INTRODUCTION
The interaction of cetaceans with fisheries is a worldwide problem and all fishing gear is believed to be involved to some extent. Amongst all the interactions, the depredation of fisheries by cetaceans (Northridge & Hoffman, Reference Northridge, Hofman, Twiss and Reeves1999; Reeves et al., Reference Reeves, Read and Notarbartolo-di-Sciara2001) is of greatest concern since it may cause negative economic consequences for the fisheries concerned. Cetaceans can cause direct damage by stealing fish from the net, damaging and spoiling fish in the net and also reducing the catch rate (Reeves et al., Reference Reeves, Read and Notarbartolo-di-Sciara2001; Lauriano et al., Reference Lauriano, Fortuna, Moltedo and Notarbartolo di Sciara2004). These factors are responsible for making fishermen angry, who in some cases, try to scare cetaceans away employing dangerous methods, such as dynamite (Reeves et al., Reference Reeves, Read and Notarbartolo-di-Sciara2001; de Stefanis, Reference de Stephanis2004) or asking for dolphin culling (Lauriano et al., Reference Lauriano, Fortuna, Moltedo and Notarbartolo di Sciara2004). These interactions can occur along any coastal areas where artisanal fisheries take place. Among cetaceans, the common bottlenose dolphin (Tursiops truncatus) is believed to be the main culprit (Reeves et al., Reference Reeves, Read and Notarbartolo-di-Sciara2001), due to its opportunistic feeding habits (Barros & Odell, Reference Barros, Odell, Leatherwood and Reeves1990; Blanco et al., Reference Blanco, Salomon and Raga2001) and its coastal distribution (Notarbartolo & Demma, Reference Notarbartolo di Sciara and Demma1994; Gannier, Reference Gannier2005) which overlaps with artisanal fisheries.
In the Mediterranean Sea this problem has been reported in several coastal zones: Greece (Conides & Papacostantinou, Reference Conides and Papaconstantinou2001), Spain (Silvani et al., Reference Silvani, Raich and Aguilar1992; Gazo et al., Reference Gazo, Gonzalvo and Aguilar2008), Tunisia (Naceur et al., Reference Naceur, Gannier, Bradai, Drouot, Bourreau, Laran, Khalfallah, Mrabet and Bdioui2004), Morocco, Libya (Hamza, personal communication), Cyprus (Reeves et al., Reference Reeves, Read and Notarbartolo-di-Sciara2001) and Italy (Cannas et al., Reference Cannas, Fadda, Lenti, Massidda and Pinna1994; Reeves et al., Reference Reeves, Read and Notarbartolo-di-Sciara2001). Despite the widespread occurrence of depredation, only few attempts have been made to evaluate the phenomenon along the Italian coasts (e.g. Sardinia: Lauriano et al., Reference Lauriano, Fortuna, Moltedo and Notarbartolo di Sciara2004; Diaz Lopez, Reference Diaz Lopez2006).
The general lack of knowledge on the real extent of depredation makes any attempt to manage or even mitigate the conflict rather difficult. Although mitigation has been attempted (see Lauriano & Bruno, Reference Lauriano and Bruno2007), so far, no conclusive evidence that it was successful has been reported.
The Italian peninsula and its islands are characterized by a conspicuous coastal zone extension with a wide range of habitats which determine a broad diversity of ad hoc fishing gear which have been adapted over generations to the local context. Accordingly, the Italian artisanal fishery is a highly diversified system and gathering complete information on the artisanal fisheries and on depredation would require a considerable effort in terms of the number of on-board observers and the number of fishing trips, with very high costs. A way to overcome these limitations is to conduct interview surveys; even if these indirect methods do not provide quantitative data (López et al., Reference López, Pierce, Santos, Gracia and Guerra2003). The in situ interviews, by means of face to face contacts with fishing crew, are a handy and reliable method to infer preliminary information (Lien et al., Reference Lien, Stenson, Carver and Chardine1994; Wise et al., Reference Wise, Silva, Ferreira, Silva and Sequeira2007).
In this study we report results from in situ interview (Lien et al., Reference Lien, Stenson, Carver and Chardine1994) made in Italy during 2002, aimed at determining the extent of the interaction between dolphins and artisanal fishery, monitoring fishing gear involved and evaluating the effects of such interaction on both fishing gear and on catches. Moreover, we attempted to derive a regional depredation ranking table.
MATERIALS AND METHODS
Study area and data collection
The fisherman interviews were carried out in 2002 at landing sites along thirteen coastal administrative subdivisions (regions) of Italy (Figure 1). A two-stage sampling regime was conducted: firstly, fishing harbours were sampled according to their representativeness; secondly, boats were randomly sampled within each fishing harbour selected. All the interviews were carried out by experienced professionals who had previous knowledge of both the fishery and the fishermen in their own area. A minimum sample of 5% of interviews was required; moreover, if less than 100 fishing boats were present in the harbour the minimum sample was set to 5 interviews. In order to ensure the representativeness of the data output, the interviews were weighted in each fishing harbour according to the ratio between the local overall fleet and the local sample dimension. Fishermen were asked to record fishing activities over the last year. Questions on fishing activities were related to: (i) fishing gear deployed according to the season and target species; and (ii) sightings of interacting fauna during fishing activities, damage caused both to the fish catches and to the fishing gear and frequency of such interactions. The interacting fauna was composed of: common bottlenose dolphin, striped dolphin (Stenella coeruleoalba), short beaked common dolphin (Delphinus delphis), loggerhead turtles (Caretta caretta), tuna-like fishFootnote 1(Thunnus spp., Euthynnus alletteratus, Auxis rochei and Sarda sarda), sun fish (Mola mola), and sharks of undetermined species. The fishing gear investigated (Nedelec & Prado, Reference Nedelec and Prado1990) were the following:
• set gillnet (GNS) and trammel nets (GTR);
• set long line (LLS); and
• encircling gillnet (GNC).
In Italian, the common names for the striped and the bottlenose dolphins are, respectively, Stenella and Tursiope, while the word ‘dolphin’ is used for the common dolphin (Notarbartolo & Cagnolaro, Reference Notarbartolo di Sciara and Calognaro1987). So, even if fishermen were able to distinguish between the different dolphin species, they more commonly use the term ‘dolphin’. Accordingly, in order to carry out the analysis we include all the dolphin species within the ‘dolphin category’ (hereafter called dolphin). Among the fishery target species, seven ‘fauna classes’ were considered, based on their main ecological niches: cephalopods, crustaceans, pelagic fish, benthic fish, nektonic–benthic fish, and nektonic fish.

Fig. 1. The Italian regions (in grey) where the survey was carried out.
In order to study the association between the interacting fauna and the fishing gear we used the Chi-square (χ2) test, which can then be evaluated on a frequency table (see Table 2). The dependency between these variables was further investigated by analysing the contribution to this index of several factors that combine the different values of the fauna classes, of the fishing gear and of the damage typologies. To this extent we used correspondence analysis (CA; Benzecri, Reference Benzecri1973), because of its ability to reveal relationships that would not be detected in a series of pair-wise comparisons of variables. Due to its geometric nature, CA could cause a loss of information; hence we fully explained the χ2 index by plotting the different values of the variables in a bi-dimensional space (a plane) if one of the two variables had three different values. Using the same approach, we also investigated the relationship between interacting fauna and GNS/GTR damage typologies.
As a further step, we took into consideration a ‘model of explanation’ between the interacting fauna and the regions (considered as explanatory variables) on gear damage. The model can usefully quantify the effects of the explanatory variables related to the damage; in this case we adopted a logistic regression (Cox & Snell, Reference Cox and Snell1989) in order to estimate the corresponding probability of damage for each class that belongs to the considered fauna and for each region. Letting π be the unknown probability of the damage (GNS/GTR and catches), the mathematical formulation of the model is:

where:
- β0
is the mean general effect (intercept);
- βRi
is the quantification of effect of each factor of the variable regions X Ri (Ri = 1,2,..10);
- βTF
is the quantification of effect of the variable tuna fish sighting X TF;
- βTF
is the quantification of effect of the variable sea turtle sighting X ST;
- βSF
is the quantification of effect of the variable sun fish sighting X SF;
- βS
is the quantification of effect of the variable shark sighting X S;
- βD
is the quantification of effect of the variable dolphin sighting X D.
Positive estimate of β i corresponds to an increase of ![]() and therefore to the probability of the damage π. βi estimates express the influence of the variables X i on the presence of damage. The goodness of fit of the logistic model with our real data was evaluated, in order to verify whether the model itself can be used as a good representation of the phenomenon. An interaction risk score was set up by ranking both the fishing gear damage frequency and the fish catch damage.
and therefore to the probability of the damage π. βi estimates express the influence of the variables X i on the presence of damage. The goodness of fit of the logistic model with our real data was evaluated, in order to verify whether the model itself can be used as a good representation of the phenomenon. An interaction risk score was set up by ranking both the fishing gear damage frequency and the fish catch damage.
Overall, 1497 interviews were carried out in 245 fishing harbours along the Italian coast (i.e. 11.09% of the artisanal fleet) (Table 1).
Table 1. Number of fishing harbours and fishing boats investigated for each region, with information on the total fleet coverage.

Table 2. Quota (%) of the distribution of damaged fishing gear and of target species, for each species of the interacting fauna.

RESULTS
Seasonal trends in fishing gear
Set gillnets and trammel nets (GNS/GTR) were the most commonly used fishing gear (80.6%), followed by long lines (21.4%) and encircling gillnets (GNC) (10.1%). GNS/GTR was the most widely used gear category in all the Italian regions and throughout the year, while LLS and GNC categories had a more marked seasonality and were used only in some regions (Figure 2). Given that several fishing gear categories can be deployed and combined in the same season by each fishing vessel, the sum of frequencies is greater than 100%.
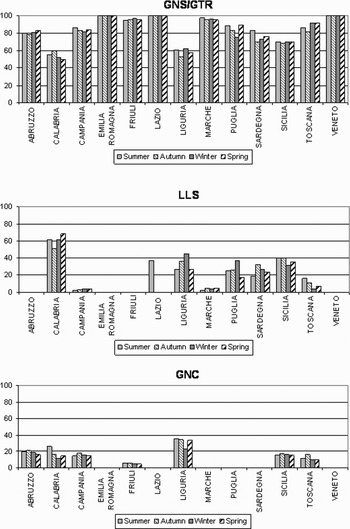
Fig. 2. Seasonal use of fishing gear within the Italian regions. GNS/GTR, set gillnet and trammel nets; LLS, long line set; GNC, encircling gillnet.
Relationships among damaged gear, target species and the ‘interacting’ fauna
The CA among the interacting fauna, the target species and the damaged fishing gear reveals that a bi-dimensional space explains 94% of the total χ2 index. A graphical representation displays the association between fauna, target species and damaged fishing gear (see Figure 3). The first axis distinguishes between the GNS/GTR and the other damaged fishing gear. As reported in Table 3 dolphins are well represented by the first axis (the square of the cosine between dolphins and the first axis is 0.94) and in the graph, they are close to GNS/GTR position. This means a high probability of dolphin–fishery interaction with such gear. Shark, crustacean, sea turtle and sun fish, instead, are well represented by the second axis, suggesting weak interactions with fishing gear. Among the target species, both the benthic and benthic–nektonic fish are projected near GNS/GTR.

Fig. 3. Plot of the damaged fishing gear, the target species and of the interacting fauna, as derived by the correspondence analysis. □, fishing gear; ▵, interacting fauna; ○, target species.
Table 3. Projections and square of cosine for the value of damaged fishing gear, target species, and interacting fauna for the correspondence analysis.

GNS/GTR damage typologies and their relationship with the interacting fauna
The relationship between the GNS/GTR damage typologies and the interacting fauna is shown in Figure 4. Because the damage typologies allow only three possibilities, CA does not involve any data reduction and explains 100% of the total χ2 index. From the resulting plot it is possible to detect an association between holes in the net caused by dolphins and sun fish which are all well represented by the second axis (see Table 4).

Fig. 4. Plot of the GNS/GTR damage typologies versus the interacting fauna, as derived by the correspondence analysis. ▵, interacting fauna; ○, target species.
Table 4. Projections and their cosine square for the value of the GNS/GTR damage typologies and the interacting fauna for the correspondence analysis.

GNS/GTR damage
By using the logistic regression model the influence of both interacting fauna and the regions in which GNS/GTR damage occurs was analysed; the results are shown in Table 5. All the variables were significant at the level of 95% except for tuna-like fish and loggerhead turtle, neither of which has a statistical influence on the GNS/GTR. The damage in the GNS/GTR is greatest in Sardinia, Campania, Friuli and Apulia. The dolphin category is principally responsible for damaging the fishing gear. The results obtained by the logistic model are significant (81.5%).
Table 5. Parameter estimate, standard error and P value for the logistic model used to analyse the influence of regions and interacting fauna on fishing gear damages.

Frequency of the interaction
Amongst the whole fleet, 66.4% of boats report GNS/GTR damage with dolphins sightings (Table 6); among the regions, Friuli and Apulia are those showing the highest values of damaged GNS/GTR (91.6% and 87.5% respectively). In Italy, 72.2% of the fishing boats report fish damaged in the GNS/GTR with corollary dolphin sightings (Table 6). The regions most affected were Sardinia and Campania. In Sardinia, frequencies of net damage were reported in 75.8% of the fleet and damage to catches was always recorded when dolphins were sighted. In Campania, interactions were registered in 83.1% of all the cases and damage to catches occurred in 93.0% of the interactions.
Table 6. Ratio (%) of the fishing boats that sighted dolphins and reported respectively GNS/GTR damaged (first column) and fish damaged in the GNS/GTR (second column).

Interaction regional ranking
By using the data on frequency of damage to fishing gear and to catch, a regional risk ranking table (Table 7) was estimated. From the table, it is evident that outstanding incidences of depredation occurred in Friuli, Campania, Sardinia and Apulia.
Table 7. Interaction regional ranking (values sorted by descending risk).

DISCUSSION
This study represents the first attempt to outline the extent of depredation in the Italian artisanal fishery, by means of investigating the fishing gear involved and the regions mostly affected. Due to the high diversity and to the complexity of the fishery along the Italian coast, depredation was inferred through in situ interviews (Lien et al., Reference Lien, Stenson, Carver and Chardine1994). Fishermen recorded their fishing activity over the previous year. The nature of the census itself constrained us to make the questionnaire as simple as possible, asking only basic and clear questions in order to avoid complex and useless answers. Accordingly, and considering that the interview data are subject to biases, it should be taken into account that this study is intended to provide a rough indication on the phenomenon and show areas where future studies should be concentrated.
The relationship between the fishing gear, their target species and the interacting fauna did display a connection between the set gillnet (GNS) and trammel nets (GNS/GTR), with the benthic and benthic–nektonic fish and dolphins; whereas the set long line (LLS) and the encircling gillnet (GNC), did not show significant dolphin interaction. Altogether, these results are consistent with general information available for the Mediterranean region, in which conflicts with dolphins were reported primarily in bottom set trammel and gill-nets (Reeves et al., Reference Reeves, Read and Notarbartolo-di-Sciara2001).
Elsewhere in the world, long line interactions are known to occur with the sperm whale (Ashford et al., Reference Ashford, Rubilar and Martin1996; Hill et al., Reference Hill, Laake and Mitchell1999), killer whale (Orcinus orca) (Nolan et al., Reference Nolan, Liddle and Elliot2000; Karpouzli & Leaper, Reference Karpouzli and Leaper2004) and other large pelagic species (Donoghoue et al., Reference Donoghoue, Reeves and Stone2002). In the Mediterranean Sea the only information available does indicate a cetacean by-catch with long lines (SEC, 2002) rather than depredation. Besides LLS, depredation in the encircling gillnet fishery is not common in Italy, although conflict is registered in southern Italy in the Euthynnus alletteratus fishery and in northern Sardinia in the anchovy (Engraulis engrasicoulus) fishery (Lauriano, unpublished data); in all cases it is reported that dolphins cause the fish schools to disperse, which annoys the fishermen. Furthermore, amongst the fishing gear that might be subjected to depredation, there is the jigging line for the mesopelagic squid, deployed in some areas only and of minor economic importance. This gear type has been reported to interact with the striped and the Risso's dolphin (Grampus griseus) in the Gulf of Naples (Notarbartolo di Sciara, personal communication) and with the bottlenose dolphin in Sicily (Lauriano, unpublished data).
Concerning the dolphin species involved in the GNS/GTR depredation, it should be noted that this fishing gear is commonly used by small vessels and is mainly concentrated along coastal areas; therefore, we can conclude that any regular depredation is likely only with the bottlenose dolphin, the only coastal delphinid with a high degree of overlap with the fishing ground of this artisanal fishery (Notarbartolo & Demma, 1994). Amongst other dolphin species, the striped dolphin shows an offshore distribution (Aguilar, Reference Aguilar2000) and interacts mostly with pelagic fisheries and the main problem is entanglement in pelagic fishery (Magnaghi & Podestà, Reference Magnaghi and Podestà1987; Silvani et al., Reference Silvani, Raich and Aguilar1992; Aguilar, Reference Aguilar2000) rather than depredation. The short beaked common dolphin, which is an epipelagic species and a mesopelagic feeder, is similarly distributed in both neritic and pelagic waters (Bearzi, et al., Reference Bearzi, Holcer and Notarbartolo di Sciara2004).
Depredation seems to represent a major problem in the northern and southern Adriatic Seas (corresponding to the Friuli and Apulia regions) and in the Tyrrhenian and Sardinian Seas (Campania and Sardinia), where the GNS/GTR is prevalent (UNIMAR, 2001). In the northern Adriatic Sea, the conflict between dolphins and fisheries has been reported at least since the second half of the 19th Century and was so acute that it promoted culling on the eastern Adriatic coast (Crnkovic, Reference Crnkovic1958), which became the main cause of dolphin mortality (Bearzi et al., Reference Bearzi, Holcer and Notarbartolo di Sciara2004). To date the bottlenose dolphin is the only cetacean in the area (Bearzi et al., Reference Bearzi, Holcer and Notarbartolo di Sciara2004) where a high interaction rate occurs in the autumn (ICRAM, Reference Giovanardi and Cornello2004) with the set bottom trammel nets, commonly deployed to catch solea (Solea vulgaris) and cuttlefish (Sepia officinalis) (UNIMAR, 2001).
In both Campania and Apulia, along the southern Tyrrhenian and southern Adriatic Seas respectively, the set bottom trammel net is the most commonly used fishing gear to catch striped red mullet (Mullus surmuletus), cod (Merluccius merluccius) and cuttlefish in the Tyrrhenian Sea, and scorpion fish (Scorpena spp.), octopus (Octopus vulgaris), cuttlefish, mullet, wrasse (Labrus spp.) and bogue (Boops boops) in the Adriatic Sea (UNIMAR, 2001). In Sardinia, the fishery is generally highly specialized and the set bottom trammel net is the main fishing gear employed on a seasonal basis to catch scorpion fish, cuttlefish (Sepia spp.), striped red mullet and lobster (Palinurus elephas) (UNIMAR, 2001). Moreover, in north-western Sardinia, interactions occurred mainly during the autumn, when the striped red mullet trammel nets were set (Lauriano et al., Reference Lauriano, Fortuna, Moltedo and Notarbartolo di Sciara2004), whilst in north-eastern Sardinia the gillnet depredation did not show inter-seasonal variation (López et al., Reference López, Pierce, Santos, Gracia and Guerra2003).
The evidence of the magnitude of the depredation in the four regions does not mean that depredation produces an equal level of impact in all areas; consequently differences in economic impact may be expected according to the respective regional fishery characteristics. As a matter of fact, when considering the economic effects of depredation, it should be noted that the economic loss might be even heavier in a monospecific fishery than in a differentiated fishery. Small scale fishery in the northern Adriatic Sea is mainly based upon a single system, yet fishing activity is normally supplemented by sea farming in internal waters or by shellfish farming in lagoons (UNIMAR, 2001). In other regions, such as Sardinia and Campania, the integrating of fishing activities with other fishing/aquaculture systems is less important, as fishery and tourism (pescaturismo) are often combined activities.
Recently, a general increase in the perception of the detrimental effect of depredation was an impetus in promoting ‘self made’ solutions which were shared between fishermen. For instance, the acoustic deterrent device (ADD) became widespread despite the international recommendations stating the need for caution when using such devices (Reeves et al., Reference Reeves, Read and Notarbartolo-di-Sciara2001; SEC 2002). Moreover, several studies carried out on the use of acoustic devices in the Mediterranean Basin (Spain (Gazo et al., Reference Gazo, Gonzalvo and Aguilar2008); Italy (Goodson et al., Reference Goodson, Datta, Dremiere and Di Natale2001); Greece (Northridge et al., Reference Northridge, Vernicos and Raitsos-Exarchopolous2003); Morocco (Zahri et al., Reference Zahri, Abid, Elouamari and Abdellaoui2004)) did not provide any conclusive evidence that ADD was effective, which eventually, even the fishermen themselves considered as an inappropriate means of mitigating depredation (Lauriano & Bruno, Reference Lauriano and Bruno2007). Moreover, from an economic point of view, the ADD could be an expensive solution to the problem, especially if no detailed information is provided on the fish species involved, on the seasonality of interactions and on the estimated economic loss due to depredation.
Conflicts frequently occur on a seasonal basis rather than all year round. Since the damage is caused during a very short period and involves just a single type of fishing gear, this should persuade fishermen to adopt a solution during a specific season rather than all year round. Future studies are needed in order to fill knowledge gaps regarding the seasonality of depredation, which was not covered by the present work.
ACKNOWLEDGEMENTS
We wish to thank the people who carried out the interviews, the fishermen and the three Italian fishery consortiums (CIRSPE, ICR Mare and Consorzio Mediterraneo) and those involved in data entry and management of raw data: S. Bruno, M. Cusimano, C. Fortuna, L. Lauricella and F. Vaiano. We would like to thank Randall R. Reeves and Giuseppe Notarbartolo di Sciara for their helpful comments and suggestions. A special thank you goes to Tundi Agardy and Mark Simmonds for help with the English grammar. We also thank the anonymous referees for their comments on this manuscript and the Executive Editor for help in revising the text.













