Introduction
Soft bottoms represent one of the most common habitats in marine environments, supporting a high productivity and, at the same time, experiencing different anthropogenic impacts (e.g. trawling) (Snelgrove, Reference Snelgrove1999; Collie et al., Reference Collie, Hall, Kaiser and Poiner2000; Tudela, Reference Tudela2004; De Juan & Demestre, Reference De Juan and Demestre2012). The diversification of sedimentary micro-habitats can also provide a variety of niches that are used by different species, including some of commercial interest, which increase the local and regional biodiversity of soft bottoms (Gray, Reference Gray2002; García Muñoz et al., Reference García Muñoz, Manjón-Cabeza and García Raso2008; Labrune et al., Reference Labrune, Grémare, Amouroux, Sardá, Gil and Taboada2008; Urra et al., Reference Urra, Gofas, Rueda and Marina2011, Reference Urra, Gofas, Rueda, Marina, Mateo-Ramírez, Antit and Salas2017). The benthic and demersal fauna of soft bottoms are generally influenced by different environmental variables such as changes in sediment characteristics (e.g. grain size, content of organic matter) and water masses, as well as by biological processes such as intra- and inter-specific interactions (e.g. predation, recruitment, etc.) that need to be studied in detail (Wildish, Reference Wildish1977; Wilson, Reference Wilson1991; Snelgrove & Butman, Reference Snelgrove and Butman1994). Preservation and management of some soft bottom communities should also be reconsidered because of the importance of some of their species and functional groups in ecosystem processes that have global importance (Snelgrove, Reference Snelgrove1998). Improving information on these communities is a key element in this process and those inhabiting circalittoral and bathyal soft bottoms have been poorly studied in some areas so far, as is the case of some high biodiversity areas within the Mediterranean Sea such as the Alboran Sea (Templado et al., Reference Templado, Guerra, Bedoya, Moreno, Remón, Maldonado and Ramos1993; Salas, Reference Salas1996; Gofas et al., Reference Gofas, Salas, Rueda, Canoura, Farias and Gil2014; Marina et al., Reference Marina, Rueda, Urra, Salas, Gofas, García Raso, Moya, García, López-González, Laiz-Carrión and Baro2015).
The Alboran Sea is located in the western Mediterranean Sea, and it is considered a biodiversity hot spot due to the biogeographic confluence of organisms from the Lusitanian, the Mauritanian and the Mediterranean region (Ekman, Reference Ekman1953; Rueda et al., Reference Rueda, Urra, Marina, Mateo and Reina-Hervás2010; García Raso et al., Reference García Raso, Gofas, Salas Casanova, Manjón-Cabeza, Urra and García Muñoz2010a; Templado, Reference Templado2011; Urra et al., Reference Urra, Gofas, Rueda, Marina, Mateo-Ramírez, Antit and Salas2017). This biological confluence is supported by the exchange of water masses and organisms (including larvae), between the Atlantic Ocean and Mediterranean Sea through the Strait of Gibraltar. Moreover, some important physical and oceanographic features of the Alboran Sea (e.g. upwellings) promote a high biological productivity area within the Mediterranean Sea (Parrilla & Kinder, Reference Parrilla and Kinder1987; Sarhan et al., Reference Sarhan, García-Lafuente, Vargas and Plaza2000; Reul et al., Reference Reul, Rodríguez, Jiménez-Gomez, Blanco, Bautista, Sarhan, Guerrero, Ruíz and García-Lafuente2005; Muñóz et al., Reference Muñóz, Reul, Plaza, Gómez-Moreno, Vargas-Yañez, Rodríguez and Rodríguez2015). An ecologically interesting area within the northern Alboran Sea is the Bay of Málaga, which harbours a high diversity of marine commercial species that has allowed the development of an intensive fishing activity targeting demersal (mainly Merluccius merluccius and Mullus barbatus barbatus), small pelagic (mainly Trachurus trachurus, T. mediterraneus, S. pilchardus, E. encrasilocus and Alloteuthis spp.) and benthic species (mainly molluscs such as Octopus vulgaris, Donax spp., Chamelea gallina and Callista chione), among others (Reina-Hervás & Serrano, Reference Reina-Hervás and Serrano1987a, Reference Reina-Hervás and Serrano1987b; Camiñas et al., Reference Camiñas, Baro and Abad2004; Robles, Reference Robles2010; Baro et al., Reference Baro, Serna-Quintero, García, Giráldez, Marina, Rueda, Gallardo-Núñez, Moya, Laiz-Carrión and García2015).
Circalittoral benthic communities (within the depth range from seagrass deepest occurrence until the shelf break) are globally exposed to anthropogenic activities, such as trawling, that can have long-lasting negative effects, especially in those environments dominated by slow-growing sessile invertebrates that are ecologically or socially important (Jennings & Kaiser, Reference Jennings and Kaiser1998; Hall, Reference Hall1999; Kaiser et al., Reference Kaiser, Clarke, Hinz, Austen, Somerfield and Karakassis2006). Moreover, there is an actual need for improving the knowledge on the composition and structure of benthic and demersal communities and of their linkage with environmental factors under the framework of current European conservation directives (e.g. Marine Strategy Framework Directive). This is of importance in the case of the Alboran Sea because it represents a marine biodiversity hot spot, but it is unfortunately exposed to a high fishing activity, maritime traffic and tourism that are known to promote shifts in habitats and associated communities, as well as in the commercial resources, as observed in this and other basins (Tillin et al., Reference Tillin, Hiddink, Jennings and Kaiser2006; Rueda et al., Reference Rueda, Marina, Urra and Salas2009; Robles, Reference Robles2010; Baeta et al., Reference Baeta, Ramón and Galimany2014; Dannheim et al., Reference Dannheim, Brey, Schröder, Mintenbeck, Knust and Arntz2014; Muñoz et al., Reference Muñóz, Reul, Plaza, Gómez-Moreno, Vargas-Yañez, Rodríguez and Rodríguez2015; Froglia et al., Reference Froglia, Gramito, Martinelli and Betulla2017). Despite all this, most previous studies of soft bottom communities of the Alboran Sea have been done on the infralittoral zone and on specific faunistic groups such as fishes, decapods and molluscs (Cano & García, Reference Cano and García1982; Luque, Reference Luque1983, Reference Luque1986; Salas et al., Reference Salas, García Raso and López-Ibor1984; Reina-Hervás & Serrano, Reference Reina-Hervás and Serrano1987a, Reference Reina-Hervás and Serrano1987b; García Muñoz et al., Reference García Muñoz, Manjón-Cabeza and García Raso2008; Urra et al., Reference Urra, Gofas, Rueda and Marina2011, Reference Urra, Marina, Salas, Gofas and Rueda2013). Therefore, information on other groups, including commercial species, is still very scarce for most of the circalittoral soft bottom communities of this basin (Cano & García, Reference Cano and García1982; Templado et al., Reference Templado, Guerra, Bedoya, Moreno, Remón, Maldonado and Ramos1993; Abad et al., Reference Abad, Preciado, Serrano and Baro2007; García Raso et al., Reference García Raso, Salas, García Muñóz and Gofas2010b; Marina et al., Reference Marina, Rueda, Urra, Salas, Gofas, García Raso, Moya, García, López-González, Laiz-Carrión and Baro2015).
This study aims to improve the knowledge on circalittoral soft bottom communities and demersal resources within the Bay of Málaga. The specific objectives of this study are: (1) to characterize the composition and structure of benthic and demersal assemblages inhabiting circalittoral soft bottoms of the bay; (2) to analyse the spatial and/or temporal patterns of the detected assemblages, as well as those of some dominant species; and (3) to study potential linkages of benthic and demersal communities with some environmental variables of the sediment and water column, as well as with the trawling activity within the bay.
Materials and methods
Study area
This study has been carried out on the continental shelf of the Bay of Málaga (N Alboran Sea), which extends from Calaburras headland (36°30′24″N 04°38′17″W) to Caleta de Vélez (36°43′33″ N 04°06′17″W) (Figure 1). The bay is characterized by a narrow littoral shelf (6 km width off Calaburras and 11 km width off Málaga harbour) with a large prodeltaic platform composed of sedimentary substrates (mainly mud and muddy fine sands). These sedimentary substrates are derived from fluvial processes, with the Guadalhorce River as the major source of sediment input in the area (De la Cruz et al., Reference De la Cruz, Hernández-Molina and Vázquez1992). Sedimentary habitats are very widespread throughout this bay, including infralittoral bottoms with well-sorted fine sands and coarse sand and gravels, all of them related to the habitat 1110 ‘Sandbanks which are slightly covered by sea water all the time’ of the Habitat Directive (92/43/EEC) (García Raso et al., Reference García Raso, Salas, García Muñóz and Gofas2010b; IEO-MAGRAMA, 2012). In the circalittoral zone the most frequent bottoms are those with fine sands, terrigenous muds and coastal detritic sediments.

Fig. 1. Location of the samples collected with benthic dredge (squares) and otter trawl (lines) in circalittoral soft bottoms of the Bay of Málaga (northern Alboran Sea).
The water masses in this bay are strongly influenced by the Atlantic surface current entering through the Strait of Gibraltar, and by the easterly water masses drifts that promote almost constant cold and nutrient-rich deep water upwellings (Arévalo & García, Reference Arévalo and García1983; Parrilla & Kinder, Reference Parrilla and Kinder1987; Cano & García Lafuente, Reference Cano and García Lafuente1991; Sarhan et al., Reference Sarhan, García-Lafuente, Vargas and Plaza2000). All these hydrological features promote high phytoplanktonic and zooplanktonic biomasses in comparison with other Mediterranean areas, with the Bay of Málaga being considered a hot spot of biological productivity within the Mediterranean Sea (Rodríguez et al., Reference Rodríguez, García and Rodríguez1982; Cortés et al., Reference Cortés, Gil and Garcia1985; García & Camiñas, Reference García and Camiñas1985; Vargas-Yáñez et al., Reference Vargas-Yáñez, García Martinez, Moya, Tel, Parrilla, Plaza, Lavín, García, Salat, López-Jurado, Pascual, García Lafuente, Gomis, Álvarez, García Sotillo, González Pola, Polvorinos, Fraile Nuez, Fernandez de Puelles and Zunino2010). This high biological productivity makes possible the presence of different fishing fleets in the Bay of Málaga (e.g. trawling, artisanal, purse seine) that target a wide variety of species, as commented on in the Introduction. Trawling occurs in different sedimentary bottoms below 50 m depth and is high in the central and eastern part of the bay, purse seine occurs mainly in the central part of the bay and artisanal fishing occurs in shallower water and close to the coast, especially in the eastern and western parts of the bay (Baro et al., Reference Baro, Serna-Quintero, García, Giráldez, Marina, Rueda, Gallardo-Núñez, Moya, Laiz-Carrión and García2015).
Sample and data collection
Hydrological and faunistic data were obtained in eight sampling stations located in circalittoral soft bottoms of the Bay of Málaga on board RV ‘Francisco de Paula Navarro’ (Figure 1), within the framework of the REMALA project. These sampling stations covered the western, central and eastern sectors of the bay, different depths within the circalittoral zone (37–81 m depth) and sediment types with a priori different proportions of mud, sand and gravels that were selected from maps of the ESPACE project.
Water column and sediment variables
Near-bottom seawater measurements (salinity and temperature) (1–2 m above seafloor) were taken seasonally (December 2013, February, May and August 2014) at each sampling station using a SBE-37-SMP MicroCAT CTD profiler. Other important variables of the water column that could influence the distribution of benthic and demersal species such as near-bottom current speed could not be obtained due to sampling limitations or a lack of information for this area. Sediment samples were collected in December 2013 with a box-corer (mouth: 10 × 17 cm; maximum penetration: 37 cm) within the framework of the TESELA project. Granulometric analyses were performed in the laboratory by sieving sediment samples of a similar weight over a column of sieves, and then weighing (dry weight) the fractions retained on each sieve. The Udden–Wentworth scale (Wentworth, Reference Wentworth1922) was used to characterize the type of sediment. The percentage of organic matter was estimated as the weight loss in samples of dry sediment (60°C for 72 h) after ignition (550°C for 4 h).
Fauna
Faunistic samples were collected combining two sampling methods targeting different organisms in each sampling station. Small epibenthic and infaunal species could only be sampled in December 2013 using a small benthic dredge (BD) (width: 42 cm; height: 22 cm; mesh size: 4 mm) (N = 8 samples). The dredge was towed at a boat speed of 1.8 knots for 5 min, resulting in a sampling area of ~117 m2 per sample. This sampled area is similar to previous studies on soft bottom benthic communities in the southern Iberian Peninsula (Urra et al., Reference Urra, Gofas, Rueda and Marina2011, Reference Urra, Marina, Salas, Gofas and Rueda2013; Marina et al., Reference Marina, Rueda, Urra, Salas, Gofas, García Raso, Moya, García, López-González, Laiz-Carrión and Baro2015). The demersal and large epibenthic species could be sampled in the same eight sampling stations but also in different seasons (December 2013, February, May and August 2014) with a GOC-73 otter trawl (OT) (N = 30 samples) with the net (mesh size: 20 mm) open laterally up to 18 m wide and vertically up to 3.2 m high. The otter trawl was towed at a boat speed of 3 knots for 30 min, resulting in a sampling area of ~50,000 m2 per haul (Bertrand et al., Reference Bertrand, Gil de Sola, Papaconstantinou, Relini and Souplet2002). Each trawling operation was controlled with SCANMAR sensors, which gave information on the geometry of both the gear and the net while operating on the seabed. For each haul, the initial and final position of the research vessel was taken for a more accurate estimation of the sampled area in each operation.
The material collected with the BD was sieved on board using a sieve column of 10, 5 and 1 mm mesh sizes, preserving each fraction in 70% ethanol until further processing. Once in the laboratory, all individuals from each fraction were identified to the lowest taxonomic level that was possible and counted using stereo microscopes. The material collected with the OT was sorted on board and all individuals were identified to the lowest taxonomic level that was possible, counted and weighed to the closest 0.5 g. Those species that could not be identified on board were coded, fixed in ethanol 70% or stored at −20°C and transported to the laboratory for further analyses. Some of the collected material has been deposited in the invertebrate and fish reference collection of the Centro Oceanográfico de Málaga (Instituto Español de Oceanografía). Scientific names for all taxa followed the nomenclature of the World Register of Marine Species (WoRMS) (http://www.marinespecies.org/).
Trawling activity
The trawling activity of the fishing fleet operating in the Bay of Málaga was analysed by using data provided by the Vessel Monitoring System (VMS), a satellite-based monitoring system designed for fisheries management and control. This system regularly provides data to the fisheries authorities on the location, course and speed of vessels larger than 12 m length. Trawling activity was quantified with a map displaying the position of every vessel that was trawling (vessel speed between 1–4 knots) in the Bay of Málaga from 2010 to 2013. Then, the number of occurrences of those vessels was counted in each of the eight sampling stations and in each season (spring, summer, autumn, winter), obtaining an estimation of the trawling activity in each sampling station and season. This provided estimations on the cumulative trawling in each sampling site and season during different years. No data on VMS were available for 2014 but similar spatial and seasonal patterns on the spatial distribution of the fishing activity were detected among the studied years.
Data analysis
The characterization of the species was carried out with data standardized to 117 m2 for benthic dredge (BD) data, and to 50,000 m2 for otter trawl (OT) data, which is similar to the sampled area with both gears. The species collected with the BD were characterized according to their abundance (N: number of individuals of each species), dominance index based on their abundance (%Da: percentage of individuals of a species from the total) and frequency of occurrence index (%Fr: percentage of samples in which the species is present). The species collected with the OT were seasonally characterized according to their N, %Da, %Fr, biomass (B) and dominance index based on their biomass (%Db: percentage of weight of a species from the total). Groups of samples detected with multivariate methods (presented in the next paragraph) and/or presenting significant differences in multivariate test according to factors (e.g. sediment, depth, season) were interpreted as different assemblages and characterized according to the species richness (S), total abundance (N) and biomass (B), the Shannon–Wiener diversity (H’: log2) (Krebs, Reference Krebs1989) and the evenness (J’) (Pielou, Reference Pielou1969). These ecological parameters and indexes were calculated using the software PRIMER v6.0 (Clarke & Warwick, Reference Clarke and Warwick2001). ANOVA (only with data displaying a normal distribution and homogeneity of variances) and non-parametric tests (Median Test, data without normal distribution) were carried out for testing the differences in those ecological parameters and indexes in relation to the potential assemblages detected with multivariate analyses and with the different seasons (only for OT samples). These statistical procedures were performed using the software SPSS v22.
Similarities between BD and OT samples were analysed separately using quantitative data (N data for BD samples and N and B data for OT samples) and the Bray–Curtis similarity index (Bray & Curtis, Reference Bray and Curtis1957). Classification and ordination of these samples was explored with group-average sorting classification (CLUSTER) and non-metric Multi-Dimensional Scaling ordination (nMDS). Distance-based permutational multivariate analyses of variance (PERMANOVA) were carried out for testing differences of groups of samples according to a priori selected factors such as depth (shallow circalittoral – less than 50 m vs intermediate circalittoral – more than 50 m depth), sediment type (muddy fine sand, muddy fine-medium sand, mixed sediments) and season (autumn, winter, spring, summer) (only for OT samples). The SIMPER (Similarity Percentage) procedure was used to identify those species that contributed to the separation of groups of samples resulting from CLUSTER or from statistical differences between groups of samples (PERMANOVA). Additionally, the relationships between environmental variables, trawling activity and fauna were studied through a Principal Component Analysis (PCA), as it provides a graphical representation of the distribution of samples in relation to the water and sediment characteristics as well as the trawling activity. Prior to the PCA analyses, these abiotic variables were screened and those which presented a correlation of more than 0.8 (after Spearman correlation analysis) were not considered further. All variables expressed in percentages were previously transformed using log (x + 1). All these multivariate analyses were executed using the software PRIMER v6.0 (Clarke & Warwick, Reference Clarke and Warwick2001).
Results
Environmental variables and trawling activity
Near-bottom seawater temperature ranged between 13.3°C in winter 2014 and 15.8°C in autumn 2013 and salinity ranged between 36.6 psu in autumn and 38.1 psu in winter. Muddy fine sand bottoms appear to currently occupy an extensive area of the Bay of Málaga, mainly off the Guadalhorce River (percentage of mud up to 50.91% at 80 m depth) and in the eastern sector at depths between 20 and 100 metres. The percentages of fine sand ranged between 30–45% in the outer zone of the continental shelf off the bay, and up to 90% off the Guadalhorce River at 50 m depth. Gravels (30–40%) are distributed in the eastern outer shelf of the bay between 50 and 100 metres depth. The percentage of organic matter in the sediment is lower in the western sector of the bay (2–3%) than in the eastern sector (3–6%), with the maxima recorded off the Guadalhorce River (7–8%).
Trawling activity is more intense off Málaga harbour and in the eastern sector (between Málaga and Torre del Mar) (>50 trawling vessels per km2 and year), especially at 50 m depth. In the outer bay, the trawling activity is less intense (between 15–40 trawling vessels per km2 and year). An overall higher activity was detected in spring and summer, with ~70% of the annual trawling activity occurring in spring and summer. Trawling activity was apparently similar in different types of soft bottoms, but the scarce hard bottoms occurring in the bay displayed low to null trawling activity.
Characterization and variability of assemblages
A total of 287 species were identified from 153,058 individuals collected with both sampling methods (Faunistic list in supplementary material). In benthic dredge samples (BD), 139 species (98 spp. exclusively collected with this method) were detected and mostly represented by small epibenthic and infaunal species such as molluscs (48 spp., 42% of all species collected with this method), crustaceans (34 spp., 30%) and annelids (30 spp., 25%) (Figure 2A, B). On the other hand, 189 spp. were detected in the otter trawl (OT) samples (148 spp. collected exclusively with this method), and mostly represented by demersal and large epibenthic species belonging to fishes (70 spp. and 76% of all individuals collected with this method), molluscs (40 spp., 11% ind.) and crustaceans (23 spp., 9% ind.) (Figure 2C, D). Fishes also represented the largest biomass collected with OT (69% Db), followed by molluscs (20% Db) (Figure 2E). Some of the collected molluscs, crustaceans, annelids and fishes displayed high frequency of occurrence in the samples (%F > 60%), whereas other taxa belonging to ascidians, poriferans, bryozoans and nemerteans were represented by few species (1–7 spp.) and individuals (<50 ind.) and low frequency of occurrence (Supplementary material).

Fig. 2. Percentages of number of species as well as abundance and biomass of individuals of different faunistic groups collected with benthic dredge (A, B) and otter trawl (C–E) in circalittoral soft bottoms of the Bay of Málaga.
Samples collected with the benthic dredge
Multivariate analyses based on abundance data revealed the presence of three groups of samples from different sediment types that were interpreted as different assemblages associated with (1) muddy fine sand bottoms at 37–47 m depth (MFS), (2) muddy gravel bottoms with fine and coarse sand at 70–75 m depth (mixed sediments, MX), and (3) muddy fine-medium sand bottoms at 49–81 m depth (MFMS) (Figure 3A). PERMANOVA reported higher differences of these groups of samples based on depth rather than on sediment type, but in both cases these differences were non-significant (PERMANOVA, factor depth: Pseudo-F = 2.03; P = 0.061; factor sediment type: Pseudo-F = 0.49; P = 0.85). SIMPER analyses showed dissimilarities higher than 70% for every pairwise comparison of these groups of samples. Differences between MX and MFMS assemblages (SIMPER, average dissimilarity (av. diss.) = 71.86%) were mainly related to a higher abundance of the annelid Spiochaetopterus cf. costarum, the decapod Philocheras bispinosus bispinosus and the gastropod Tritia ovoidea in MFMS and of the gastropods Alvania testae and Sorgenfreispira brachystoma in MX. Differences between MX and MFS assemblages (av. diss. = 72.98%) were mainly related to a higher abundance of A. testae, S. brachystoma, P. bispinosus bispinosus as well as the bivalves S. commutata and Venus nux in MX, and of the annelid Sternaspis scutata in MFS. Differences between MFMS and MFS assemblages (av. diss. = 71.73%) were mainly due to a higher abundance of S. cf. costarum, P. bispinosus bispinosus, T. ovoidea and the gastropod Turritella communis in MFMS. A similar composition but with some species displaying different dominances was also detected in the top-dominant lists for three assemblages detected in CLUSTER (Table 1). Nevertheless, these three assemblages also displayed exclusive species such as the decapod Macropodia tenuirostris and the gorgonian Eunicella filiformis in MFS, the decapod Athanas amazone, the sea-pen Pennatula rubra and the bivalve Serratina serrata in MX and the decapod Hyppolite leptometrae, which could represent a new record for the Spanish waters and the Western Mediterranean, the gorgonian Spinimuricea cf. atlantica and the bivalve Acanthocardia aculeata in the MFMS.
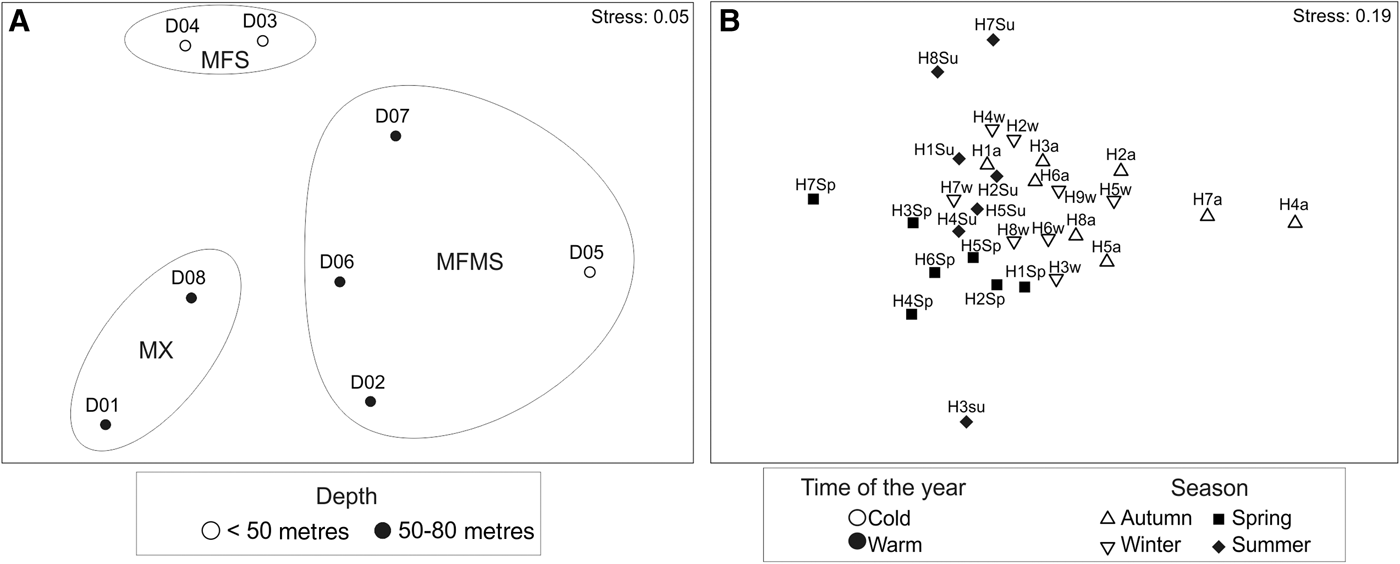
Fig. 3. Non-metric multidimensional scaling ordination of samples collected in circalittoral bottoms of the Bay of Málaga using the benthic dredge (A) and otter trawl (B), based on the Bray–Curtis similarity index and abundance data. Circles represent groupings of samples detected in the CLUSTER. MFMS: muddy fine-medium sand; MFS, muddy fine sand; MX, muddy gravels with fine and coarse sand; D, dredge; H, haul; a, autumn; w, winter; Sp, spring; Su, summer.
Table 1. Top 15 dominant species in the different types of assemblages detected in CLUSTER and nMDS using the data of benthic dredge in the circalittoral bottoms of the Bay of Málaga

Total number of individuals collected (N) and percentage of abundance (%Da) are also indicated. MFMS, muddy fine-medium sand (37–47 m depth); MFS, muddy fine sand (49–81 m depth); MX, muddy gravels with fine and coarse sand (70–75 m depth).
Regarding ecological parameters and indexes, species richness (S) and abundance (N) values were higher for the MFMS assemblage but did not display significant differences between CLUSTER groupings that were interpreted as different assemblages (one-way ANOVA: S, F = 1.28; N, F = 2.78, P > 0.05 in both cases) (Figure 4A, B). The Shannon–Wiener diversity index (H′) did not display significant differences (Median test: χ 2 = 4.0, P > 0.05), but the evenness (J′) index values were significantly higher at MFS assemblages (Median test: χ 2 = 8.0, P < 0.05) (Figure 4C, D). Based on all these results and the lack of significant differences in most tests, the three potential assemblages detected in CLUSTER could be interpreted to belong to three different facies of the same benthic community that display some small differences in their structure.

Fig. 4. Species richness, abundance, Shannon–Wiener diversity index (Diversity index), evenness index and biomass values (the latter only for otter trawl samples) for potential assemblages identified from benthic dredge samples (A–D), and for seasonal samples collected with otter trawl (E–I), on circalittoral bottoms from the Bay of Málaga. Mean + SE. MFMS: muddy fine-medium sand; MFS: muddy fine sand; MX: muddy gravels with fine and coarse sand.
In the PCA, the first two components accounted for 67% of the total variance. The variability along the PC1 axis was mainly explained by an increase in mud content (%M) and percentage of organic matter (%OM), as well as by a decrease in seawater temperature (T), while the PC2 axis was explained by a decrease in the percentages of medium sand (%MS), coarse sand (%CS) and gravel contents (%Gr) as well as by an increase in percentage of fine sand content (%FS) (Figure 5A).

Fig. 5. Results of the Principal Component Analysis (PCA) ordination carried out with data from samples collected with the benthic dredge (A) and the otter trawl (B). Abiotic parameters included FishAct, fishing activity; %Gr, gravels; %CS, coarse sand; %MS, medium sand; %FS, fine sand; %M, mud; %OM, organic matter. D, dredge; H, haul; a, autumn; w, winter; Sp, spring; Su: summer.
Samples collected with the otter trawl
Multivariate analyses displayed some scattering in the ordination of the samples in relation to seasons of the year, but the CLUSTER did not display clear groups of samples related to seasons, depths or sediment types (Figure 3B). PERMANOVA analyses revealed significant differences in relation to season of the year but not to sediment types or depths (PERMANOVA, factor season: Pseudo-F = 1.95; P < 0.005; factors sediment type and depth: Pseudo-F < 1.35; P > 0.05). A similar pattern was found using biomass (B) data (not displayed in figure), with significant differences between groups of samples according to seasons but not to sediment types or depths (PERMANOVA. factor season: Pseudo-F = 1.67; P < 0.005; factors sediment type and depth: Pseudo-F < 1.38; P > 0.05). Although differences between all seasons were detected, the strongest differences occurred between autumn with the remaining seasons.
The fish Capros aper and the decapod Plesionika heterocarpus with higher abundances in autumn as well as the cephalopod Alloteuthis spp. with higher abundance in winter contributed mostly to the dissimilarity between autumn and winter (SIMPER, av. diss. = 74.91%); C. aper and Alloteuthis spp. with higher abundances in autumn mostly contributed to the dissimilarity between autumn and spring (av. diss. = 87.10%); the fishes C. aper and Trachurus trachurus, Alloteuthis spp. with higher abundances in winter and the flatfish Buglossidium luteum with higher abundances in spring mostly contributed to the dissimilarity between winter and spring (av. diss. = 81.77%); Alloteuthis spp. and P. heterocarpus with higher abundances in summer and C. aper and T. trachurus with higher abundances in winter mostly contributed to the dissimilarity between summer and winter (av. diss. = 79.19%); and finally Alloteuthis spp., P. heterocarpus and the fishes Aphia minuta with higher abundances in summer and B. luteum with higher abundances in spring mostly contributed to the dissimilarity between spring and summer (av. diss. = 83.72%).
The dominant species were similar between seasons and included the fishes C. aper (79,564 ind., 13.3% Db) and T. trachurus (8458 ind., 18.4% Db), molluscs such as the cephalopods Alloteuthis spp. (14,179 ind., 3.1% Db), Sepia elegans (240 ind., 0.16% Db) and Octopus vulgaris (194 ind., 11% Db), decapods such as P. heterocarpus (8109 ind., 12.3% Db) and Liocarcinus depurator (2850 ind., 4.1% Db), and the cnidarian Alcyonium palmatum (231 ind., 3% Db) (Table 2). But these species displayed different dominances between seasons following a similar pattern as in the SIMPER analyses.
Table 2. Top 15 dominant species found in different seasons (December 2013–August 2014) using otter trawl in the circalittoral bottoms of the Bay of Málaga

Total number of individuals (N) and biomass (B) collected (sum over all samples of each season and the total), as well as percentage of abundance (%Da) and of biomass (%Db), are also indicated.
Values of S, N and B did not display significant seasonal differences (one-factor ANOVA: S, F = 0.46; Median test: N, χ 2 = 2.79; B, χ 2 = 2.79, P > 0.05 respectively) (Figure 4E, F, I). The high abundance reached by C. aper in autumn (79.1% Da, 30% Db) affected N, H′ and J′ values, with N displaying a peak value and H′ and J′ displaying low values in this season. Nevertheless, seasonal differences of both H′ and J′ values were non-significant (Median test: H′, χ 2 = 2.79; J′, χ 2 = 3.05, P > 0.05 respectively) (Figure 4G, H). In contrast, some dominant species displayed significant seasonal changes in N and/or B values, with maxima in cold seasons (autumn–winter) such as the fishes C. aper (Median test: N, χ 2 = 12.14; B, χ 2 = 9.86, P < 0.05 respectively), Trachurus mediterraneus (Median test: N, χ 2 = 9.86; B, χ 2 = 9.86, P < 0.05 respectively) and Merlucius merlucius (Median test: N, χ 2 = 9.29, P < 0.05), or in warm seasons (spring–summer) such as the cephalopod Alloteuthis spp. (Median test: N, χ 2 = 9.29, P < 0.05) and the fish A. minuta (Median test: N, χ 2 = 14.58, P < 0.005; B, χ 2 = 11.27, P < 0.05). Based on these results and as observed for the BD data, the community did not display large spatial differences, but it displayed some seasonal changes in the structure of the dominant species.
In the PCA, the first two components accounted for 60% of the total variance and the variability along the PC1 axis was explained by an increase in %MS, %CS and %Gr and a decrease in %FS, while the PC2 axis was explained by an increase in %M and %OM, and a decrease in T (Figure 5B).
Discussion
The present study reports a high number of benthic and demersal species (287 fish and invertebrate species) inhabiting circalittoral soft bottoms (down to 81 m depth) of the Bay of Málaga (northern Alboran Sea). A similar number was reported by Marina et al. (Reference Marina, Rueda, Urra, Salas, Gofas, García Raso, Moya, García, López-González, Laiz-Carrión and Baro2015) (218 species of molluscs, decapods, echinoderms and fish) on circalittoral soft bottoms (down to 72 m depth) at the Special Area of Conservation (SAC) ‘Calahonda-Castell de Ferro’ (NE Alboran Sea), but in that case sampling was only done with a benthic dredge. At infralittoral soft bottoms of the Alboran Sea, García Muñoz et al. (Reference García Muñoz, Manjón-Cabeza and García Raso2008) and Urra et al. (Reference Urra, Gofas, Rueda and Marina2011) documented 60 decapod species and 234 molluscan species, respectively, at the SAC ‘Calahonda’ (NW Alboran Sea) using a benthic dredge, which suggests that the biodiversity of infralittoral soft bottoms of the northern Alboran Sea is higher than that detected in circalittoral ones. Nevertheless, the present study and those cited above detected a higher biodiversity on soft bottoms when compared with similar ones in other parts of the European margin (Van Hoey et al., Reference Van Hoey, Degraer and Vincx2003; Moreira et al., Reference Moreira, Quintas and Troncoso2005; Koulori et al., Reference Koulori, Dounas, Arvanitidis, Koutsoubas and Eleftheriou2006; Lourido et al., Reference Lourido, Gestoso and Troncoso2006; Bolam et al., Reference Bolam, Eggleton, Smith, Mason, Vanstaen and Rees2008; Labrune et al., Reference Labrune, Grémare, Amouroux, Sardá, Gil and Taboada2008; Mastrototaro et al., Reference Mastrototaro, Giove, D'Onghia, Tursi, Matarrese and Gadaleta2008). This highlights the important contribution of the biodiversity of the Alboran Sea in the European context as a result of its geographic location between different biogeographic regions, the heterogeneity and complexity of the habitats existing in the area, and the importance of local oceanographic and hydrological processes (García Raso et al., Reference García Raso, Gofas, Salas Casanova, Manjón-Cabeza, Urra and García Muñoz2010a, Reference García Raso, Salas, García Muñóz and Gofas2010b; Robles, Reference Robles2010). The benthic and demersal community of the Bay of Málaga could also be favoured by a high availability of nutrients and variety of food sources in the bay due to (i) the presence of almost permanent upwellings along the coast of Málaga (Sarhan et al., Reference Sarhan, García-Lafuente, Vargas and Plaza2000), and (ii) inputs from rivers, mostly the Guadalhorce River. These inputs of fine and organic particles would compensate for the relative sedimentary homogeneity of the circalittoral bottoms of the bay, and could play an important role in the distribution of the benthic communities at a small scale as detected in the PCA using BD data. These bottoms with high content of fine sand and mud generally display lower species richness than detritic bottoms with coarser sediments as observed in infralittoral and circalittoral bottoms of the Iberian Peninsula (García Muñoz et al., Reference García Muñoz, Manjón-Cabeza and García Raso2008; Urra et al., Reference Urra, Gofas, Rueda and Marina2011; Martins et al., Reference Martins, Quintino and Rodrigues2013; Marina et al., Reference Marina, Rueda, Urra, Salas, Gofas, García Raso, Moya, García, López-González, Laiz-Carrión and Baro2015). On the other hand, the influence of bottom currents and upwellings in the northern Alboran Sea, driven partly by its orography and the influx of Atlantic waters via the Strait of Gibraltar, seem to promote the existence of deeper faunistic components in shallower environments of the bay (e.g. Alvania testae, a typical species of the bathyal zone but dominant in the MX facies at 70–75 m depth). This phenomenon would explain the occurrence of the MX facies in the Bay of Málaga, which is similar to the ‘unstable bottom assemblage’ found by Marina et al. (Reference Marina, Rueda, Urra, Salas, Gofas, García Raso, Moya, García, López-González, Laiz-Carrión and Baro2015) within the SAC ‘Calahonda-Castell de Ferro’, both of them usually found in the mid-low circalittoral zone of the Mediterranean Sea (Zavodnik et al., Reference Zavodnik, Vidakovic and Amoureux1985; Somaschini et al., Reference Somaschini, Martini, Gravina, Belluscio, Corsi and Ardizzone1998).
The combination of different sampling methods for the collection of organisms with different lifestyles (e.g. demersal, epibenthic, infaunal) in different bottom types and seasons (only OT), increases the possibility of capturing a large number of the species that occur in the bay. Considering all this, this study has increased the knowledge on different faunistic groups that were only previously studied in shallower bottoms of the bay such as fishes (Reina-Hervás & Serrano, Reference Reina-Hervás and Serrano1987a, Reference Reina-Hervás and Serrano1987b) or infauna (Cano & García, Reference Cano and García1982). Furthermore, the study has improved the information on the presence and distribution of rare species, such as the decapod Hippolyte leptometrae, previously cited in the Bay of Biscay and in a few Mediterranean locations (Ledoyer, Reference Ledoyer1969; Udekem d'Acoz, Reference Udekem d'Acoz1996, Reference Udekem d'Acoz2007), and the gorgonian Spinimuricea cf. atlantica that seems to be a common component of some circalittoral soft bottoms of the northern Alboran Sea (Rueda and Moya-Urbano, pers. comm.) and could represent a first record for this area (Carpine & Grasshoff, Reference Carpine and Grasshoff1975).
The lack of strong differences in the sediment types as well as in fauna collected with the benthic dredge in the Bay of Málaga suggests that the potential benthic assemblages identified with multivariate methods could be interpreted as different facies of a single benthic community. The analyses of the epibenthic and demersal fauna collected with the otter trawl also suggested a single community that only changed significantly with the seasons but not in relation to different sediment types of the bay. The studied community could correspond to the ‘Biocénose des vases terrigenes cotieres’ described by Pérès & Picard (Reference Pérès and Picard1964) because a large number of species belonging to different facies of this same community have been found as dominants in this study such as Goneplax rhomboides, Sternaspis scutata, Turritella communis or Leptopentacta tergestina. Pérès & Picard (Reference Pérès and Picard1964) detected some facies within this community such as ‘Facies de vases gluantes’, ‘Facies des formes pivotantes’ and ‘Facies des formes sessiles’ that are characterized by some habitat-forming species such as Veretillum cynomorium, Pennatula sp. and Alcyonium palmatum. These soft bottom octocorals were also detected in the Bay of Málaga but in low numbers, probably due to the intense trawling activity which may have caused their decline over decades as detected in other areas (Ruiz-Pico et al., Reference Ruiz-Pico, Serrano, Punzón, Altuna, Fernández-Zapico and Velasco2017). Other studies in the Alboran Sea and the European margin have found facies of a single community in areas without strong environmental changes, and sometimes these facies are transitions between infralittoral and circalittoral zones or small sedimentary changes (Zavodnik et al., Reference Zavodnik, Vidakovic and Amoureux1985; Somaschini et al., Reference Somaschini, Martini, Gravina, Belluscio, Corsi and Ardizzone1998; Labrune et al., Reference Labrune, Grémare, Amouroux, Sardá, Gil and Taboada2008; Marina et al., Reference Marina, Rueda, Urra, Salas, Gofas, García Raso, Moya, García, López-González, Laiz-Carrión and Baro2015). The facies of the studied circalittoral bottoms could be related to small changes in the sediment (e.g. mud content, organic matter), which have been widely documented to influence the structure and distribution of benthic assemblages along European coastal areas (Moreira et al., Reference Moreira, Quintas and Troncoso2005; Cosentino & Giacobbe, Reference Cosentino and Giacobbe2008; Urra et al., Reference Urra, Gofas, Rueda and Marina2011; Martins et al., Reference Martins, Quintino and Rodrigues2013; Marina et al., Reference Marina, Rueda, Urra, Salas, Gofas, García Raso, Moya, García, López-González, Laiz-Carrión and Baro2015).
In the PCA, the linkage observed between the epibenthic and demersal assemblage and the medium and coarse sand as well as the gravel contents may indicate that minor spatial variability may still occur within the bay, which may influence the occurrence of different facies. Regarding this, Damalas et al. (Reference Damalas, Maravelias, Katsanevakis, Karageorgis and Papaconstantinou2010) investigated fish-habitat associations for 25 non-commercial demersal species in the Aegean sea (E Mediterranean Sea), and reported that most species were associated with specific substratum characteristics (e.g. dry weight percentage of sand-gravel, carbonate content). Sediments with high content of coarse sand and gravels seem to promote rich benthic communities and a wider variety of food sources for demersal species than those sediments with high mud content (Zenetos et al., Reference Zenetos, Christianidis, Pancucci, Simboura and Tziavos1997). Linkages between sediment-related variables and epibenthic and demersal fauna have been documented in other areas of the Mediterranean Sea (Massutí et al., Reference Massutí, Reñones, Carbonell and Oliver1996; Massutí & Reñones, Reference Massutí and Reñones2005; Abad et al., Reference Abad, Preciado, Serrano and Baro2007; Damalas et al., Reference Damalas, Maravelias, Katsanevakis, Karageorgis and Papaconstantinou2010). Moreover, some authors have suggested that the plume of a river can enhance primary production through stratification between marine and fresh waters and wind-induced vertical mixing, increasing ground fish production through the food web (Gaertner et al., Reference Gaertner, Mazouni, Sabatier and Millet1999). Unlike the demersal and epibenthic community, the benthic community of the benthic dredge samples seems to be mainly linked to mud and organic matter contents in the PCA, with high contents of these variables in sampling stations close to the Guadalhorce River. This may highlight the effect of the river for the infauna and small epifauna in the Bay of Málaga, because some deposit-feeders were also dominant in stations located off the mouth of the river (e.g. Abra nitida and Sternaspis scutata). These little sedimentological changes could promote the different facies within the same benthic communities by making some species more dominant than others when the sediment is coarser or muddier.
In the epibenthic and demersal community, changes were only significant regarding seasons and these are probably due to changes of the water masses characteristics and the biology of the species. Seawater temperature, salinity and primary production are environmental drivers that may have a local effect on the biology of several fishes and invertebrates, inducing temporal changes in their populations and, therefore, in the composition and structure of the community (Witman et al., Reference Witman, Cusson, Archambault, Pershing and Mieszkowska2008; Kordas et al., Reference Kordas, Harley and O'Connor2011; Smyth & Elliott, Reference Smyth, Elliott, Solan and Whiteley2016). For example, the squid Alloteuthis spp. displayed maximum abundances in summer in the Bay of Málaga, and this could be due to its seasonal migrations to coastal areas for breeding between spring and autumn (Pierce et al., Reference Pierce, Allcock, Bruno, Bustamante, González, Guerra and Jereb2010). Seasonal horizontal movements could also explain abundance peaks of the small goby Aphia minuta in the bay during spring and summer. This goby (known locally as ‘chanquete’ and overexploited during the last decades) generally occurs in infralittoral bottoms during winter and early spring but moves to circalittoral ones from late spring to autumn (Iglesias & Morales-Nin, Reference Iglesias and Morales-Nin2001). In contrast, the boarfish Capros aper, a planktivorous species and an indicator of high productivity areas (Lopes et al., Reference Lopes, Murt and Cabral2006), displayed an abundance peak in autumn when high values of primary and secondary production generally occur in the bay (Rodríguez et al., Reference Rodríguez, García and Rodríguez1982; Ramírez et al., Reference Ramírez, Cortés, Mercado, Vargas-Yañez, Sebastián and Liger2005). The commercial mackerel (T. mediterraneus) displayed peaks in autumn and winter but usually reproduce in spring and summer months (Junta de Andalucía, 2001), so this could be the result of the post-recruitment as observed in other Mediterranean areas (Massutí & Reñones, Reference Massutí and Reñones2005; Catalán et al., Reference Catalán, Jiménez, Alconchel, Prieto and Muñoz2006).
Bays and gulfs with river inputs have generally been found to represent areas with high demersal productivity (Gaertner et al., Reference Gaertner, Mazouni, Sabatier and Millet1999; Damalas et al., Reference Damalas, Maravelias, Katsanevakis, Karageorgis and Papaconstantinou2010). The abundance and biomass of commercial species collected in this study do not seem to be high, which could be a result of the overfishing experienced in the Bay of Málaga by several fishing fleets (trawling fishing fleet, purse-seine fishing fleet and artisanal fishing fleet) (Baro et al., Reference Baro, Serna-Quintero, García, Giráldez, Marina, Rueda, Gallardo-Núñez, Moya, Laiz-Carrión and García2015). Regarding this, the minimum abundance and biomass values of epibenthic and demersal species were generally recorded in spring and summer, along with increases in the trawling activity within the bay. This trawling activity may not only impact commercial species, but also those sensitive habitat-forming species which were found in low numbers in the bay (e.g. sea-pens), and this contradicts some current directives such as the EU Marine Strategy Framework Directive (Van Hoey et al., Reference Van Hoey, Borja, Birchenough, Buhl-Mortensen, Degraer, Fleischer, Kerckhof, Magni, Muxika, Reiss, Schröder and Zettler2010; IEO-MAGRAMA, 2012; Baro et al., Reference Baro, Serna-Quintero, García, Giráldez, Marina, Rueda, Gallardo-Núñez, Moya, Laiz-Carrión and García2015). The importance of the Bay of Málaga as a nursery and recruitment spot for many small pelagic commercial species (e.g. Engraulis encrasicolus (Linnaeus, 1758), Sardina pilchardus (Walbaum, 1792) is well known (Abad & Giráldez, Reference Abad and Giráldez1990; Marina et al., Reference Marina, Tendero, Rodríguez, Laiz, Serna-Quintero, García and Baro2014). Moreover, the wide variety of faunistic components and demersal species of commercial interest should also be considered for the potential implementation of a fishing reserve with some specific fishing regulations. This may help (1) to avoid and/or decrease the possible effects of overfishing on marine resources, ecosystems and communities, and (2) to improve the quality of the benthic and demersal components of the ecosystem in line with an Ecosystem Approach to Fisheries Management (Goñi, Reference Goñi1998; Hall, Reference Hall1999). Moreover, the creation of a fishing reserve with non-fishable areas would increase the knowledge of the impact suffered by fishing activities in the past and the capacity of recovery of communities and resources in the future.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315419000766.
Acknowledgements
We thank the collaboration of the captains and crew members of the RV ‘Francisco de Paula Navarro’ and ‘Isla de Alborán’, as well as of colleagues from the Instituto Español de Oceanografía who helped in the expeditions and processing of some samples. We also thank the Secretaría General de Pesca from the Spanish Government for providing the VMS data for this study.
Financial support
This study was developed under the collaboration agreement between the ‘Consejería de Agricultura, Pesca y Desarrollo rural’ and the ‘Consejería de Economía, Innovación, Ciencia y Empleo’ of the Junta de Andalucía (Spain) and the Instituto Español de Oceanografía (IEO), within the framework of the research projects entitled ‘Estudio previo para la protección, ordenación y determinación de una reserva de pesca en el área marítima de la Bahía de Málaga’ (REMAN-REMALA), and ‘Transporte de sedimentos en la plataforma continental de Andalucía oriental: Observación multiescalar, modelado y simulación numérica’ (TESELA).









