INTRODUCTION
Early embryogenesis of echinoderms is marked by the development of the hyaline layer that coats the external surface of embryos and is thought to protect the embryo, play an important role in embryo cell adhesion, stabilize the blastomeres during morphogenesis, provide positional information and regulate nutrient intake (Noll et al., Reference Noll, Matranga, Cervello, Humphreys, Kuwasaki and Adelson1985; Cervello & Matranga, Reference Cervello and Matranga1989; Campbell & Crawford, Reference Campbell and Crawford1991). Previous studies have shown that formation of the hyaline layer occurs due to exocytosis of extracellular embryonic matrix (ECM) by the cortical granules (Crawford & Abed, Reference Crawford and Abed1986; Reimer & Crawford, Reference Reimer and Crawford1995; Maghsoodi & Crawford, Reference Maghsoodi and Crawford2005). However, there are some other structures, such as the secretory vacuoles and secretory vesicles situated along the apical periphery of embryonic cells (Crawford et al., Reference Crawford, Campbell and Reimer1997; Pang et al., Reference Pang, Crawford and Maghsoodi2003; Maghsoodi & Crawford, Reference Maghsoodi and Crawford2005) and the relation of these to ECM secretion remains unknown.
Moreover, the yolk granules have to be considered in terms of ECM. It is known that in the sea urchin a substance that appears to be synthesized by limited proteolysis of yolk granules is located in the ECM and is responsible for specific intercellular adhesion (Gratwohl et al., Reference Gratwohl, Kellenberger, Lorand and Noll1991). Besides, a 32-kDa protein component of the embryonic ECM, HCl-32, was found in sea urchin yolk granules (Mayne & Robinson, Reference Mayne and Robinson1998). Probably the yolk granules of sea stars may also contain ECM components and this should be highlighted.
In this work we intend to study whether in the gastrula of the starfish Pisaster ochraceus the hyaline layer epitope could be contained in the cortical granules, secretory vacuoles, secretory vesicles as well as granules of yolk.
MATERIALS AND METHODS
Rearing of embryos
Ripe adult P. ochraceus collected from Saanich Inlet, British Columbia were kept in tanks of running seawater at the aquatic facility of the University of Victoria. The animals were kept under constant low light conditions to inhibit spawning and were fed with mussels. When cultures were desired the gonads were removed surgically from the adults and the animals were immediately euthanized by placing them in a –20°C freezer. The ovaries were washed with filtered seawater and placed in seawater containing 1-methyladenine for 75–90 minutes to mature the eggs. Once the germinal vesicles had broken down (indicating egg maturity) the mature eggs were washed by pipetting them into 300 ml of freshly aerated, filtered seawater and allowing them to settle. The process was repeated twice. Finally sufficient eggs to cover 1/2 the bottom of a 400 ml plastic beaker were suspended in 300 ml of seawater.
Testis were placed in plastic Petri dishes and allowed to contract to release ‘dry’ sperm. Sufficient ‘dry’ sperm was suspended in freshly aerated filtered seawater to create a slightly turbid suspension. The sperm were checked for activity using a Leitz phase contrast microscope and each beaker of eggs was fertilized with 5–10 drops of the active sperm suspension. The contents of the beakers were mixed using a meat baster and placed in a 12°C incubator. Once the eggs had settled (1–2 hours) most of the seawater was poured off and replaced with fresh aerated, filtered seawater and the eggs were allowed to develop at 12°C. The embryos were examined daily with a Zeiss Jena dissecting microscope until the mid-gastrula stage (Figure 1) was reached.

Fig. 1. A semithin section through the gastrulae of the starfish Pisaster ochraceus stained with anti-hyaline layer antibodies and studied by fluorescent light microscopy. Staining is seen in the hyaline layer, lining of the archenteron and blastocoel. Arrowheads show the hyaline layer-positive granules located in the cytoplasm of the embryonic cells; ar, archenteron; arl, lining of archenteron; b, blastocoel; ec, ectoderm embryonic cells; hl, hyaline layer.
Purification of hyaline layer
The ECM of the hyaline layer was purified in accordance with a routine protocol (see Reimer & Crawford, Reference Reimer and Crawford1995). Cultures grown as above were harvested at the gastrula stage by gentle centrifugation (speed 3 on a clinical centrifuge for 3 minutes). The packed embryos were stored frozen at –20°C. The previously stored gastrulae were thawed quickly and solubilized in an equal volume of extraction buffer (TBS with 1% Brij 56 (polyoxethylene 10 cetyl ether (Sigma)) containing the protease inhibitors 1 mM iodoacetamide, 1 mM PMSF (phenylmethlysulphonyl flouride) (Sigma), 1 µg/ml pepstatin A (Sigma), homogenized at 4°C with 10 plunges of a Dounce tissue homogenizer followed by sonication on a Fisher probe sonic dismembrenator for 15 seconds at 45% power. The homogenate was then extracted for 30 minutes at 4°C, centrifuged at 100,000 × g for 1 hour. It was then dialysed against 20 mM Tris–HCL, 0.5 M NaCl, pH 7.4 containing the same protease inhibitor listed above overnight at 4°C. To prepare an antigen preparation that was enriched in ECM the dialysed extract was applied to a 10 ml Concanavalin A–Sepharose 4B column that had been equilibrated with 10 column volumes of TBS buffer and washed with 3 volumes of elution buffer (20 mM Tris–HCL, 0.5 M NaCl, pH 7.4 containing 0.5 M α-methyl mannoside). The column was washed with 10 column volumes of washing buffer (20 mM Tris–HCL, 0.5 M NaCl, pH 7.4) and eluted with 5 ml of the elution buffer. The antigen preparation was then concentrated to 4 mg/ml of protein mixed with an equal volume of Freund's incomplete adjuvant.
Preparation of antiserum
The hyaline layer antiserum was generated in accordance with a routine protocol (see Reimer & Crawford, Reference Reimer and Crawford1995). Antigens were isolated from gastrula larvae and injected intraperitonealy into 4 week old BALB/c mice. One month following the first injection the mice were boosted by second injection. Five days later, antiserum from a tail bleed was diluted in 1/100 in PBS-blotto. For control this antiserum part was used as a primary antibody to stain a 1 µm thick section of a P. ochraceus gastrula that had been fixed by freeze substitution and embedded in LR White (Polysciences, Warrington, Pennsylvania). The slide was then rinsed three times in PBS-blotto and stained with FITC labelled goat anti-mouse IgG diluted 1:256 in PBS-blotto for a further 1 hour at room temperature and observed on a fluorescence microscope. Controls consisted of sections in which non-immunized mouse serum was substituted for that from the immunized mouse.
Spleens were harvested from animals that showed positive staining and hybridomas were produced according to published methods (Reimer, Reference Reimer1994). Clones were checked for antibody activity by staining sections as described above using the tissue culture medium from each isolated clone as the primary antibody. Clones that exhibited a strong staining reaction with the hyaline layer were then expanded into 10 cm plastic Petri dishes. Once the cultures had reached logarithmic growth, the tissue culture medium was harvested, the cells were removed by centrifugation (800 × g for 10 minutes) and the media were stored at –20°C. For staining of sections the media were used directly.
Freeze substitution
Immunogold studies were performed on material fixed using the freeze substitution technique (Campbell et al., Reference Campbell, Crawford and Reimer1991; Crawford & Burke, Reference Crawford, Burke, Ettensohn, Wessel and Wray2004). Embryos for immunogold studies were reared and concentrated as described above. For fixation, the embryos were incubated in seawater containing 15% 2, 3 butanediol (Sigma) for 15–20 minutes in a conical centrifuge tube at 4°C. During this time the embryos settled to the bottom of the tube and most of the seawater was removed. Roughly 1 µl of the concentrated embryos was pipetted on to a freeze fracture grid held in very fine tipped cross-closing forceps. As much of the remaining seawater as possible was removed from the grid with the tip of a piece of filter paper. The grid was then plunged into liquid propane in liquid nitrogen. Following this 3–4 grids were rapidly transferred to absolute ethanol saturated with Alcian blue (Marivac/Caneco, Halifax, Nova Scotia) at –85°C and maintained at –85°C for 5 days to undergo substitution. The grids were then slowly warmed to room temperature and embedded in LR White (Polysciences, Warrington, Pennsylvania). Embryos were cut from the polymerized blocks, oriented and mounted on aluminium stubs with epoxy glue, sectioned and mounted on slot grids as described below.
Transmission electron microscopy
Gastrula stage larvae were concentrated by gentle centrifugation (250 × g for 1 minute) and the surplus water was removed by pipette. The cell suspension was fixed in 2.5% glutaraldehyde buffered with 0.2 M cacodylate buffer (pH 7.4). The osmomolarity of solution was adjusted to 1250 milliosmoles by adding NaCl. The materials were then post-fixed for 2 hours with 1% OsO4 buffered with seawater. After dehydration in alcohol and acetone, the fixed cell suspension was embedded in Epon in large flat moulds. The Epon was polymerized for 48 hours at 60°C and the embryos were located using a dissecting microscope, cut out of the plate with a coping saw, oriented and mounted on the metal stubs. The embryos were then sectioned on a Sorvall Porter-Blum ultramicrotome MT-1 using a diamond knife. The sections were collected on metal slot grids covered by Formvar (Polysciences, Warrington, Pennsylvania) film, stained with uranyl acetate and lead citrate and photographed on a Hitachi H-7000 TEM.
Immunological staining
Material for immunofluorescence studies was sectioned at 1 µm and mounted on glass slides. The sections were incubated for 10–15 minutes in phosphate buffered saline containing 0.2% Carnation instant milk powder (PBS-blotto). They were then treated for 1 hour at room temperature in 1–2 drops of anti-hyaline layer hybridoma supernatant (full strength tissue culture medium in which high concentrations of hybridoma cells had been grown) and rinsed in two 10–15 minute changes of PBS-blotto. Following this the sections were incubated in FITC goat anti-mouse serum diluted 1:256 in PBS-blotto for 1 hour, rinsed in PBS and mounted in Gelvatol (Mosanto). Controls consisted of a normal (non-immunized) mouse serum in place of the anti-hyaline layer tissue culture medium.
The grids were incubated on droplets laid out on sheets of Parafilm (Fisher Scientific, Ottawa) in a moist chamber. The grids were then incubated for 10 minutes on two changes of PBS-blotto. This was followed by 1 hour incubation in the primary antibody which consisted of anti-hyaline layer hybridoma supernatant. The grids were then washed in PBS-blotto and incubated for 1 hour in the secondary goat anti-mouse IgG (Sigma) antibody conjugated to colloidal gold (size 20–25 nm) prepared according to the methods of Slot & Geuze (Reference Slot and Geuze1985). Following this the grids were washed in PBS followed by a rinse in distilled water. They were further stained with uranyl acetate and lead citrate and photographed as above. Controls were made by substituting normal mouse serum instead of primary antibody solution.
RESULTS
Histological sections of gastrulae show strong reaction of anti-hyaline layer antibody with the hyaline layer which surrounds the outside of the embryo and lining the archenteron. Besides, it reacted with some ECM material within the blastocoel. Moreover, the antibody interacted with numerous small granules in the cytoplasm of ectoderm cells and cells of archenteron (Figure 1). An analysis by transmission electron microscopy (TEM) has shown specific reaction of antibodies with substances of the hyaline layer (Figure 2A), intercellular spaces (Figure 2B) and fibrils of the blastocoel matrix (Figure 2C).

Fig. 2. Transmission electron microscopy showing the extracellular sites of hyaline layer epitope localization in the gastrula of the starfish Pisaster ochraceus. The gastrulae stained by anti-hyaline layer antibodies are labelled with colloidal gold in the areas of (A) hyaline layer, (B) intercellular spaces and (C) blastocoel; the colloidal gold particles are shown by arrowheads; ec, ectoderm embryonic cells; b, blastocoel; is, intercellular space; ps, perivitelline space.
Examination of the embryonic cells using TEM demonstrated the random presence of cortical granules. These granules consisted of dense material patches located in more electron-lucent sphere (Figure 3A). Immunogold studies showed that these granules labelled with the anti-hyaline layer antibody and that the label was located both in the electron-lucent and electron-dense areas (Figure 3B).

Fig. 3. Transmission electron microscopy showing the intracellular sites of hyaline layer epitope localization in the gastrula of the starfish Pisaster ochraceus. (A) A cortical granule; (B) a cortical granule labelled with anti-hyaline layer antibodies; the colloidal gold particles are shown by an arrow; (C) a secretory vacuole prior to secretion in the apex of the ectodermal cell; (D) the secretory vacuole stained by anti-hyaline layer antibodies; the colloidal gold particles are marked by arrow. Note that the perivitelline space is very heavily labelled; the colloidal gold particles in perivitelline space are pointed by arrowheads (D); (E) the secretory vesicles prior to secretion in the apex of the ectodermal cell; (F) the secretory vesicles stained by anti-hyaline layer antibodies; the colloidal gold particles are marked by arrow; the colloidal gold particles in perivitelline space are shown by arrowhead; cg, cortical granule; ps, perivitelline space; va, secretory vacuole; ve, secretory vesicle.
The secretory vacuoles that are often seen contacting cell surface (Figure 3C) were labelled by antibody which also stains the extracellular matrix (Figure 3D). The secretory vesicles that are often seen along cell surface (Figure 3E) were also labelled with hyaline-layer specific antibodies (Figure 3F).
Both the ectodermal cells and cells of archenteron contain a large number of yolk granules that are peculiar in having either compact or loosened contents (Figure 4A). The loosened granules were frequently observed in close association or even direct contact with cell membrane in both the lateral (Figure 4B) and basal parts of the cells (Figure 4C). The immunogold technique demonstrated that these yolk granules labelled with anti-hyaline layer antibodies (Figure 4D). The darker staining yolk granules having a compact content did not label with this antibody (Figure 4E).
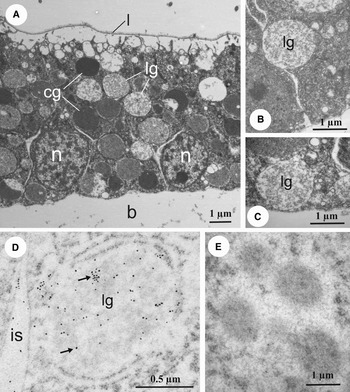
Fig. 4. The yolk granules in the gastrulae of the starfish Pisaster ochraceus. (A) Transmission electron microscopy (TEM) of ectodermal cells showing the samples of compact yolk granules and loosened yolk granules; (B, C) TEMs of two ectodermal cells showing loosened yolk granules; the first is located close to the basolateral cell border while the second lies at the base of the cell in close proximity to the blastocoel; (D) the loosened yolk granules label with anti-hyaline layer antibodies; the colloidal gold particles are marked by arrows; (E) the compact yolk granules are negative in terms of anti-hyaline layer antibodies; b, blastocoel; cg, compact yolk granules; is, intercellular space; lg, loosened yolk granules; l, vitelline layer; n, nucleus.
DISCUSSION
In the present study of P. ochraceus, it has been observed that anti-hyaline layer antibodies react with the hyaline layer and also label ECM material within the intercellular spaces and blastocoel. This observation is tenable in light of ultrastructural investigations showing that in the sea stars the ECM forms not only the hyaline layer but also fills up the inner space of embryo (Reimer & Crawford, Reference Reimer and Crawford1995; Pang et al., Reference Pang, Crawford and Maghsoodi2003; Maghsoodi & Crawford, Reference Maghsoodi and Crawford2005).
The granules which were found in both the ectodermal cells and cells of archenteron have a similar ultrastructural appearance to the cortical granules of the egg and early embryo (Reimer & Crawford, Reference Reimer and Crawford1995). As shown in the present report, they were labelled with antibodies and this fact corroborates an affinity of cortical granules to hyaline layer.
It is known that the apical parts of gastrula embryonic cells exhibit vacuoles and vesicles which are proposed to perform secretion (Crawford et al., Reference Crawford, Campbell and Reimer1997; Pang et al., Reference Pang, Crawford and Maghsoodi2003; Maghsoodi & Crawford, Reference Maghsoodi and Crawford2005; Reunov & Crawford, unpublished). The present immunocytochemical study showed that both structures were labelled with anti-hyaline layer antibodies. Thus, the result presumably confirms a role of secretory vacuoles and secretory vesicles in secretion of ECM substance.
The cytoplasms of the cells of the gastrula are filled with granules which are thought to contain yolk (Reimer, Reference Reimer1994; Reimer & Crawford, Reference Reimer and Crawford1995). The yolk granules exhibit different electron densities and the majority of them are characterized by compact material. However, some of electron-light granules show loosened content. Immunocytochemical studies have demonstrated that compact yolk granules were not stained by anti-hyaline layer antibodies. So, material in these granules is probably not involved with the contents of the ECM. On the other hand, the electron-lucent yolk granules displayed dispersed contents staining with antibodies. It is likely that ECM molecules containing the hyaline layer epitope appeared in these yolk granules that therefore may participate in secretion.
Thus, it seems possible that in the starfish the yolk granules participate in ECM production. This conclusion is in accordance with information obtained for sea urchins. Indeed, in addition to the generally accepted version describing sea urchins yolk granules as storage organelles which contain protein and lipid compounds utilized by the embryo during development (Yokota & Kato, Reference Yokota and Kato1988; Leanne & Lennarz, Reference Leanne and Lennarz1989; Yokota et al., Reference Yokota, Kato and Mita1993) there is an opinion that the sea urchin yolk granules are multifunctional organelles containing materials that participate in various processes in addition to the nutritive role (see for review Mallya et al., Reference Mallya, Partin, Valdizan and Lennarz1992; Hayley et al., Reference Hayley, Perera and Robinson2006). It is proposed that these processes could include extracellular export of ECM proteins (Gratwohl et al., Reference Gratwohl, Kellenberger, Lorand and Noll1991; Mayne & Robinson, Reference Mayne and Robinson1998, Reference Mayne and Robinson2002). It seems that in the sea stars and sea urchins the yolk granules perform some sort of ECM secretion and more detailed analysis will be needed to determine the ultrastructural and molecular features of this phenomenon.
To summarize, we have found that the hyaline layer epitope provides a signal in various parts of the P. ochraceus gastrula and takes place in distinct cell structures. We have revealed that this epitope is contained in such ‘traditional’ secretory structures as the cortical granules and besides it was detected in the secretory vacuoles, secretory vesicles and loosened yolk granules. A total molecular compound confined to each secreting structure still has to be investigated but affinity of all of these to ECM secretion became obvious. The reason for diversity of secreting structures is not readily apparent. According to Pang et al. (Reference Pang, Crawford and Campbell2002) the hyaline layer appears to be complex in nature and its formation involves multi-stage secretion. It could be suggested that successive phases of the secretion process might be connected with activation of appropriate secreting structures and focused ultrastructural study is required to verify this hypothesis.
ACKNOWLEDGEMENT
This work was supported by NSERC for B.J. Crawford.






