INTRODUCTION
Despite their ecological and economic importance, coral reefs worldwide are increasingly threatened by anthropogenic activities. Over the past 50 years, coral reefs have suffered from substantial declines in coral cover, habitat complexity and biodiversity (e.g. Bruno & Selig, Reference Bruno and Selig2007; Burke et al., Reference Burke, Reytar, Spalding and Perry2011). In some locations, coral cover has already been reduced by 50% or more since the 1980s (Bruno & Selig, Reference Bruno and Selig2007; De'ath et al., Reference De'ath, Fabricius, Sweatman and Puotinen2012). Declines in spatially competitive scleractinian corals can provide populations of less competitive benthic taxa with an opportunity to expand, and there are increasing reports of substantial reef community shifts (e.g. McManus & Polsenberg, Reference McManus and Polsenberg2004; Norström et al., Reference Norström, Nyström, Lokrantz and Folke2009). While the majority of the literature has focused on shifts from coral to macroalgal-dominated communities, many other groups of benthic taxa can potentially benefit from coral declines (Mumby, Reference Mumby2009; Norström et al., Reference Norström, Nyström, Lokrantz and Folke2009; Cheal et al., Reference Cheal, MacNeil, Cripps, Emslie, Jonker, Schaffelke and Sweatman2010; Bell et al., Reference Bell, Davy, Jones, Taylor and Webster2013). Bioeroding sponges are one such group, and are reportedly becoming more abundant on some degraded reefs in the Caribbean (e.g. Rützler, Reference Rützler2002; Lόpez-Victoria & Zea, Reference López-Victoria and Zea2004), the eastern Pacific (Carballo et al., Reference Carballo, Bautista, Nava, Cruz-Barraza and Chávez2013), and on the Great Barrier Reef (GBR) (Schönberg & Ortiz, Reference Schönberg and Ortiz2009). For example bioeroding sponges occupy up to 46% of sampled substrate on bleached reefs in the Mexican Pacific (Carballo et al., Reference Carballo, Bautista, Nava, Cruz-Barraza and Chávez2013) and increased in abundance by 150% on the Great Barrier Reef (GBR) after two major bleaching events (Schönberg & Ortiz, Reference Schönberg and Ortiz2009). Many bioeroding sponge species appear to be resilient to some of the stressors that are detrimental to corals (reviewed in Schönberg et al., Reference Schönberg, Fang, Carballo, Bell and Carballo2017a, Reference Schönberg, Fang, Carreiro-Silva, Tribollet and Wisshakb), and some species are aggressive spatial competitors (e.g. Lόpez-Victoria & Zea, Reference López-Victoria and Zea2005; Chaves-Fonnegra & Zea, Reference Chaves-Fonnegra, Zea, Custódio, Lôbo-Hajdu, Hajdu and Muricy2007; González-Rivero et al., Reference González-Rivero, Yakob and Mumby2011).
A wide range of organisms contribute to bioerosion (e.g. Glynn, Reference Glynn and Birkeland1997; Wisshak & Tapanila, Reference Wisshak and Tapanila2008; Schönberg et al., Reference Schönberg, Fang, Carreiro-Silva, Tribollet and Wisshak2017b), and infestation levels of different boring taxa can vary depending on environmental conditions (e.g. Tribollet et al., Reference Tribollet, Decherf, Hutchings and Peyrot-Clausade2002; Tribollet & Golubic, Reference Tribollet and Golubic2005). However, sponges regularly generate the majority of internal reef bioerosion and often represent 60–90% of macroborer activity (e.g. Risk et al., Reference Risk, Sammarco and Edinger1995; Mallela & Perry, Reference Mallela and Perry2007). Rates of erosion can be very high, with some species capable of removing over 20 kg of calcareous substrate per m2 sponge surface area a year (Schönberg, Reference Schönberg2002; Calcinai et al., Reference Calcinai, Bavestrello, Cerrano, Gaggero, Wisshak and Tapanila2008). Furthermore, under certain environmental conditions that favour high abundances of bioeroding sponges, and in combination with reduced coral cover/calcification, the balance between calcification and reef erosion could potentially be altered by sponge bioerosion, resulting in net carbonate erosion (e.g. Nava & Carballo, Reference Nava and Carballo2008; Perry et al., Reference Perry, Spencer and Kench2008; Kennedy et al., Reference Kennedy, Perry, Halloran, Fine, Carricart-Ganivet, Iglesias-Prieto, Form, Wisshak, Schönberg and Mumby2013). Moreover, bioerosion has been identified as a principal driver of the loss of reef structural complexity (e.g. Alvarez-Filip et al., Reference Alvarez-Filip, Gill, Dulvy, Perry, Watkinson and Côté2011). This is important, as three-dimensional reef structure provides critical habitat, maintaining densities and biomass of reef organisms, and is central to ecosystem services that support tourism and shoreline protection (e.g. Enochs & Manzello, Reference Enochs and Manzello2012; Graham & Nash, Reference Graham and Nash2012).
Given the potentially negative consequences of increases in bioeroding sponge abundances on reefs, it is important to understand the factors that influence their distributions and assemblage composition. As bioeroding sponges are dependent on calcareous substrates, the availability of suitable substrate has been identified as a key factor influencing their abundance (Chaves-Fonnegra et al., Reference Chaves-Fonnegra, Zea and Gómez2007; Carballo et al., Reference Carballo, Bautista-Guerrero and Leyte-Morales2008; Schönberg & Ortiz, Reference Schönberg and Ortiz2009; Schönberg, Reference Schönberg2015a). Other important environmental and abiotic factors that have been previously shown to influence bioeroding sponge abundance include eutrophication (e.g. Holmes et al., Reference Holmes, Edinger, Limmon and Risk2000; Chaves-Fonnegra et al., Reference Chaves-Fonnegra, Zea and Gómez2007; Nava et al., Reference Nava, Ramírez-Herrera, Figueroa-Camacho and Villegas-Sanchez2014), light availability (e.g. Lόpez-Victoria & Zea, Reference López-Victoria and Zea2005), and sedimentation and turbidity (e.g. Muricy, Reference Muricy1991; Nava & Carballo, Reference Nava and Carballo2013; see summary in Schönberg, Reference Schönberg, Wisshak and Tapanila2008). Many of these environmental parameters can also be associated with reef degradation. Previous studies have shown that eutrophication and high chlorophyll concentrations are associated with low coral recruitment and species richness, and promote macroalgal abundance (e.g. De'ath & Fabricius, Reference De'ath and Fabricius2010; Fabricius, Reference Fabricius, Dubinsky and Stambler2011). In addition to eutrophication, reefs that are subject to excessive terrestrial run-off are typically characterized by highly turbid water with high sedimentation rates. High turbidity reduces light availability for photosymbiotic hard corals and has been associated with reduced coral growth rates (Crabbe & Smith, Reference Crabbe and Smith2005), reduced coral diversity (e.g. De'ath & Fabricius, Reference De'ath and Fabricius2010) and higher disease prevalence (Pollock et al., Reference Pollock, Lamb, Field, Heron, Schaffelke, Shedrawi, Bourne and Willis2014). Sedimentation can further stress and kill corals through either smothering or complete burial and has been associated with large declines in coral cover and diversity at impacted sites (see Fabricius, Reference Fabricius2005; Erftemeijer et al., Reference Erftemeijer, Riegl, Hoeksema and Todd2012 for reviews).
In this study we hypothesized that coral mortality, and subsequent increases in the availability of dead calcareous substrate, promotes bioeroding sponge abundance. Furthermore, we hypothesized that bioeroding sponges are resilient to the environmental stressors (specifically eutrophication, turbidity and sedimentation) that can contribute towards coral mortality. To test this hypothesis we investigated the influence of different biotic and abiotic factors on the abundance and assemblage composition of bioeroding sponges on Indonesian reefs. Recognizing related patterns will provide an increased understanding of how reef degradation may affect bioeroding sponge abundances and how future reefs might function if degradation continues.
MATERIALS AND METHODS
Study area
This study was conducted within the Wakatobi Marine National Park (Wakatobi) in south-east Sulawesi, Indonesia. The Wakatobi supports a high diversity of marine species and was gazetted as a Biosphere Reserve by UNESCO in 2012. The region is also home to over 90,000 people who depend on the local reefs for food and other resources (Cullen et al., Reference Cullen, Pretty, Smith and Pilgrim2007).
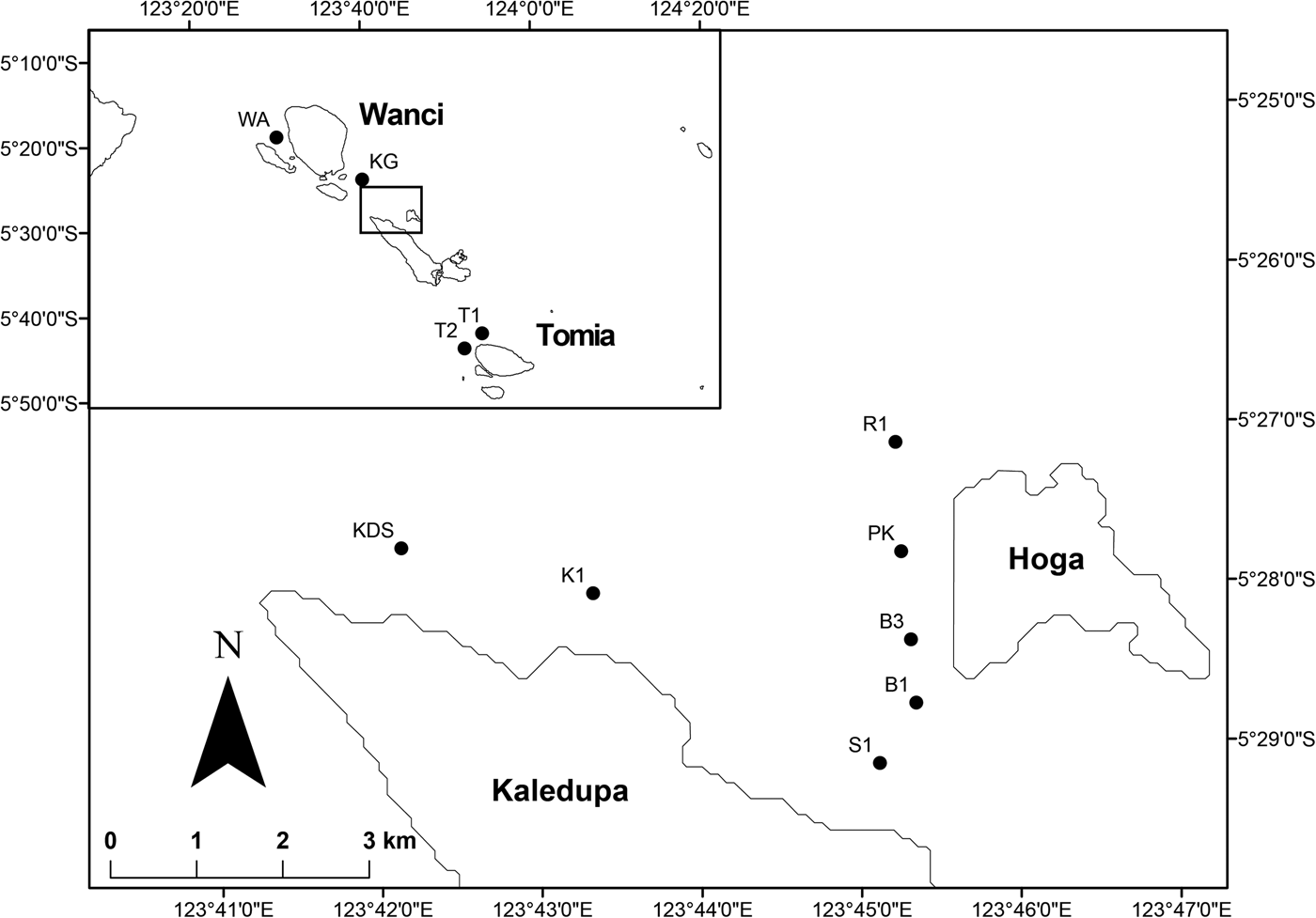
Surveys were conducted at seven sites on the fringing reefs around the islands of Hoga and Kaledupa in March–April 2014, and then continued in June 2014 to include another four sites around the islands of Wanci and Tomia (Figure 1). Survey sites were chosen to represent a variety of reef types, environmental conditions and levels of reef degradation. The initial seven ‘core’ sites included three steep-wall reefs (>70° inclination): Buoy 1 and 3, and Ridge 1; three sloping reefs (50–70°): Kaledupa 1, Kaledupa Double Spur and Pak Kasims; and Sampela 1, a more gently sloping reef adjacent to a stilted Bajo village, which is considered to be highly degraded, sedimented and turbid (Bell & Smith, Reference Bell and Smith2004; McMellor & Smith, Reference McMellor, Smith, Clifton, Unsworth and Smith2010; Powell et al., Reference Powell, Smith, Hepburn, Jones, Berman, Jompa and Bell2014). The additional four sites were again gently sloping reefs: Karang Gurita, a coral atoll located off the island of Wanci; Tomia 1 and 2 off the island coast of Tomia, which appear relatively healthy with high cover of branching corals; and Wanci Harbour, a turbid and sedimented reef within Wanci Harbour.

Fig. 1. Site map of the survey region in the Wakatobi (top left) and the core survey sites around the islands of Hoga and Kaledupa. Sites Buoy 1 and 3, Kaledupa 1 and Kaledupa Double Spur, Karang Gurita, Pak Kasim, Ridge 1, Sampela 1, Tomia 1 and 2, and Wanci Harbour are abbreviated as B1, B3, K1, KDS, KG, PK, R1, S1, T1, T2 and WA, respectively.
Sponge species identification
Initial surveys were conducted across the seven core sites between depths of 3–20 m, to identify the locally dominant bioeroding sponge species. Bioeroding sponges are difficult to identify in situ, and new studies in previously un-surveyed regions have often discovered new species (e.g. Calcinai et al., Reference Calcinai, Bavestrello and Cerrano2005). Sponges encountered during these surveys were initially identified as different operational taxonomic units (OTUs) based on recognizable external morphology and erosion chamber macro-characteristics. To create a functional context, pulse amplitude modulated fluorometry (DIVING-PAM with red excitation light, Walz, Iffeltrich) was used to detect photoactivity of in situ light-adapted sponges by measuring fluorescence yield; subsequent tissue examination then identified the presence of symbiotic Symbiodinium. Eight different OTUs were designated in this manner and three ethanol-preserved representative samples of each were transported back to the Victoria University of Wellington for species confirmation using spicule analysis. Full taxonomic results will be published elsewhere.
Bioeroding sponge abundance and benthic composition
Bioeroding sponge abundances and substrate characteristics were assessed in situ using line intercept transects. Surveys were conducted at 5 and 10 m depths for each of the initial seven ‘core’ sites in March–April 2014. Due to logistical constraints, surveys were conducted at just 10 m depth for the four additional sites surveyed in June 2014. At each site and depth, six 10 m transects were haphazardly deployed running parallel to the reef contours with a minimum separation of 10 m between replicate surveys. Sponge abundances and substrate composition were recorded by visually inspecting ten 1 m subsamples on each transect; along each transect subsample, the total linear distance of each sponge species and substrate type that intercepted the measuring tape were recorded (after English et al., Reference English, Wilkinson and Baker1994; Schönberg, Reference Schönberg2015a). Schönberg (Reference Schönberg2015a) found linear distance to be proportional to areal extent, therefore our linear distributions were treated as a proxy for percentage benthic cover. Substrate types were listed as ‘live coral’, ‘live other’, ‘‘dead substrate’ and ‘sand’. The dead exposed substrate category included recently dead coral, coral rubble or calcareous platform that was un-colonized by other taxa, except for short filamentous algal turf.
Environmental variables
Environmental conditions at each site were characterized in terms of chl-a concentration, turbidity, sedimentation and water flow (for full details of methods see Marlow et al., Reference Marlow, Smith, Werorilang and Bellin press). Average chl-a concentration and turbidity levels for the seven core sites were determined from multiple deployments of a XR-420 multi-channel data logger in 2010, 2014 and 2015. Due to time constraints, no chl-a or turbidity data were collected from the four additional sites around Tomia and Wanci. Sediment deposition and retention were indirectly evaluated in March and June 2014 by measuring depth of ‘settled sediment’ along 30 m transects at each site and depth (following Bell & Turner, Reference Bell and Turner2000). Mean proportional current velocity for the seven core sites was quantified in June–August 2014 using multiple 24 h deployments of plaster of Paris casts, commonly referred to as ‘clods’ (Doty, Reference Doty1971; Jokiel & Morrissey, Reference Jokiel and Morrissey1993).
Statistical analysis
All univariate statistical analyses were performed in SPSS (version 21; IBM) and multivariate analysis using the PRIMER-E v6 software package with the permutational multivariate analysis of variance (PERMANOVA) add on (Clarke & Gorley, Reference Clarke and Gorley2006).
The average abundance of each bioeroding sponge species across each transect was calculated from the linear extent of each of the ten 1 m subsamples and used as a proportional indicator for percentage cover (see Schönberg, Reference Schönberg2015a). Previous studies have consistently found that bioeroding sponge abundance correlates with the availability of dead calcareous substrate (Chaves-Fonnegra et al., Reference Chaves-Fonnegra, Zea and Gómez2007; Carballo et al., Reference Carballo, Bautista-Guerrero and Leyte-Morales2008; Schönberg & Ortiz, Reference Schönberg and Ortiz2009). Therefore analysis of environmental drivers of bioeroding sponge abundance should use abundance data that are standardized to the local availability of dead substrate (Schönberg, Reference Schönberg2015a). After finding similar correlations in the Wakatobi (see below), total bioeroding sponge abundance and individual species abundances were standardized to available dead substrate (including that which the sponges already occupy).
The lack of chl-a, turbidity or water movement data from the four additional sites meant that the analysis of interactions between environmental variables and boring sponges only incorporated data from the core sites. Due to the considerable differences in environmental conditions between Sampela 1 and the other sites, and the tendency for this site to drive collinearity amongst variables, the analysis of the core sites was conducted twice, once including Sampela 1 and once without.
BIOERODING SPONGE ABUNDANCE AND DIVERSITY
Differences in total bioeroding sponge abundance and species diversity (Shannon's index; H’) between sites and depths were tested using a two factorial general linear model (GLM). Where significant differences were found Tukey's or Gabriel's (in the case of unequal sample sizes) post hoc procedures were used to identify where the differences occurred. The influence of individual environmental/benthic factors on bioeroding sponge abundance and diversity were assessed with Pearson's product-moment correlation coefficients. To determine whether these same factors constituted stressors for corals, environmental/benthic data was also correlated against coral cover using Pearson's product-moment correlation coefficients. Data were square-root or log transformed to meet the necessary assumptions of equal variance and normality for both tests. For any data that failed to meet these assumptions despite transformation, non-parametric tests were used (a Kruskal–Wallis or Spearman's Rank test).
ENVIRONMENTAL CHARACTERIZATION
Environmental characterization of each site was achieved using a multivariate analysis within PRIMER. All analyses were based on resemblance matrices calculated from normalized data using Euclidean-Distance similarity coefficients. Draftman's plots of all pairwise correlations were checked for collinearity and skewness in the abiotic and biotic data; these suggested that fourth-root transformation was appropriate for an increase in normality for all the variables. Substrate and environmental differences between sites and depths were examined using one and two-factor PERMANOVAs (site and depth as factors with 7 levels or site as factor with 11 levels), and results were graphically displayed through principal coordinate analysis (PCO). The inter-site differences in abiotic and biotic variables were characterized with Pearson's correlations (>0.4) between PCO axes and the individual abiotic/biotic components.
BIOERODING SPONGE ASSEMBLAGE PATTERNS
All biological assemblage data analyses were based on resemblance matrices using Bray–Curtis similarity coefficients calculated from square-root transformed data. Due to the general sparseness or patchiness of bioeroding sponge abundances and the complete absence on one transect, a dummy species with the abundance value of 0.5 was added to each transect in order to diminish the erratic behaviour of the Bray–Curtis coefficient (Clarke et al., Reference Clarke, Somerfield and Chapman2006). To identify those species that were contributing towards any assemblage dissimilarity between sites or depths, a SIMPER analysis was performed. Associations between bioeroding sponge assemblage structure and abiotic/biotic variables were investigated in a distance-based multiple linear regression model (DISTLM), which is a non-parametric permutation-based procedure that enables significance testing of explanatory variables against multivariate response variables (Anderson et al., Reference Anderson, Gorley and Clarke2008). In order to find the most parsimonious model, Akaike's Information Criterion (AIC), a step-wise procedure, adjusted for small sample sizes (AICc), was used (Burnham & Anderson, Reference Burnham and Anderson2004). As with univariate correlations, the DISTLMs were repeated twice, once with the outlier site Sampela 1 and once without. DISTLM outputs were represented graphically with a distance-based redundancy ordination (dbRDA), including a 2D scatterplot to identify differences between sites and 2D bubble plots to identify differences in species abundances. The influence of the predictor variables selected by the DISTLM on the abundance of individual species that were contributing the greatest difference between sites were tested with Spearman's Rank correlations.
RESULTS
Bioeroding sponges
TAXA PRESENT
The Wakatobi bioeroding sponge fauna consisted of eight distinct species. Six of these sponges were characterized at the species level: Cliona cf. schmidtii (Ridley, 1881), Cliona orientalis Thiele, 1900, Cliothosa hancocki (Topsent, 1888), Cliothosa cf. aurivillii (Lindgren, Reference Lindgren1897), Spheciospongia cf. vagabunda (Ridley, 1884) and Zyzzya criceta Schönberg, Reference Schönberg2000. Two species were allocated to the Cliona viridis (Schmidt, 1862) species complex, but were not identified further (Cliona aff. viridis sp. A & B). The abbreviations cf. and aff. are used to indicate that a species is either similar and possibly conspecific (cf.) or similar but likely different (aff.) to a previously described species. Three species, C. orientalis and C. aff. viridis sp. A and B, were hosts to photosynthetic dinoflagellates (Symbiodinium spp.).
ABUNDANCE, DISTRIBUTION AND DIVERSITY
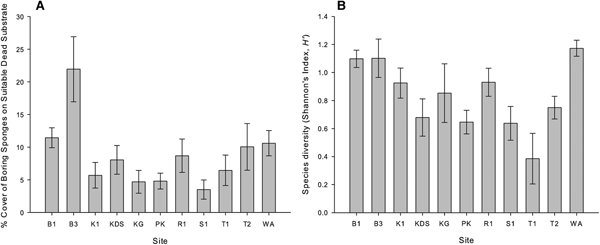
Bioeroding sponges occupied an average of 3.1% (±0.21 SE) of the total reef area, and total abundance varied significantly among sites (GLM, F (10,108) = 5.721, P < 0.001), but not between the depths of 5 and 10 m (GLM, F (1,108) = 0.242, P = 0.624). Abundance was highest at Wanci Harbour (4.9% of total reef area ± 0.63 SE) and lowest at Tomia 1 (1.3% of total reef area ±0.31 SE).
After standardizing sponge abundance to dead substrate availability (standardized bioeroding sponge abundance herein referred to as % DS), bioeroding sponges were found to occupy an average of 8.9% DS (±0.7 SE) across all sites. There was a significant difference between sites (GLM, F (10,108) = 17.366, P < 0.001) with up to 21.9% DS (±2.7 SE) at Buoy 3 and as low as 3.5% DS (±0.8 SE) at Sampela 1 (Figure 2A). There was also a significant difference between depths (GLM, F (10,108) = 10.445, P = 0.002) with 9.7% DS (±0.5 SE) at 10 m depth and 7.7% DS (±0.6 SE) at 5 m depth.
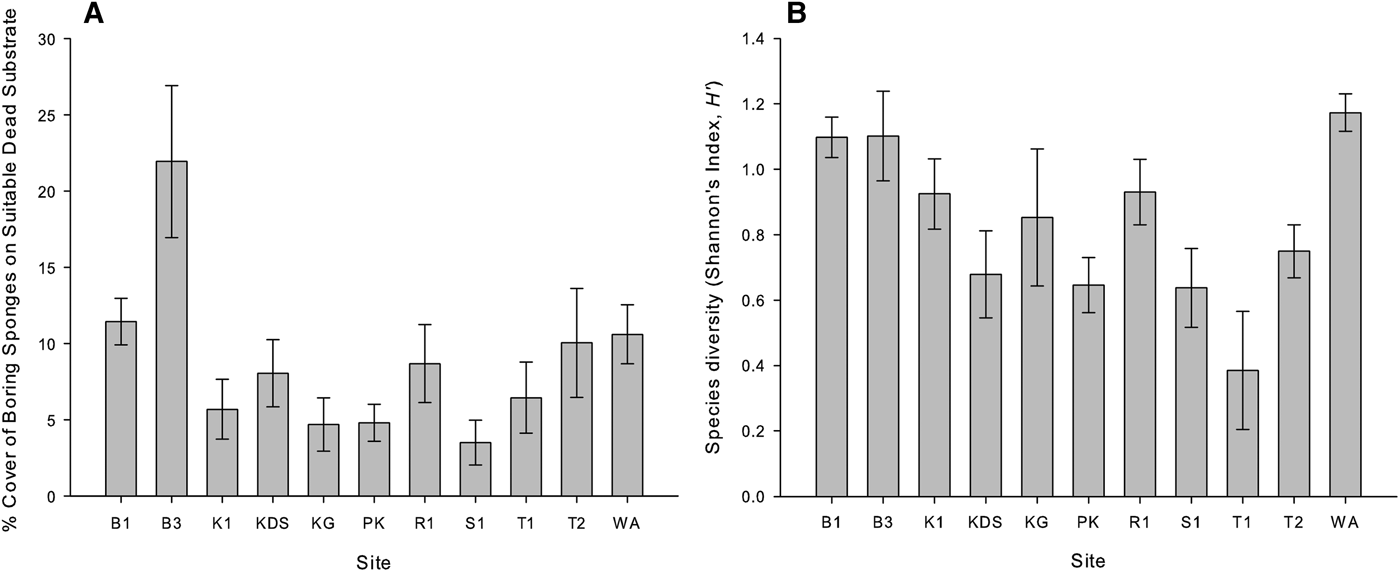
Fig. 2. Bioeroding sponge distribution patterns per sample site and across all depths. (A) Total sponge abundance (standardized to substrate). (B) Species diversity. Bar height represents mean ± SE. See Figure 1 for site abbreviations.
Species diversity of the bioeroding sponges differed significantly among the sites, but not between depths (Kruskal–Wallis, P = 0.003 and P = 0.986, respectively). Species diversity was highest at Wanci Harbour and Buoys 1 & 3 (H’ = 1.173, 1.098 and 1.102, respectively), and lowest at Karang Gurita and Tomia 1 (H’ = 0.380 and 0.385, respectively; Figure 2B).
ASSEMBLAGE COMPOSITION
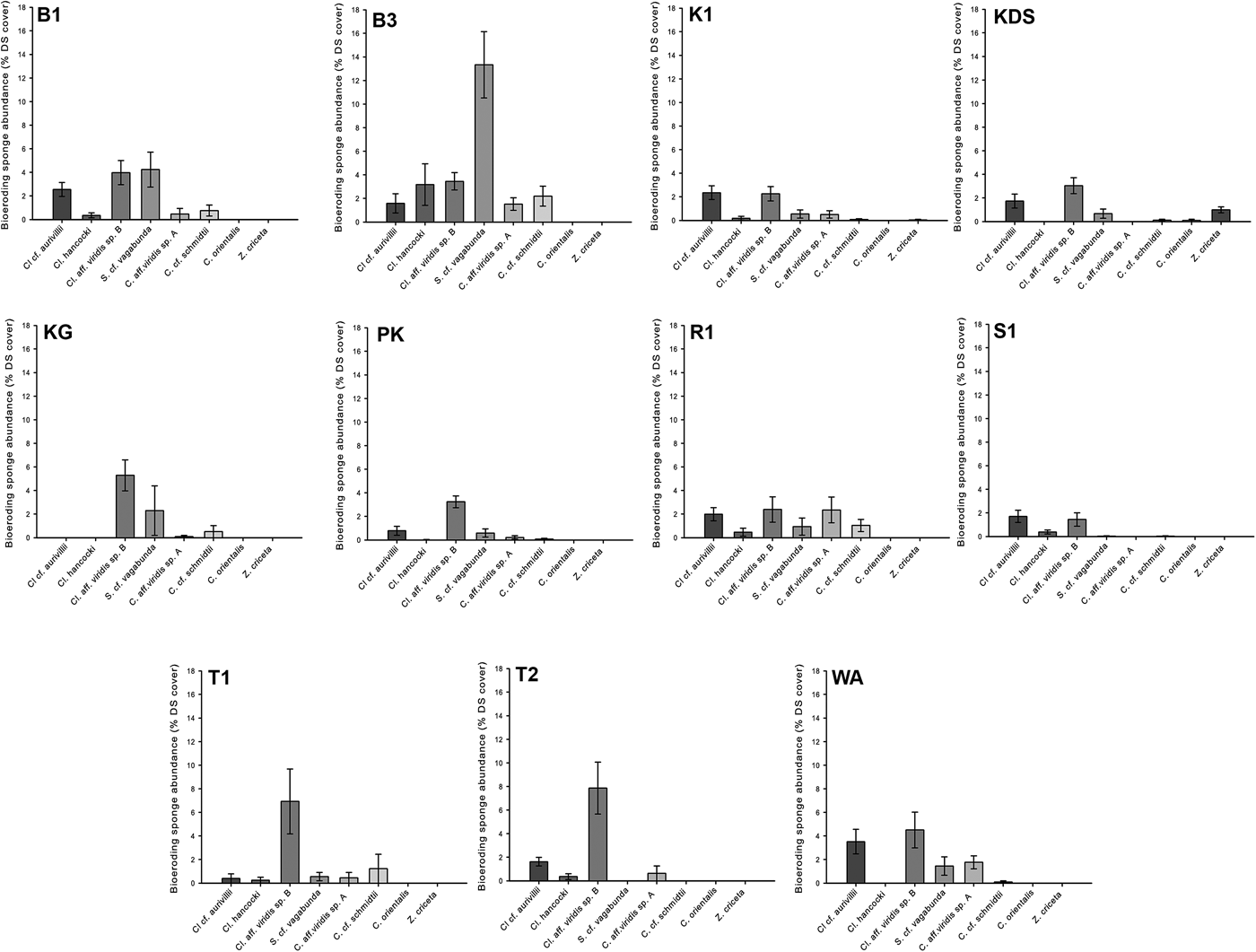
Although the field surveys recognized eight bioeroding sponge species within the Wakatobi assemblage, 81.8% of the abundance of the sponges was represented by only three species; C. aff. viridis sp. B (39.1%), Cl. cf. aurivillii (23.2%) and S. cf. vagabunda (19.5%). Sponge assemblage composition was significantly different among sites (PERMANOVA, Pseudo-F = 5.1843, P = 0.001; Figure 3), and between 5 and 10 m depths (PERMANOVA, Pseudo-F = 6.3, P = 0.001). Given this influence of depth on assemblage composition, further multivariate analysis was restricted to the seven core sites, which included survey data from both depth categories.

Fig. 3. Bioeroding sponge species abundance (standardized to substrate availability) at each survey site. Bar height represents mean ± SE. See Figure 1 for site abbreviations.
The three largest inter-site differences in assemblage composition occurred between Buoy 3 vs Sampela 1, Kaledupa Double Spur and Kaledupa 1 (80.0, 70.1 and 68.6%, respectively), and were largely driven by differences in the abundance of S. cf. vagabunda. This species was relatively abundant at Buoy 3 (average abundance of 13.3% DS and within-site assemblage similarity contribution of 58.0%), but had a low abundance at Sampela 1 (<0.1% DS), Kaledupa Double Spur (0.7% DS) and Kaledupa 1 (0.5% DS), contributing 40.7, 35.3 and 37.8% towards inter-site assemblage dissimilarity, respectively.
The majority (68.1%) of the 52.6% difference in species composition between depths was attributed to depth-related variation in the abundance of three most common species, C. aff. viridis sp. B, Cl. cf. aurivillii and S. cf. vagabunda. Variation in Cl. cf. aurivillii abundance contributed 25.7% of the assemblage difference; average abundance (2.6% DS) was higher at 5 m than at 10 m (1.1% DS). The reverse was true for S. cf. vagabunda; average abundance at 5 m (1.5% DS) was almost threefold lower than at 10 m (4.3% DS), accounting for 19.1% of the variation in assemblage composition between depths. Variation in the abundance of C. aff. viridis sp. B accounted for 23.4% of variation, and although average abundance was similar at 5 and 10 m depths (2.6 and 3.1% DS, respectively), there was high within-group variation.
Environment
SUBSTRATE
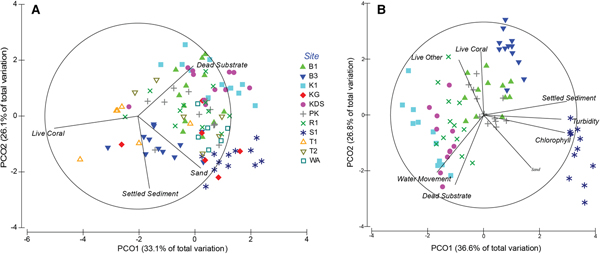
Substrate composition did not differ significantly with depth (PERMANOVA, Pseudo-F = 1.7118, P = 0.143) but differed significantly among the sites (PERMANOVA, Pseudo-F = 9.1056, P = 0.001; Table 1), with 59.2% of the variation being explained by the principal coordinate analysis (PCO; Figure 4A). Tomia 1 and Buoy 3 were characterized by hard corals; Kaledupa Double Spur and Kaledupa 1 by dead substrate; Karang Gurita, Sampela 1 and Wanci Harbour by sand; and the other four sites were more heterogeneous in their substrate composition (Figure 4A; Table 1).
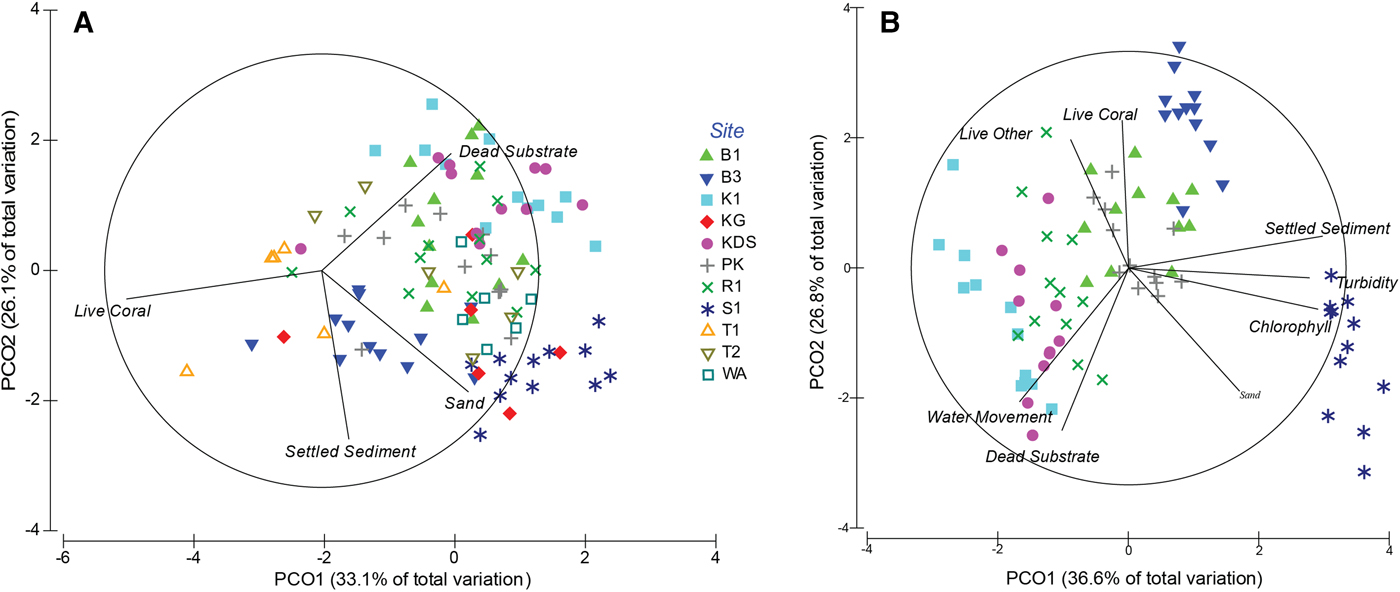
Fig. 4. Two dimensional representation of site similarities with respect to their multivariate physical and water quality parameters (principal coordinate analysis, PCO). (A) Comparison of all 11 study sites with regards to substrate composition. (B) Comparison between the seven core study sites including substrate composition and selected environmental factors as chosen for this study. Overlaid vectors represent components that have a Pearson's correlation of greater than 0.4 with either of the PCO axes. See Figure 1 for site abbreviations.
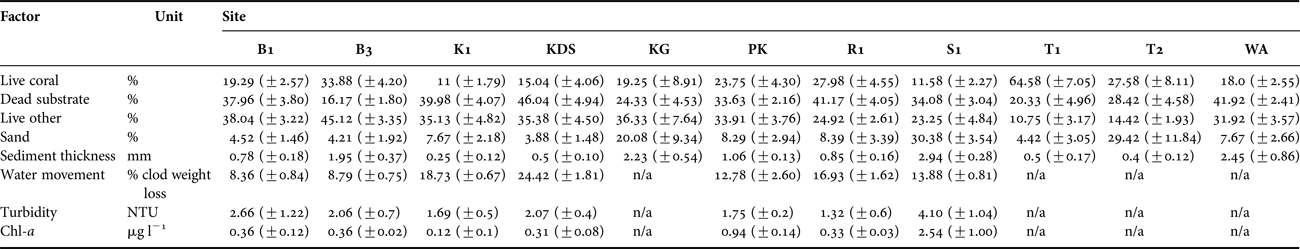
Table 1. Summary table of substrate and environmental characteristics of each site. Standard errors shown. See Figure 1 for site abbreviations. Percentages refer to proportional linear extent along intercept transects.
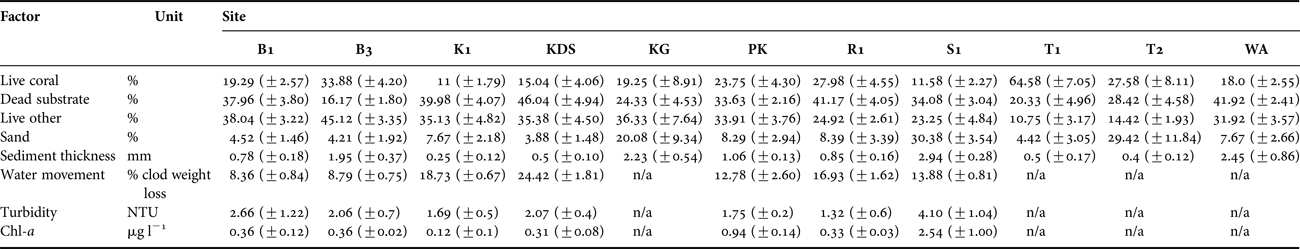
CHL-A, TURBIDITY, SETTLED SEDIMENT AND WATER FLOW
Environmental conditions (chl-a, turbidity, settled sediment, water flow) varied significantly among the core sites (PERMANOVA, Pseudo-F = 26.572, P = 0.001), but not between depths. The two axes of the PCO together explained 63.4% of the variation, further characterizing the core sites by demonstrating: elevated chl-a concentration, settled sediment and turbidity at Sampela 1; low flow rates at Buoys 1 and 3; high flow rates at Kaledupa 1, Kaledupa Double Spur and Ridge 1; and comparatively heterogeneous environmental conditions at Pak Kasim (Figure 4B; Table 1).
How did the environment affect bioeroding sponges?
SPONGE ABUNDANCE AND DIVERSITY
Overall, total bioeroding sponge abundance was positively correlated with availability of dead calcareous substrate (Pearson's, r = 0.345, P < 0.001), negatively correlated with the abundance of sandy substrate (Pearson's, r = −0.199, P < 0.039), and showed no significant correlation with either live coral or other live cover (Table 2).
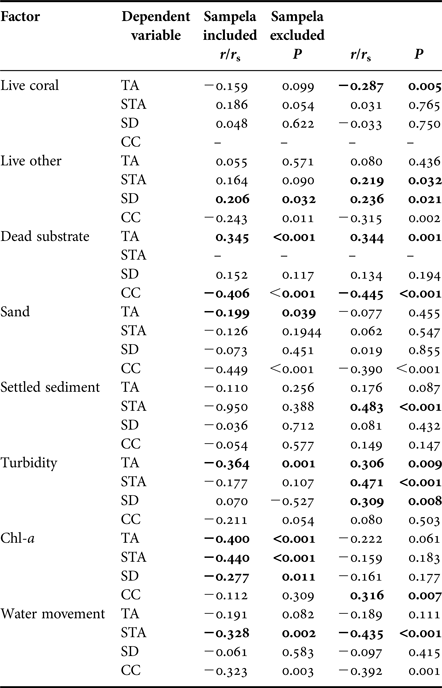
Table 2. Results of bivariate correlations comparing total sponge abundance (TA), substrate standardized abundance (STA), bioeroding sponge species diversity (SD) and coral cover (CC) with substrate characteristics and environmental factors. Abundance correlations using Pearson's and species diversity correlations using Spearman's Rank.
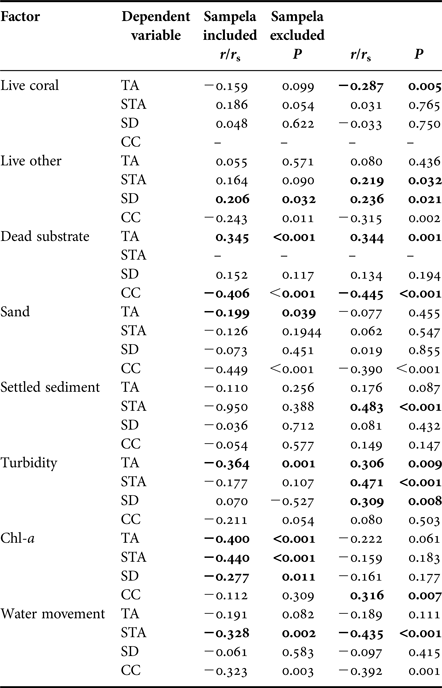
Significant correlations in bold.
Abundance (% DS) decreased on reefs with higher water movement and on those with high chl-a concentrations (Table 2). Omitting Sampela 1, data still showed that within the remaining core sites water movement correlated negatively with % DS, but greater depth of settled sediment and turbidity were positively correlated with % DS and chl-a concentration had no influence (Table 2). The abundance of different substrate types (with the exception of dead substrate) also showed no significant correlation with % DS of these sponges (Table 2).
Sponge species diversity increased with increasing proportional contribution of ‘other live’ benthos, regardless of whether or not Sampela 1 was included in the analysis. However, a significant negative correlation with chl-a concentration only occurred when Sampela 1 was included in the analysis, and a significant negative correlation with turbidity only when Sampela 1 was omitted (Table 2).
ASSEMBLAGE COMPOSITION
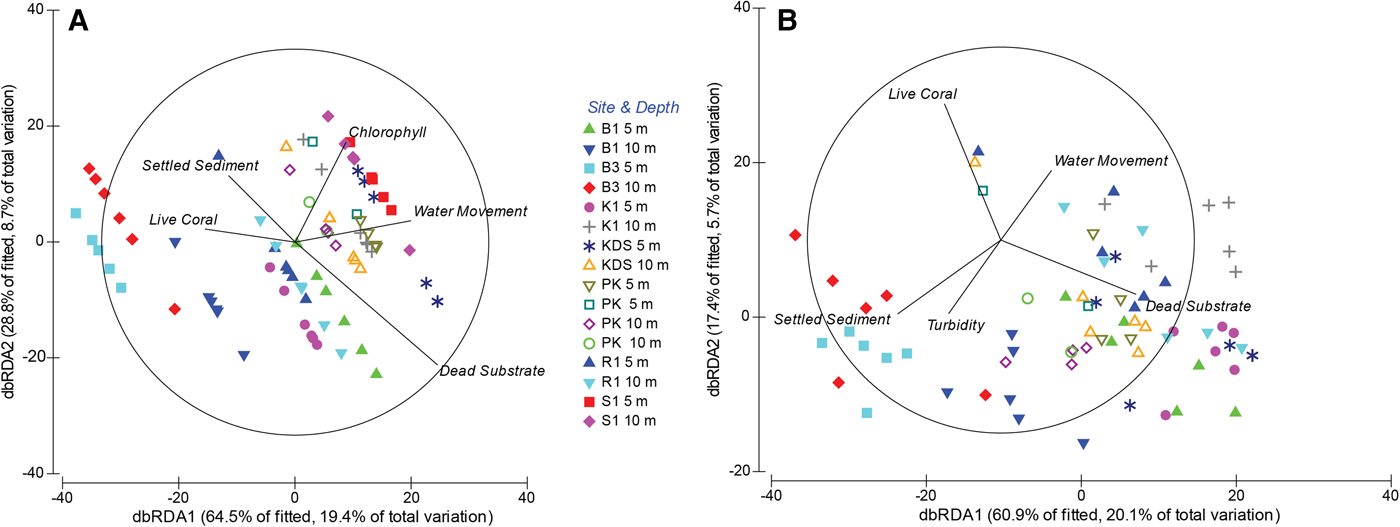
The best sponge assemblage model in DISTLM explained 30.1% of variation in assemblage structure based on non-substrate-standardized abundance: dead substrate (14.0%), chl-a concentration (6.4%), water movement (4.9%) and our proxy for sedimentation, thickness of settled sediment (4.9%) (Figure 5A). Removal of the Sampela 1 data increased the total explanation value slightly to 33.0%. The explanatory power of dead substrate (16.1%), water movement (5.2%) and settled sediment (4.0%) changed little, there was no explanatory value for chl-a concentration, and changes in turbidity (4.2%) and live coral cover (3.5%) were found to have only slight influence (Figure 5B).
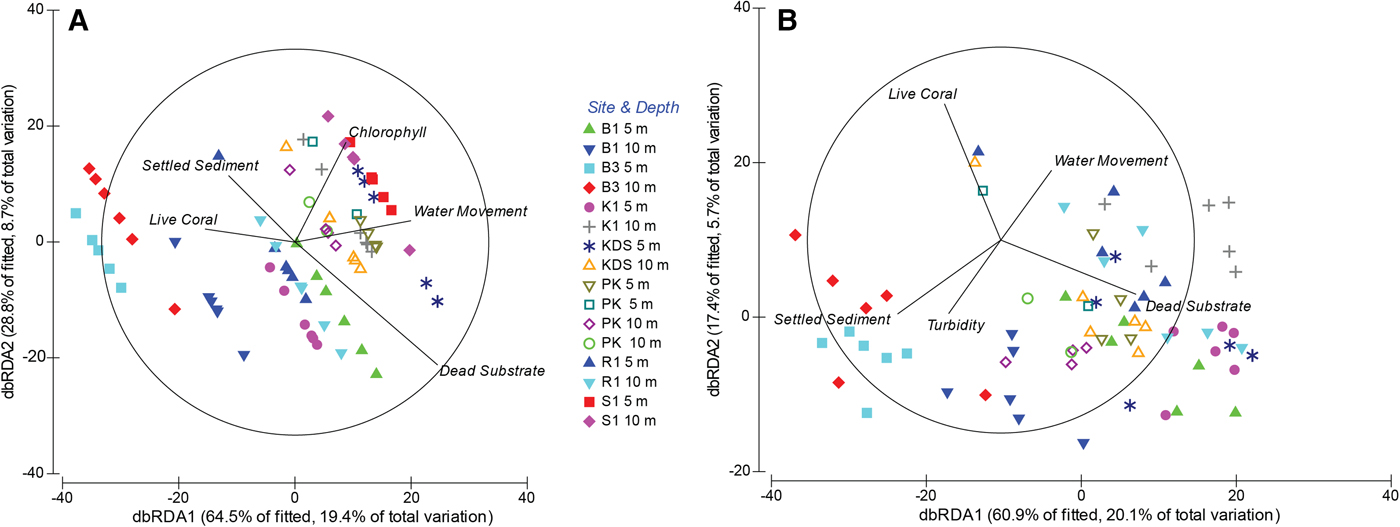
Fig. 5. Distance based redundancy analysis (dbRDA) ordinations of fitted models for assemblage composition. (A) Seven core sites and both depth categories. (B) Core sites and both depth categories with the omission of Sampela 1. See Figure 1 for site abbreviations.
When abundance was standardized to dead substrate availability, 23.9% of assemblage structure variation was explained by environmental variables including water movement (9.3%), settled sediment (8.2%) and chl-a concentration (6.4%). The removal of Sampela 1 from this analysis explained a similar amount of variation (24.4%), but the explanatory value of settled sediment was greater (14.8%), water movement became less important (4.4%), chl-a concentration no longer had an effect, and turbidity was found to be more important (5.2%).
INDIVIDUAL SPECIES
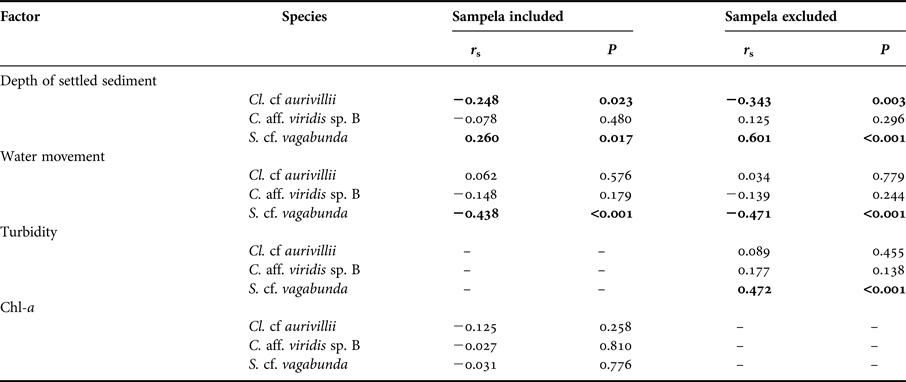
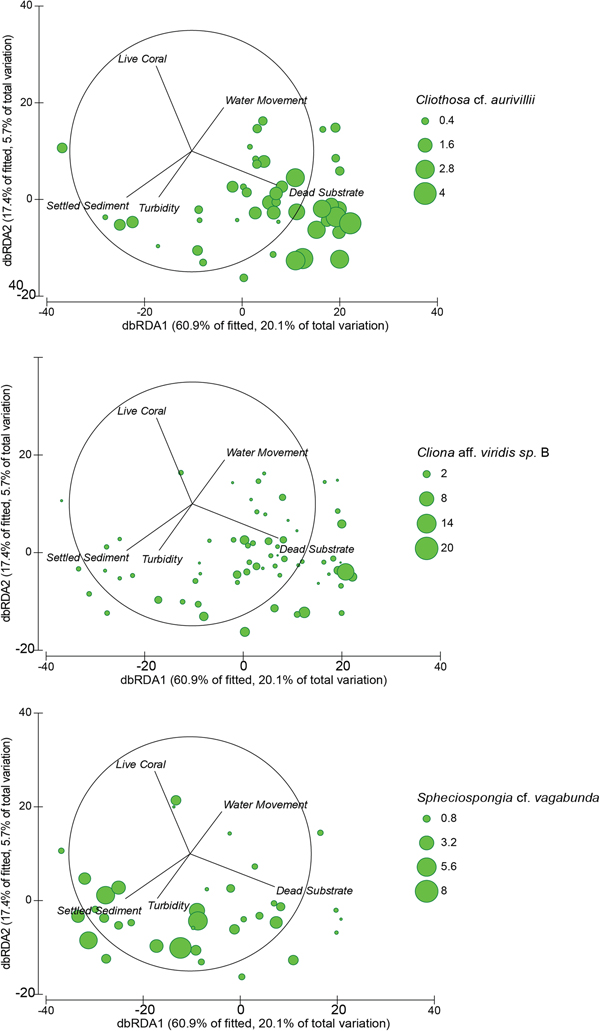
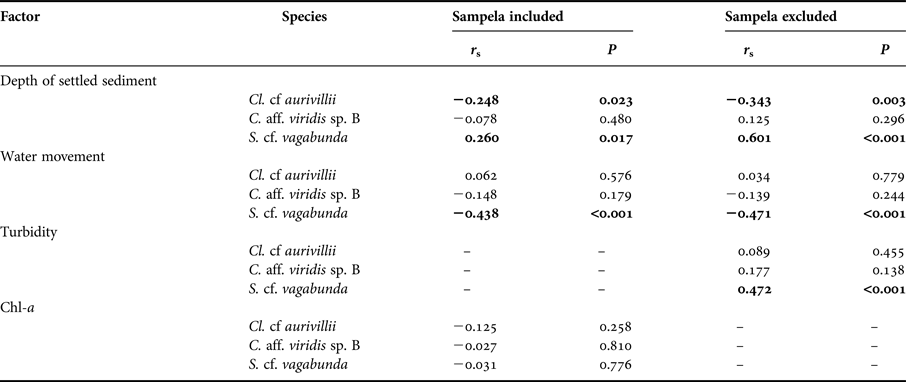
The abundance of dead substrate correlated positively with two of the three dominant boring sponge species: C. aff. viridis sp. B and Cl. cf. aurivillii (Spearman's Rank, r s = 0.245, P = 0.011 and r s = 0.552, P < 0.001, respectively; Figure 6). However, the abundance of S. cf. vagabunda had an inverse relationship with dead substrate (Spearman's Rank, r s = −0.299, P = 0.002) (Figure 6). When the abundance of these species was standardized to dead substrate availability their individual relationships with the explanatory variables identified by the DISTLM were mixed. None of the variables correlated with C. aff. viridis sp. B % DS, Cl. cf. aurivillii % DS correlated negatively with the depth of settled sediment, and S. cf. vagabunda % DS correlated positively with the depth of settled sediment but negatively with water movement (Appendix). Omitting Sampela 1 changed these relationships very little, but revealed a stronger relationship between S. cf. vagabunda % DS and settled sediment, as well as a positive correlation with turbidity (Appendix).
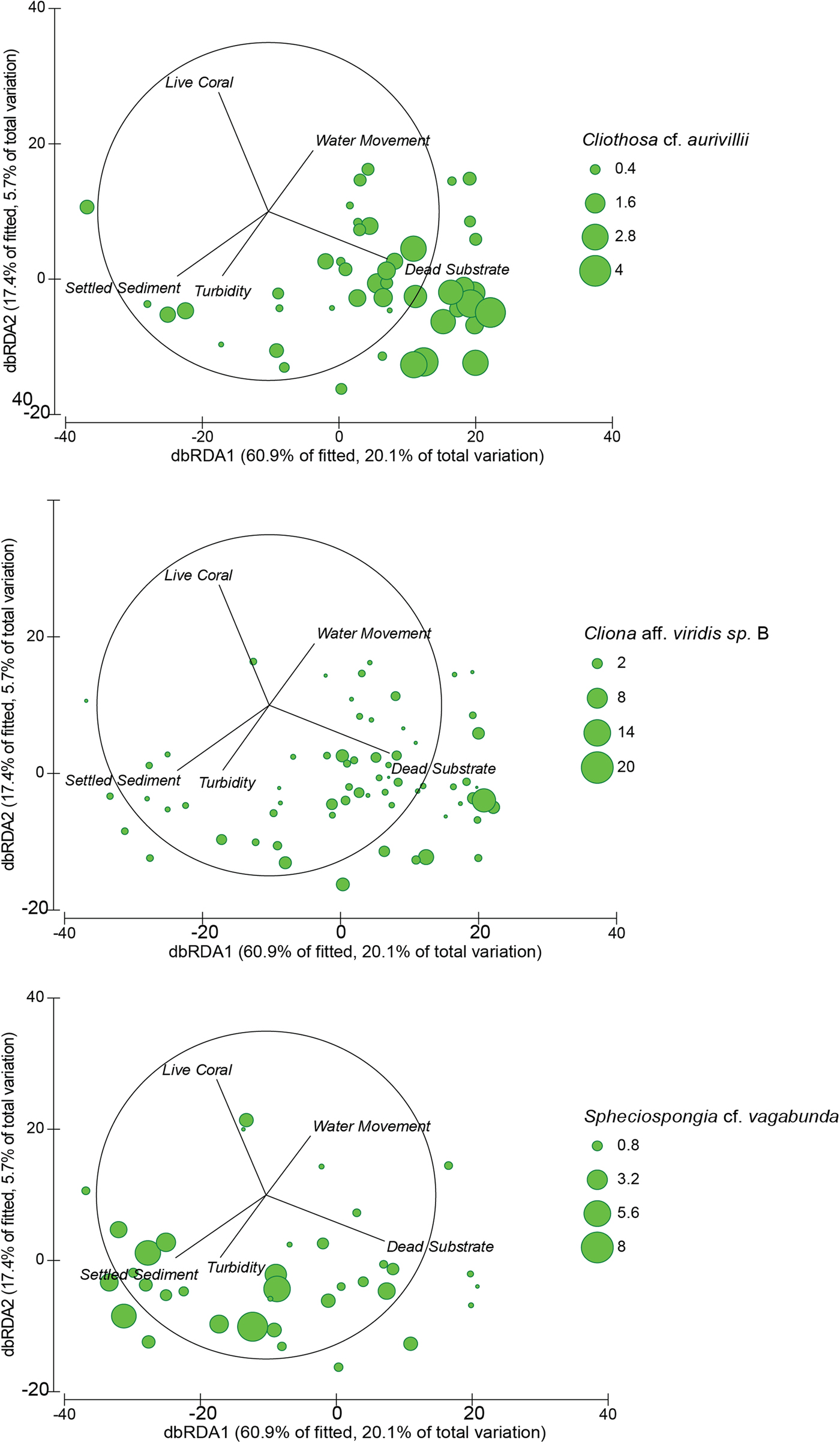
Fig. 6. Distance based redundancy analysis (dbRDA) ordinations of fitted models for assemblage composition of the seven core sites (both depth categories), with the omission of Sampela 1. Overlapping 2D bubbles represent the abundance Cliothosa cf. aurivillii (top), Cliona aff. viridis sp. B (middle) and Spheciospongia cf. vagabunda (bottom).
Hard corals
Hard coral cover was highest at Tomia 1 (64.58% of total reef area ± 7.05) and lowest at Kaledupa 1 and Sampela 1 (11% ± 1.79 and 11.58% ± 2.27 of total reef area, respectively; Table 2). Coral cover was significantly negatively correlated with the abundance of dead substrate, live ‘other’, sand and water movement (Table 2). When omitting Sampela 1 from the analysis, coral cover remained negatively correlated with all these factors, but a positive correlation with chl-a also emerged (Table 2).
DISCUSSION
By identifying putative environmental drivers of bioeroding sponge abundance, this study provided an opportunity to assess how different bioeroding sponges respond to environmental variability using a model reef system. We hypothesized that factors that can lead to coral mortality result in increased availability of dead calcareous substrate, and that such factors are thus beneficial for bioeroding sponges. Our results showed that dead substrate availability was greater on reefs with low coral cover. Furthermore, the availability of this suitable dead substrate was correlated with overall bioeroding sponge abundance, and was the best predictor of differences in assemblage composition. However, bioeroding sponge abundance was lowest on a sedimented reef, despite high availability of dead substrate, demonstrating a limited resilience to sedimentation in some bioeroding sponge species. This indicates that not all forms of coral mortality, and subsequent increases in dead substrate availability, are beneficial for bioeroding sponges. Finally, inter-species variation in abundance with respect to environmental factors illustrated how largely ubiquitous patterns cannot easily be generalized across the entire taxon group.
Abiotic and biotic influences on bioeroding sponges
The availability of dead calcareous substrate was one of the most important factors causing differences in bioeroding sponge abundance and assemblage composition on Wakatobi reefs. This is consistent with earlier studies in Indonesia (Holmes et al., Reference Holmes, Edinger, Limmon and Risk2000), on the Australian GBR (Schönberg, Reference Schönberg2001, Reference Schönberg2015a; Schönberg & Ortiz, Reference Schönberg and Ortiz2009), in the Mexican Pacific (Nava & Carballo, Reference Nava and Carballo2013) and in the Caribbean (Callahan, Reference Callahan2005; Chaves-Fonnegra et al., Reference Chaves-Fonnegra, Zea and Gómez2007). This observation is a consequence of the sponges’ endolithic habit that creates a dependency on calcareous structures (e.g. Schönberg, Reference Schönberg, Wisshak and Tapanila2008). Nevertheless, this relationship was not uniform across all species studied, and the association was primarily observed in the papillate Cliothosa cf. aurivillii and Cliona aff. viridis sp. B. Both species dominated at three sites with a high occurrence of dead substrate and little live coral. Conversely, Spheciospongia cf. vagabunda was more common where dead substrate availability was low, but coral cover was relatively high. This suggests that biological requirements vary between these species, which could be due to different levels of dependency on endolithic life style. Some Spheciospongia spp. are facultative endoliths, known to develop into fleshy or massive morphologies that are largely independent of the endolithic habit (Schönberg et al., Reference Schönberg, Fang, Carreiro-Silva, Tribollet and Wisshak2017b). Cl. aurivillii was originally described as occurring in a free-living, as well as in an endolithic form (Lindgren, Reference Lindgren1897), but recent reports only observed the endolithic form (e.g. Calcinai et al., Reference Calcinai, Azzini, Bavestrello, Cerrano, Pansini and Thung2006). We do not know enough about Cliona aff. viridis sp. B to assess how tightly it is associated with calcareous substrates, but in Wakatobi it was only observed in endolithic form. Furthermore, dispersal capabilities of bioeroding sponges are thought to vary significantly, from the extremely limited (<10 m) to the extensive (>10 km) (Mariani et al., Reference Mariani, Uriz and Turon2000; Bautista-Guerrero et al., Reference Bautista-Guerrero, Carballo and Maldonado2010, Reference Bautista-Guerrero, Carballo, Aguilar-Camacho and Sifuentes-Romero2016; Chaves-Fonnegra et al., Reference Chaves-Fonnegra, Feldheim, Secord and Lopez2015). Thus, depending on species-specific traits, distribution patterns can be further decoupled from substrate availability, as some recruits may settle close to the parent sponge rather than evenly spreading out across all available substrate (Callahan, Reference Callahan2005).
As many bioeroding sponges are heterotrophic (or at least partly), they are reliant on particulate and dissolved organic carbon to meet their energetic demands (Mueller et al., Reference Mueller, de Goeij, Vermeij, Mulders, van der Ent, Ribes and van Duyl2014). Nutrient availability, typically measured as chl-a, has consequently repeatedly been shown to correlate with bioeroding sponge abundance and erosion rates (e.g. Rose & Risk, Reference Rose and Risk1985; Holmes et al., Reference Holmes, Edinger, Limmon and Risk2000; Holmes et al., Reference Holmes, Ortiz and Schönberg2009). However, in our study we found no correlation between bioeroding sponge abundance and chl-a concentration when data from Sampela 1 was excluded from the analysis. The lack of a clear nutrient stimulus may be explained by the generally low and similar chl-a concentrations at most sites (other than Sampela 1). Similar results have been found elsewhere in Indonesia; Holmes et al. (Reference Holmes, Edinger, Limmon and Risk2000) found that bioeroding sponge occupation of massive corals correlated with chl-a concentration on polluted Javan reefs but not in Ambon where chl-a was less concentrated and more consistent across sites. The majority of our chl-a measurements deployments took place during the dry season (June–August) when chl-a concentration is at its highest (Tadjuddah et al., Reference Tadjuddah, Mustafa, Pangerang and Yasidi2012). However, the broad scale seasonal upwellings that are responsible for this peak in chl-a (Wyrtki, Reference Wyrtki1961), might also mask inter-site variation in chl-a concentration from local sources. These are likely to be more pronounced in the wet season when chl-a levels are often higher on coastal reefs (e.g. Lapointe et al., Reference Lapointe, Barile and Matzie2004; Devlin et al., Reference Devlin, Da Silva, Petus, Wenger, Zeh, Tracey, Álvarez-Romero and Brodie2013). The negative correlation between total bioeroding sponge abundance and chl-a concentration, when including Sampela 1 (which had the highest chl-a concentration), appears inconsistent with the concept of nutrient enrichment being beneficial to these sponges. However, Sampela 1 was not only characterized by high chl-a levels, we found it to be the most turbid and sedimented of the surveyed sites (as is consistent with previous descriptions of this reef (see Crabbe & Smith, Reference Crabbe and Smith2002, Reference Crabbe and Smith2005; Hennige et al., Reference Hennige, Smith, Perkins, Consalvey, Paterson and Suggett2008, Reference Hennige, Smith, Walsh, McGinley, Warner and Suggett2010; Biggerstaff et al., Reference Biggerstaff, Smith, Jompa and Bell2015, Reference Biggerstaff, Smith, Jompa and Bell2017)). In these circumstances, interactions with other factors, such as sedimentation, can mask the effects of nutrient enrichment, leading to different net responses (e.g. Holmes et al., Reference Holmes, Edinger, Limmon and Risk2000; Reis & Leão, Reference Reis, Leão, Moosa, Soemodihardjo, Soegiarto, Romimohtarto, Nontji, Soekarno and Suharsono2000; Chaves-Fonnegra et al., Reference Chaves-Fonnegra, Zea and Gómez2007; Holmes et al., Reference Holmes, Ortiz and Schönberg2009; Nava et al., Reference Nava, Ramírez-Herrera, Figueroa-Camacho and Villegas-Sanchez2014). Low abundances of bioeroding sponges in eutrophic environments that experience excessive sedimentation or suspended particle concentrations have also been found in other studies. For example, Edinger et al. (Reference Edinger, Limmon, Jompa, Widjatmoko, Heikoop and Risk2000) noted that bioeroding sponges were rare on reefs in the highly eutrophic Jakarta Bay, which the authors attributed to high sedimentation levels. Nava & Carballo (Reference Nava and Carballo2013) came to a similar conclusion when they observed no relationship between the distribution of boring sponges and chl-a concentration on Pacific Mexican reefs.
Sedimentation can influence sponge distribution patterns by preventing settlement, by impeding vital physiological functions or by controlling survival and therefore selecting for adapted species over others (Bell et al., Reference Bell, McGrath, Biggerstaff, Bates, Bennett, Marlow and Shaffer2015; Schönberg, Reference Schönberg2016a, Reference Schönbergb). Bioeroding sponge assemblage compositions in sedimented environments are consequently structured by the respective differences in species sediment tolerance (Carballo et al., Reference Carballo, Sanchez-Moyano and García-Gómez1994; Nava & Carballo, Reference Nava and Carballo2013). Some bioeroding species are particularly tolerant of high levels of sediment deposition, becoming covered by a thin layer of sediment, or are even adapted to live mostly buried in sediments (endopsammic; Schönberg, Reference Schönberg2016a). Sediment tolerance mechanisms in bioeroding sponge include fistular projections above the sediment layer, self-cleaning surfaces and temporary oscula closure (Schönberg, Reference Schönberg2015b, Reference Schönberg2016a; Pineda et al., Reference Pineda, Strehlow, Kamp, Duckworth, Jones and Webster2017a). However, even these tolerant species are thought to require a patch of free substrate for larval settlement (Schönberg, Reference Schönberg2016a). In our study the impact of sedimentation primarily emerged as reduced abundance and species richness at the sedimented site, Sampela 1. Previous work by Crabbe & Smith (Reference Crabbe and Smith2005) found sedimentation rates were up to 3.8× higher at Sampela 1 than on other local reefs and Biggerstaff et al. (Reference Biggerstaff, Smith, Jompa and Bell2017) estimated average Sampela sedimentation rates to be 4.40 (±0.30) mg cm−2 d−1, which is comparable to other Indo-Pacific reefs that are considered to be highly sedimented (e.g. Golbuu et al., Reference Golbuu, Van Woesik, Richmond, Harrison and Fabricius2011; Bannister et al., Reference Bannister, Battershill and de Nys2012). One species that was absent from Sampela 1 was the endolithic, phototrophic Cliona orientalis. Recent observations identified this species as comparatively tolerant of sediment deposition and able to maintain clean surfaces (Büttner & Siebler, Reference Büttner and Siebler2013; Schönberg, Reference Schönberg2015b; Pineda et al., Reference Pineda, Strehlow, Sternel, Duckworth, Haan, Jones and Webster2017b). However, under extreme and long-term sediment pressure including high concentrations of suspended particles, C. orientalis experienced bleaching and mortality (Pineda et al., Reference Pineda, Strehlow, Kamp, Duckworth, Jones and Webster2017a, Reference Pineda, Strehlow, Sternel, Duckworth, Jones and Websterc), which might attest to its dependency on its dinoflagellate symbionts (Fang et al., Reference Fang, Schönberg, Mello-Athayde, Hoegh-Guldberg and Dove2014, Reference Fang, Schönberg, Hoegh-Guldberg and Dove2017). Cliona orientalis’ sediment intolerance appears to relate to the quality of suspended particles; on the GBR it displayed more severe responses to applications of mud than sand and was less abundant in reef areas with a higher proportion of fine sediments (Büttner & Siebler, Reference Büttner and Siebler2013; Ramsby et al., Reference Ramsby, Hoogenboom, Whalan, Webster and Thompson2017). Over 30% of settled sediment at Sampela 1 is comprised of particles finer than 63 µm (Biggerstaff et al., Reference Biggerstaff, Smith, Jompa and Bell2017), suggesting that the sediment regime is locally preventing the persistence or even the establishment of C. orientalis. The high concentration of fine sediment could also explain the near-absence of S. cf. vagabunda at Sampela 1, which contradicts the positive correlation between this species’ abundance and settled sediment depth. Several species within the genus Spheciospongia are known to benefit from living embedded in sediments (Schönberg, Reference Schönberg2016a) and S. cf. vagabunda was most abundant at Buoys 1 and 3, which had moderately high levels of sedimentation. However, previous work at Buoys 1 and 3, has shown the settled sediment composition to be much coarser than at Sampela 1 (Bell & Smith, Reference Bell and Smith2004). High levels of fine sediments, suspended or deposited, seem to be a problem for many sponges, bioeroding or otherwise (Schönberg, Reference Schönberg2001, Reference Schönberg2016a; Schönberg & Ortiz, Reference Schönberg and Ortiz2009). With little selective control over filtering intake (Reiswig, Reference Reiswig1971), sponges are more susceptible to clogging by finer particles, thereby reducing pumping, feeding efficiency and other biological processes (see Bell et al., Reference Bell, McGrath, Biggerstaff, Bates, Bennett, Marlow and Shaffer2015). Fine sediments are also more associated with terrestrial inputs (Bannister et al., Reference Bannister, Battershill and de Nys2012) and once settled are more likely to be resuspended by currents, tides and waves (Ogston et al., Reference Ogston, Storlazzi, Field and Presto2004), contributing to reef turbidity.
Low levels of particle suspension (turbidity) in the water column were beneficial for the Wakatobi bioeroding sponges and may have brought about nutrient enrichment (as per Holmes et al., Reference Holmes, Edinger, Limmon and Risk2000). However, evidence from Sampela 1 suggested that excessive turbidity may have limited the abundance of bioeroding sponges as a community. Turbidity levels at the Sampela 1 are within the range that is considered stressful to corals (Cooper et al., Reference Cooper, Ridd, Ulstrup, Humphrey, Slivkoff and Fabricius2008) and has already been associated with low coral growth rates at Sampela 1 (Crabbe & Smith, Reference Crabbe and Smith2005; Hennige et al., Reference Hennige, Smith, Perkins, Consalvey, Paterson and Suggett2008). Sponges also have the capacity to be negatively affected by high turbidity, either by direct impairment of the pumping and filtration apparatus or indirectly through shading (Pang, Reference Pang1973; Bell et al., Reference Bell, McGrath, Biggerstaff, Bates, Bennett, Marlow and Shaffer2015; Schönberg, Reference Schönberg2016b). The absence of two of the three locally abundant phototrophic zooxanthellate species at the sampled depths at Sampela 1 would support the latter. On the GBR, C. orientalis (one of the absent species) is most abundant at ~5 m depth on clear-water reefs, but at more turbid sample sites it dominates in the upper subtidal and intertidal regions (Bergman, Reference Bergman1983, as C. viridis; Schönberg, Reference Schönberg2001). Extensive work on the impacts of dredging on sponges in the GBR by Pineda et al. (Reference Pineda, Strehlow, Duckworth, Doyle, Jones and Webster2016, Reference Pineda, Strehlow, Kamp, Duckworth, Jones and Webster2017a, Reference Pineda, Strehlow, Sternel, Duckworth, Haan, Jones and Websterb, Reference Pineda, Strehlow, Sternel, Duckworth, Jones and Websterc) has also shown that C. orientalis bleaches when severely light limited and when exposed to high concentrations of suspended sediments for intermediate periods. The Symbiodinium within the bleached C. orientalis are thought to survive through parasitism of their host (Fang et al., Reference Fang, Schönberg, Hoegh-Guldberg and Dove2017) and therefore the host is unlikely to survive prolonged periods of excessive turbidity. However, light requirements are likely species specific, as other zooxanthellate clionaids appear capable of persisting in conditions of greatly reduced light availability (Rosell & Uriz, Reference Rosell and Uriz1991).
In total no more than 33% of assemblage variability was explained by our environmental/substrate variables and no more than 16% of variability was explained by any one variable. These low explanatory values are partly a reflection of inter-species differences in environmental requirements and resilience, but are also likely due to the importance of unmeasured variables such as slope, sediment grain size or PAR (photosynthetic active radiation). Furthermore, the highest values for chl-a, settled sediment and turbidity all occurred at Sampela 1, likely driving collinearity and making interpretation of the impacts of these individual factors difficult. As these factors naturally occur concurrently on reefs impacted by terrestrial run-off (Fabricius, Reference Fabricius2005), confident in situ analysis of the individual effects of these factors can be problematic. This is potentially best achieved using experimental manipulations. For example Pineda et al. (Reference Pineda, Strehlow, Duckworth, Doyle, Jones and Webster2016, Reference Pineda, Strehlow, Kamp, Duckworth, Jones and Webster2017a, Reference Pineda, Strehlow, Sternel, Duckworth, Haan, Jones and Websterb, Reference Pineda, Strehlow, Sternel, Duckworth, Jones and Websterc) investigated the impacts of dredging on a variety of common GBR sponges (including C. orientalis) using separate light attenuation, settled sediment and suspended sediment based ex situ experiments. However as the results from our paper demonstrate, these experiments need to be tailored to the relevant species or risk extrapolation to species with different biological requirements.
Coral cover and stressors
Hard corals covered ~25% of reef area in the Wakatobi. This is slightly higher than the regional average (Bruno & Selig, Reference Bruno and Selig2007), but lower than the 32% cover recorded at similar depths in the Wakatobi in the mid-1980s (Soekarno, Reference Soekarno1989). In the absence of appropriate time-series data it is impossible to attribute this decline to any particular source of coral degradation, but our study found no discernible impact of either sedimentation, turbidity or chl-a concentration on hard coral cover. This suggests that these particular coral stressors are not currently significant regulators of coral cover across the Wakatobi. The exception to this may be at a small selection of sites, such as Sampela 1. The lack of any evidence for this in our analysis may be due to the generally low levels of sedimentation, turbidity and chl-a across most sites and the collinearity of these variables at Sampela 1. Nevertheless, these run-off associated stressors do constitute a genuine threat to many reefs in Indonesia (Edinger et al., Reference Edinger, Limmon, Jompa, Widjatmoko, Heikoop and Risk1998; Edinger & Risk, Reference Edinger and Risk2013).
Coral mortality appeared to promote dead substrate availability, with an inverse correlation between the cover of these respective substrate types. Elsewhere, coral cover declines have been causally linked with increases in macroalgal cover (Hughes et al., Reference Hughes, Szmant, Steneck, Carpenter and Miller1999; McManus & Polsenberg, Reference McManus and Polsenberg2004), but in the Wakatobi macroalgae is uncommon (J. Marlow, personal observations). Wakatobi reefs appear to be neither dominated by coral nor macroalgae, instead the majority of the reef area is comprised of dead substrate (33%) and other live benthos (30%), as is found across much of the Indo-Pacific (Bruno et al., Reference Bruno, Sweatman, Precht, Selig and Schutte2009).
Implications for reef management
Bioeroding sponges in the Wakatobi occupied 8.9% of the available dead reef substrate, which is somewhat lower than found on other reef systems; e.g. 20–81% in the Mexican Pacific and 4–30% of dead substrate on the GBR (Schönberg & Ortiz, Reference Schönberg and Ortiz2009; Nava & Carballo, Reference Nava and Carballo2013). The high abundances on these reefs and other bioeroding sponge increases on degraded coral reefs are primarily attributed to the sponges’ ability to exploit newly available calcareous habitat released by coral mortality (e.g. Lόpez-Victoria & Zea, Reference López-Victoria and Zea2005; Schönberg & Ortiz, Reference Schönberg and Ortiz2009; Schönberg et al., Reference Schönberg, Fang, Carreiro-Silva, Tribollet and Wisshak2017a). In the absence of baseline data, the spatial patterns of bioeroding sponge assemblages in this study could not be conclusively associated with coral mortality and prevented the recognition of a temporal sequence of events related to reef degradation. However, our findings of a negative correlation between coral cover and dead substrate, and the importance of the availability of this dead substrate for bioeroding sponges, supports our hypothesis that coral mortality can promote the abundance of bioeroding sponges in the region. Nevertheless the importance of dead substrate availability differed between species and had no overall association with sponge diversity. Importantly, most of the local species had an apparent low resilience to excessive sediment deposition and turbidity. This suggests that on reefs degraded by terrestrial run-off, some bioeroding sponge species may be prevented from exploiting increases in substrate availability. In these environments, the species assemblage is likely to be dominated by fewer resilient species. This has already been observed in the eastern Pacific, where Nava & Carballo (Reference Nava and Carballo2013) found that although habitat availability promoted bioeroding sponge abundance, resilience to scouring by moving sediment was an important determinant of assemblage composition. In conclusion, not all factors that negatively affect corals are of benefit to bioeroding sponges. It is therefore vital to obtain a better understanding of the biological traits of bioeroding organisms, and of how they sustain or reduce the ecosystem health when reef function is stressed or degraded.
ACKNOWLEDGEMENTS
The present study was funded by a Victoria University of Wellington doctoral scholarship awarded to Joseph Marlow and facilitated by Operation Wallacea who provided travel funds and access to research facilities on Hoga, Indonesia. We would also like to thank all the volunteer and Indonesian staff at Operation Wallacea who helped facilitate this study. Joseph Marlow conducted this research as an exchange student of Hasanuddin University and as such under the research permit issued to Hasanuddin University from the Indonesian Ministry of Research and Technology (RISTEK).
APPENDIX
Table A1. Results of bivariate correlations (Spearman's Rank, rs) comparing the standardized abundance of individual species of bioeroding sponges with environmental factors. Only the three most dominant species are shown.