INTRODUCTION
Their unique biodiversity puts coral reefs among the most important marine ecosystems in the world. Scleractinian corals, the habitat-founding species of coral reefs are threatened by global warming, ocean acidification and various anthropogenic stressors and some predictions assume that most of them will be lost within the next 100 years (Hughes et al., Reference Hughes, Baird, Bellwood, Card, Connolly, Folke, Grosberg, Hoegh-Guldberg, Jackson, Kleypas, Lough, Marshall, Nystroem, Palumbi, Pandolfi, Rosen and Roughgarden2003; Donner et al., Reference Donner, Skirving, Little, Oppenheimer and Hoegh-Guldberg2005; Veron et al., Reference Veron, Hoegh-Guldberg, Lenton, Lough, Obura, Pearce-Kelly, Sheppard, Spalding, Stafford-Smith and Rogers2009). At present, it is difficult to develop strategies to reverse the decline of reefs as many physiological responses of corals to stress are not clearly understood (Hughes et al., Reference Hughes, Graham, Jackson, Mumby and Steneck2010). Molecular biological methods including genomic and proteomic approaches hold a specific promise to provide new insights into the stress physiology of corals (Barneah et al., Reference Barneah, Benayahu and Weis2006; Desalvo et al., Reference Desalvo, Voolstra, Sunagawa, Schwarz, Stillman, Coffroth, Szmant and Median2008). However, to fully exploit the power of these techniques, experimental setups are required that allow physiological experiments under tightly controlled conditions. Such manipulations may include alteration of pH, CO2, aragonite saturation, temperature, light intensity and quality, nutrient levels, and quantity and quality of available food. Although field studies offer the benefit that they are conducted on corals in their natural environment, a tight control of the aforementioned factors is difficult to achieve, in particular, in long term experiments. Land-based culture systems, providing optimal growth conditions to which the corals are fully acclimatized, allow technically sophisticated experiments in close proximity to the laboratory infrastructures required for advanced molecular biological, biochemical or optical analyses (D'Angelo et al., Reference D'Angelo, Denzel, Vogt, Matz, Oswald, Salih, Nienhaus and Wiedenmann2008). The view is sometimes expressed that corals cannot be kept in a healthy state in captivity over longer periods of time (Leewis & Janse, Reference Leewis and Janse2008) and that they are difficult to grow (Weis et al., Reference Weis, Davy, Hoegh-Guldberg, Rodriguez-Lanetty and Pringle2008). However, tremendous progress has been made over the last two decades in improving aquarium technology (Leewis & Janse, Reference Leewis and Janse2008). Consequently, there is mounting evidence that the natural behaviour of the animals is not substantially altered by life in well-managed aquarium systems. Recent studies showed for instance, that corals acclimatized to favourable aquarium conditions show their natural coloration as a result of a light driven-expression of genes encoding green fluorescent protein (GFP)-like proteins (D'Angelo et al., Reference D'Angelo, Denzel, Vogt, Matz, Oswald, Salih, Nienhaus and Wiedenmann2008). In contrast, the loss of this coloration was observed in corals transferred from the reef into aquaria for short term experiments (Bay et al., Reference Bay, Ulstrup, Nielsen, Jarmer, Goffard, Willis, Miller and Van Oppen2009). Most recently, we and others showed that the diversity of zooxanthellae from both corals and sea anemones is maintained in captivity over years (Smith et al., Reference Smith, Pinzón and LaJeunesse2009; Hartle-Mougiou et al., Reference Hartle-Mougiou, D'Angelo, Smith, Burt, West and Wiedenmann2012), and the tissue content of photosynthetic pigments shows the same increase under low light conditions that is commonly observed in the field (Falkowski & Dubinsky, Reference Falkowski and Dubinsky1981; Oswald et al., Reference Oswald, Schmitt, Leutenegger, Ivanchenko, D'Angelo, Salih, Maslakova, Bulina, Schirmbeck, Nienhaus, Matz and Wiedenmann2007). Moreover, increasing numbers of coral species are reproductively active in aquaria (Leewis & Janse, Reference Leewis and Janse2008). Finally, the fact that corals survive for decades in aquaria demonstrates that their major physiological processes function well in captivity (Leewis & Janse, Reference Leewis and Janse2008; Hartle-Mougiou et al., Reference Hartle-Mougiou, D'Angelo, Smith, Burt, West and Wiedenmann2012). We have been able to supply experimental corals from the same mother colonies for more than a decade. The establishment of such model strains has the benefit that a constantly growing database can be gathered for the individual strains that facilitate future experiments (Weis et al., Reference Weis, Davy, Hoegh-Guldberg, Rodriguez-Lanetty and Pringle2008). For instance, the knowledge about the optimal levels of light or current required by certain species or strains greatly facilitates the design of experimental set-ups that prevent undesired stress that might arise from an unsuitable illumination or flow regime.
We utilized our >20 years' experience of keeping marine aquaria to develop a land-based mesocosm concept for physiological studies of reef-building corals. The system is designed to combine maximal flexibility for experimentation while offering stable conditions for long term observations and production of replicate colonies. Applying this concept, we have observed growth rates of ~5% per week for several species including Seriatopora caliendrum and Acropora microphthalma (Figure 1). These values are comparable to those reported for acroporids in their natural habitat (Jones & Berkelmans, Reference Jones and Berkelmans2010). This system has already allowed us to study the response of corals to different light qualities and quantities (Oswald et al., Reference Oswald, Schmitt, Leutenegger, Ivanchenko, D'Angelo, Salih, Maslakova, Bulina, Schirmbeck, Nienhaus, Matz and Wiedenmann2007; D'Angelo et al., Reference D'Angelo, Denzel, Vogt, Matz, Oswald, Salih, Nienhaus and Wiedenmann2008), to determine timescales of host pigment turnover (Leutenegger et al., Reference Leutenegger, D'Angelo, Matz, Denzel, Oswald, Salih, Nienhaus and Wiedenmann2007), to elucidate the regulation of genes encoding GFP-like fluorescent proteins (D'Angelo et al., Reference D'Angelo, Denzel, Vogt, Matz, Oswald, Salih, Nienhaus and Wiedenmann2008) and to stock a high diversity of species enabling screening for novel marker proteins for biomedical imaging (Wiedenmann et al., Reference Wiedenmann, Schenk, Rocker, Girod, Spindler and Nienhaus2002, Reference Wiedenmann, Ivanchenko, Oswald, Schmitt, Rocker, Salih, Spindler and Nienhaus2004; Kredel et al., Reference Kredel, Oswald, Nienhaus, Deuschle, Roecker, Wolff, Heilker, Nienhaus and Wiedenmann2009). Here, technical details of the set-up are provided.
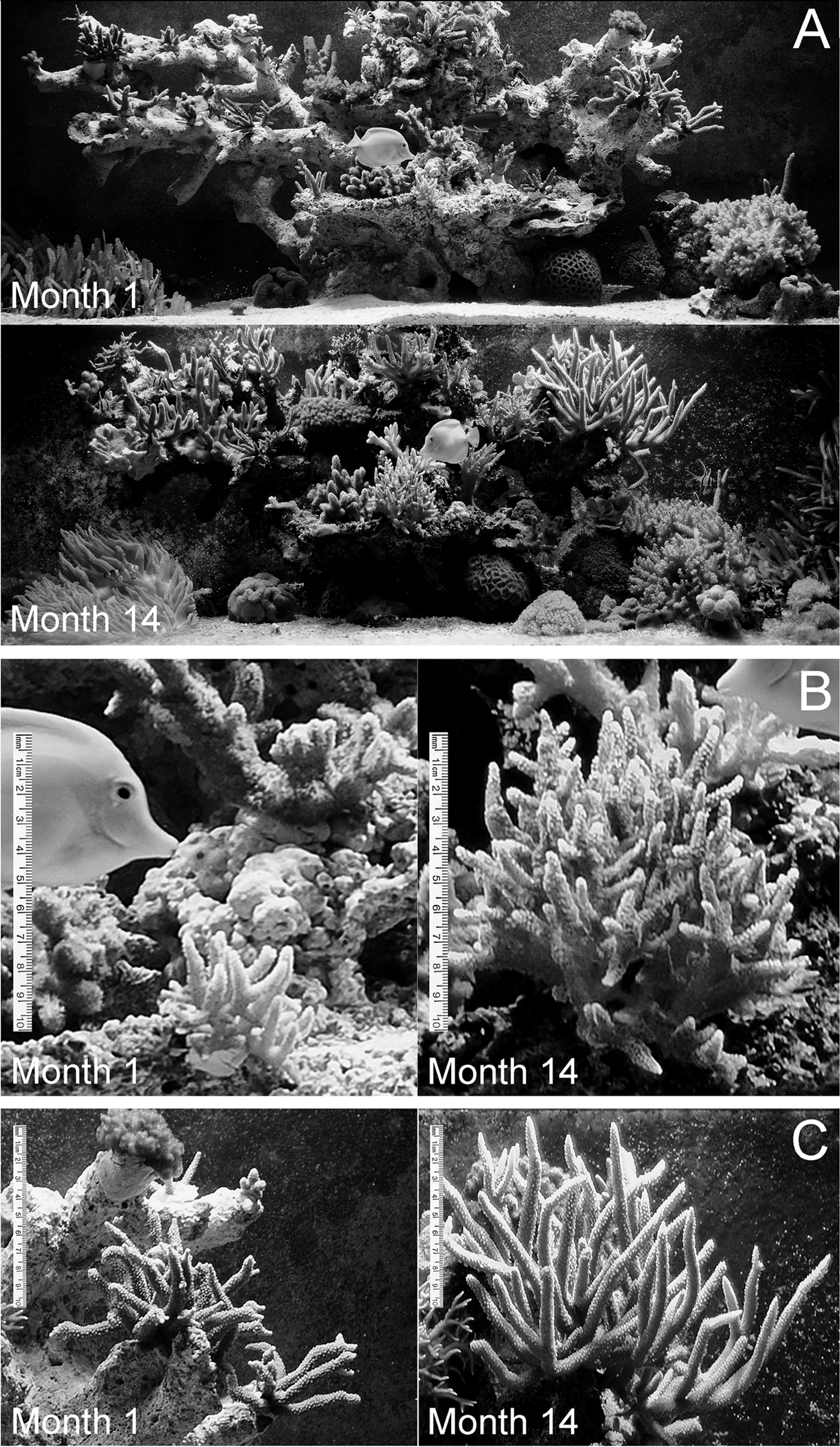
Fig. 1. Application of the ‘Crate Stack Filter' concept to the display tank in the reception area of the National Oceanography Centre, Southampton. (A) Images of the tank immediately after stocking with coral fragments (upper image) and 14 months later (lower image). Colonies of Seriatopora caliendrum (B) and Acropora microphthalma (C) show fast growth over 14 months. The same colonies are shown after being added to the tank (left panels) and 14 months later (right panels).
GENERAL CONSIDERATIONS AND SET-UP
The system is set up from components made of polyvinyl chloride (PVC) or polypropylene. Compared to glass tanks, these materials allow easy drilling for connectors or other fittings, they are lighter, less prone to damage and leakage and substantially cheaper. Although our mesocosm is designed to minimize the risk of spillages, the system is set up on the lowest floor to avoid damage to the interior fittings of the building in the event of a major leakage. Flooding risk is further reduced by fitting an elevated threshold in the door. A drain in the floor is included to take up spilled water. Our system is built from three identical modules that are interconnected by pipework (Figure 2C). Each module contains all components required for a self-sustaining operation so that parts of the mesocosm can be isolated for experimentation. Each module consists of two levels: an upper level including a holding tray and experimental or display tanks; and a lower level with reservoir and filtration tanks. Each unit contains a water-body of ~750 l. An independent module circulating ~2000 l is used to operate the display tank in the reception area of the National Oceanography Centre, Southampton (Figure 1). Note that the connecting pipes are fitted at the upper water level. This reduces the risks that the whole system drains in case of a leaking seal and allows single tanks to be emptied for cleaning. It proved useful to use oversized diameters (~63.5 mm) of the pipework to reduce the risk of blockage and facilitate cleaning. In our system, the lower level consists of three connected PVC bins that take up the filter units and technical equipment (protein skimmer, heaters and pump). The water level in the bin containing the supply pump is adjusted low enough to take up backed-up water that drains from the pipework of the system (~15 l) when the supply pump is stopped. A fourth bin is connected and used to top up evaporated water or to perform water changes. The flow through this tank is controllable and kept low. This will ensure that freshwater or newly made seawater enters the system slowly, thereby avoiding sudden changes in the water conditions.

Fig. 2. Overview of the experimental mesocosm in the Coral Reef Laboratory at the National Oceanography Centre, Southampton. (A) Photographic overview; (B) schematic side view of a single module showing the main supply pump (1) that distributes the water via the feed pipe (2) and fitted cut-off valves (3) over the experimental tanks (4), the holding tray (5) and the first reservoir bin (7). A drainpipe (6) transports the water from (4) and (5) to the filtration bins (8). These bins contain stacks of closed crates filled with coral sand (9), reef rocks (10), and mollusc shells (11). The last bin in the chain (12) accommodates heaters, a protein skimmer (13) and the supply pump (1). Approximate volumes of the different containers are given; (C) schematic aerial view over three modules. In the central module, the experimental tanks are replaced by a display tank. The modules are communicating by pipes connecting the bins number (7) and small supply pump (14) ensuring constant circulation. The other bins of the lower levels are not shown to increase clarity.
TECHNICAL COMPONENTS
Here, the technical components of the mesocosm are described. The named brands have worked reliably for long periods of time in our system. However, the selection is not the result of a systematic comparison and other brands offering similar functions might be equally suitable.
Lighting
Optimal light conditions are essential for successful coral culture. Our system is equipped with metal halide lamps suspended from cable trays above the tanks, operated at a 12h/12h light/dark cycle. A single 250 W lamp is sufficient to create high light conditions with a photon flux up to 250 µmol*m−2*s−1 in the central area of the holding tray. A gradient in light intensity towards the margins of the tank is often desirable to accommodate the different light demand of different species (Table 1). Higher light levels of up to 700 µmol*m−2*s−1 in the centre can be reached by using a 400 W lamp and, if the distance to the water is increased, a large area can be provided with a photon flux of ~200 µmol*m−2*s−1. Good coral growth has been obtained with burners providing a high amount of blue light (Aqualine 10000 (13000K), Aqua Medic, Germany). Burners are changed at least every 12 months. New burners have a higher output—the distance of lamps to the water surface is increased accordingly to avoid sudden changes in light intensity. Afterwards, the lamp is gradually lowered over a period of 2–3 weeks.
Table 1. Coral species suitable for physiological experiments in captivity.

1, weekly growth rates of 3–5% are considered fast; 2, if the fragments have to be produced in-house from a few mother colonies; 3, reference (D'Angelo et al., Reference D'Angelo, Denzel, Vogt, Matz, Oswald, Salih, Nienhaus and Wiedenmann2008); 4, reference (Kenkel et al., Reference Kenkel, Traylor, Wiedenmann, Salih and Matz2011); 5, reference (Meyer et al., Reference Meyer, Aglyamova, Wang, Buchanan-Carter, Abrego, Colbourne, Willis and Matz2009); 6, reference (Leewis & Janse, Reference Leewis and Janse2008); 7, reference (Oswald et al., Reference Oswald, Schmitt, Leutenegger, Ivanchenko, D'Angelo, Salih, Maslakova, Bulina, Schirmbeck, Nienhaus, Matz and Wiedenmann2007).
Deep water conditions could be simulated in our experimental tanks with blue fluorescent tubes (T5 NARVA blue 2, Tunze, Germany) or blue light-emitting diode strips (AquaBeam 500; 12 W, Reef Blue, TMC London, UK).
Water movement
A single supply pump (Ocean Runner 3500, Aqua Medic, Germany) ensures a constant circulation of the water body in each module at a flow rate of 3500 l/h. The pump is operated in a submerged mode to reduce the risk of spillage in case of connections becoming loose. An Ocean Runner 1200 pump (Aqua Medic, Germany) provides a circulation of 1200 l/h that connects the individual modules (Figure 2C). The holding tray and the experimental units require further pumps to create a favourable water flow. A single controllable flow pump is fitted in each holding tray with an output of 3000–13000 l/h (Turbelle® stream 6105, Tunze, Germany). The experimental tanks are operated with a Turbelle® nanostream® 6015 (Tunze, Germany; flow rate 1800 l/h).
Heating
Two 300 W precision heaters (Jäger, Germany) are fitted per module in the reservoir bins. The two heaters ensure that the temperature of the system is kept at the desired set value between 23 and 28°C even if the outside temperature drops below 20°C. A 25 W heater is fitted in each holding tray as backup for the case that the flow of warm water from the reservoir bins is stopped due to a failure of the supply pumps. Each experimental tank is also equipped with a 300 W precision heater (Jäger, Germany). This heating capacity is sufficient to adjust the water body of 40 l contained in the experimental tanks to temperatures of >33°C while maintaining a flow-through (80 l/h) with the system water. Temperature controllers (Tunze, Germany) are used to run temperature gradients. One pair of fans (Aquawind, Tunze, Germany) is kept in reserve for each holding tray for cooling of the water when outside temperatures reach exceptionally high values.
Filtration
We have developed a filtration concept that we termed the ‘Crate Stack Filter’. An important aspect about this concept is that it avoids any mechanical filtration by which the water is forced through a filter matrix. Instead, stacks of both closed and vented PVC crates are established in the reservoir bins that are gently washed by the constant water flow through the units. The crates are filled with different substrates to create different habitats for bacteria. The diversity of microorganisms is further supported by the gradient of water motion and oxygen availability. In the first bin, the top crate contains a 15 cm deep bed of coral sand and coral gravel with grain sizes between 0.5 mm and 0.5 cm obtained through the ornamental trade. The vented crate in the middle contains reef rocks, whereas the lowest crate is filled with a mixture of intact gastropod and bivalve shells. In the second bin, the same materials are used. However, the crates are differently stacked. The crate with the coral sand resides on the bottom of the bin, topped by the shell crate and reef rock crate. As the tank connectors are localized at the water level, the water motion and oxygen levels drop in deeper areas of the bin. Therefore, the distinct substrates are exposed to varying oxygen concentrations. If aerobic processes have to be supported, oxygen supply and circulation can be increased by using air diffuser stones situated at the bottom of the filtration bins. Several slow growing sponges are cultivated on top of the uppermost crates. Organic detritus that settles on the bottom of the reservoir bins and in other areas with slow water movement is siphoned out of the system as part of the water changes. The filter materials are rinsed in seawater once a year to remove organic detritus and partially replaced. The filtration bins are covered with opaque lids to prevent alga growth. A protein skimmer (H&S Type A-150-F2001; H&S, Germany) is fitted in the reservoir bin that is also harbouring the supply pump.
Water
Seawater is made from artificial salt mixtures (Tropic Marin Pro Reef, TMC London, UK) and demineralized water. Salinity is adjusted to 31‰ for Indo-Pacific species. Corals from the Arabian Gulf are kept in an isolated compartment with a salinity of 41‰. Every week, 5% of the system's water is replaced by freshly prepared seawater. However, the mesocosm can be operated for at least two months without requiring water changes. No obvious detrimental effects on the inhabitants were observed over such periods of incubation in the same water. Evaporation loss is compensated with demineralized water.
Calcium and magnesium ions are supplied to compensate the constant loss of these elements that results from calcification of corals and coralline red algae. Calcium and magnesium values are routinely monitored using test kits (Salifert, Holland). Magnesium ions are provided in the form of MgCl2 and MgSO4 solutions (Fossa & Nilsen, Reference Fossa and Nilsen2010). Calcium ions are added by dosing calcium chloride and sodium hydrogen carbonate solutions (Pawlowsky, Reference Pawlowsky1994) according to the formula:
Macronutrients
Phosphate and nitrate levels in the system are routinely measured using the High Sensitivity Phosphate Test (Rowa, Germany) and the Salifert NO3 Profi Test (Salifert, Holland). If required by the experimental approach, nanomolar level nitrate and phosphate analyses are undertaken using liquid wave guide approaches (Patey et al., Reference Patey, Rijkenberg, Statham, Stinchcombe, Achterberg and Mowlem2008). Build-up of increased phosphate levels are prevented using Rowaphos phosphate removal matrix (Rowa, Germany). If desired, phosphate and nitrate levels are lowered by adding ethanol or alcohols (1–5 ml/2000l/d) to the system (Mrutzek & Kokott, Reference Mrutzek and Kokott2004; Wiedenmann, Reference Wiedenmann2005). An increase in macronutrient levels is achieved by dosing sodium nitrate or -phosphate solutions with the help of peristaltic pumps. A constant low-level dosage proved to be more efficient in reaching and maintaining elevated nutrient levels compared to single, high-level doses.
Food
Corals are fed with frozen rotifers or crushed Artemia. When the holding tray is heavily stocked, food is supplied three times per week at a density of ~1 g (frozen weight) per 135 l. However, corals also grow in our system without dedicated feeding. Fish are fed with frozen Artemia once a day.
Control of algae
In each holding tray, one Zebrasoma flavescens prevents growth of turf alga. Moreover, self-sustaining populations of the gastropods Euplica sp. (E. versicolor/E. scripta) and Stomatella varia are kept to control algal films that are not accessible to the fish.
Corals and other cnidarians
The Coral Reef Laboratory propagates and studies more than 40 species of cnidarians. The stock includes representatives of actiniaria, ceriantharia, octocorallia, corallimorpharia and sclearactinia including 12 Acropora species. The collection covers corals from different geographical regions including Caribbean Sea, Fiji, Great Barrier Reef and various Indo-Pacific locations. Corals from the Arabian Gulf are kept in an isolated compartment at higher salinity. In our experience, a few species offer particularly favourable features for experimentation. A selection of model corals with different growth morphologies from different ecological niches are compiled in Table 1.
Other inhabitants
A number of fish species are kept in the display tanks and in the case of Amphiprion percula and Pterapogon kauderni also propagated for teaching purposes. Cleaner shrimps (Lysmata amboinensis) and several coral crabs are also on display. Aside from these wilfully added species, a high diversity of polychaetes, isopods, amphipods, tanaids, and brittle stars inhabit the system having entered initially via reef rocks and live corals.
CONCLUSION
The mesocosm introduced here provides a highly flexible platform for experimentation with reef corals. The stability of the chemical and physical water parameters enables long-term observations and technically challenging experiments. Sophisticated experimental approaches that combine physiological experiments with state-of-the-art molecular and optical analyses are facilitated by the proximity to the excellent research infrastructure at the National Oceanography Centre, Southampton/University of Southampton.
ACKNOWLEDGEMENTS
We thank Stephen Hayward (SOES technical superintendent) for his support in the technical realization of the mesocosm project. The experimental set-up was funded by the German Research Foundation (DFG Wi1990/2-1) and NERC (NE/H012303/1). We acknowledge the Tropical Marine Centre, London (UK) and Tropic Marin, Wartenberg (Germany) for sponsoring the Coral Reef Laboratory at the National Oceanography Centre, Southampton.





