and these females started to spawn when temperature reached 17.8°C (Flores & Paula, Reference Flores and Paula2002a).
The GSI values for 91 specimens of P. marmoratus ranged from 2.67 to 17.35%, with a mean value of 12%. Minimum W and CW of females with egg caught during the study were 2.08 g and 1.7 cm.
The fecundity ranged from 8989 to 151,578 eggs/crab, with an average value of 57,814.53 ± 3349 eggs/crab. These values are within the range of existing literature. For instance, Kocataş (Reference Kocataş1971) reported the average fecundity as 30,000 eggs/crab from samples caught in the Aegean Sea. Selimoğlu (Reference Selimoğlu1997) found the average fecundity of crabs from the Black Sea to be 43,700 eggs/crab.
The egg diameter ranged between 328.40 µm and 423.38 µm (mean 365.83 ± 1.97 µm) (Table 6), being in harmony with the reported values. Kocataş (Reference Kocataş1971) and Selimoğlu (Reference Selimoğlu1997) reported the mean egg diameters as 400 and 340 µm, respectively. A similar value (338 µm) was also found for crabs in Portugal by Flores & Paula (Reference Flores and Paula2002a). In a smaller sized crab species (Liocarcinus navigator) in the Black Sea, average egg diameter was found as 347.2 µm (Aydın et al., Reference Aydın, Karadurmuş and Erbay2012). The relationships between fecundity and CW and W are shown in Figure 8.

Fig. 8. (A) Relationships between CW and egg number; (B) relationship between W and egg number.
Table 6. Mean egg number and diameter according to size classes of ovigerous female Pachygrapsus marmoratus from the Southern Black Sea.

The number of eggs increased with size of females, as can be seen in Figure 9. This finding is consistent with those found in various crustaceans, such as Microphrys bicornutus (Carmona-Suarez, Reference Carmona-Suarez2013), Chaceon affinis (Tuset et al., Reference Tuset, Espinosa, García-Mederos, Santana and González2011), Porcellio siculoccidentalis (Montesanto et al., Reference Montesanto, Pizzo, Caruso and Lombardo2012) and Atya scabra (Almeida et al., Reference Almeida, Mossolin and Luz2010).

Fig. 9. Relationship between egg number and size-classes of females.
The highest correlation coefficient was obtained between CW and CL in both sexes (Table 7). There were also strong correlations between W, CW and CL for females and males. Positive correlation coefficients, albeit weak, between egg number and size parameters of females are in line with the regression model between egg number and female size. Conversely, there was no correlation between egg diameter and size parameters.
Table 7. Correlation coefficients among various variables for male and female.

**, correlation is significant at the 0.01 level (2-tailed); *, correlation is significant at the 0.05 level (2-tailed).
CONCLUSIONS
The present results indicate that males have a higher growth rate than females in P. marmoratus. Spawning takes place within a short period. The highest correlation coefficient among the variables obtained from males and females was between CW and CL. Egg number was closely associated with size-classes of marbled crab. The findings will constitute an important database for future studies of P. marmoratus and of other crab species.


















