INTRODUCTION
This is the fourth paper in the series on Ampharetidae from Japan, following the reports on the genus Ampharete Malmgren, Reference Malmgren1866 (Imajima et al., Reference Imajima, Reuscher and Fiege2012), genera with elevated or modified notopodia (Imajima et al., Reference Imajima, Reuscher and Fiege2013), and Amphicteis Grube, Reference Grube1850 and related genera (Reuscher et al., Reference Reuscher, Fiege and Imajima2014). Here we report 12 species in seven genera. One of the genera, Orochi gen. nov., is new to science. Of the 12 species eight are new, including Amage ehlersi sp. nov., A. longitorus sp. nov., Glyphanostomum hesslei sp. nov., Lysippe nipponica sp. nov., Melinnopsis augeneri sp. nov., M. mcintoshi sp. nov., Orochi palacephalus gen. et sp. nov., and Samytha annenkovae sp. nov. and two of them are new records from Japan, including Amage cf. adspersa (Grube, Reference Grube1863) and Glyphanostomum pallescens (Théel, Reference Théel1879). In the discussions to the respective generic diagnoses we comment on the synonymies of numerous genera suggested by Jirkov (Reference Jirkov2011). A comprehensive list of Ampharetidae from Japan, along with the respective references is included (Table 1). Furthermore, we provide a short conclusion that summarizes our new findings in the four papers published in this series.
Table 1. List of all Ampharetidae species recorded from Japan, along with references of the records. Abbreviation: OD, original description.

MATERIALS AND METHODS
The specimens examined in this study were collected between 1965 and 2008 from 57 stations from depths ranging from the subtidal to the deep sea around Japan. The geographic extent of the sampling area ranged from off Okinawa Sima in the south to Kyushu and the north-western coast of Honshu and the Chishima Trench off the Hokkaido east coast in the north. Samples were taken using various types of dredges and trawls and sieved on board. Specimens were fixed in 7% formaldehyde seawater solution and preserved in 70% ethanol. Preserved specimens were examined using stereo and compound microscopes. Drawings were made using a camera lucida.
The completeness of specimens is indicated in the text as: cs (complete specimen) and af (anterior fragment).
The schematic figures of the anterior ends were prepared in Adobe Illustrator. Types and other specimens are deposited in the following institutions: National Museum of Nature and Science, Tokyo (NSMT) and Senckenberg Museum Frankfurt (SMF). Full details for the material deposited at Senckenberg can be found at http://sesam.senckenberg.de/.
SYSTEMATICS
Ampharetidae Malmgren, Reference Malmgren1866
Ampharetinae Malmgren, Reference Malmgren1866
Amage Malmgren, Reference Malmgren1866
TYPE SPECIES
Amage auricula Malmgren, Reference Malmgren1866
SYNONYMS
Paramage Caullery, Reference Caullery1944
Egamella Fauchald, Reference Fauchald1972
Mexamage Fauchald, Reference Fauchald1972
GENERIC DIAGNOSIS
Prostomium with middle lobe surrounded by inflated lobe, lacking glandular ridges. Buccal tentacles smooth. Two to four pairs of cirriform branchiae. Segment II usually without chaetae, or exceptionally with minute chaetae. Thorax with 11–14 uncinigers. Modified or intermediate segments absent. Abdomen with rudimentary notopodia.
REMARKS
The synonymies of Egamella, Mexamage, and Paramage with Amage, that were suggested, but not discussed in detail by Jirkov (Reference Jirkov2011), are accepted here. The two latter genera differ from Amage by the presence of vestigial notopodia that do not carry notochaetae in anterior segments. We consider this insufficient for the erection of new genera. Fauchald (Reference Fauchald1972) seemed to be unaware of the genus Paramage when he described Mexamage, as both genera are characterized by the same trait. The monotypic genus Egamella is unusual for possessing only two pairs of branchiae, but otherwise its only species E. quadribranchiata Fauchald, Reference Fauchald1972 resembles a typical species of Amage.
We disagree with the synonymy of Phyllampharete Hartman & Fauchald, Reference Hartman and Fauchald1971 that was suggested by Jirkov (Reference Jirkov2011) because its shape of prostomium, shape and arrangement of branchiae, presence of very long dorsal cirri in abdominal neuropodia, and shape of uncini differ from Amage.
We consider the presence of digitiform abdominal notopodial rudiments to be a diagnostic character of the genus. Therefore, we doubt that the species A. septemdecima Schüller & Jirkov, Reference Schüller and Jirkov2013 belongs to Amage. In addition, the branchial arrangement of the species differs from Amage, but resembles the arrangement found in species of Lysippe. The crenulated lower lip and the high number of teeth in thoracic uncini are also indicative of a closer affiliation of this species with Lysippe.
Amage cf. adspersa (Grube, Reference Grube1863)
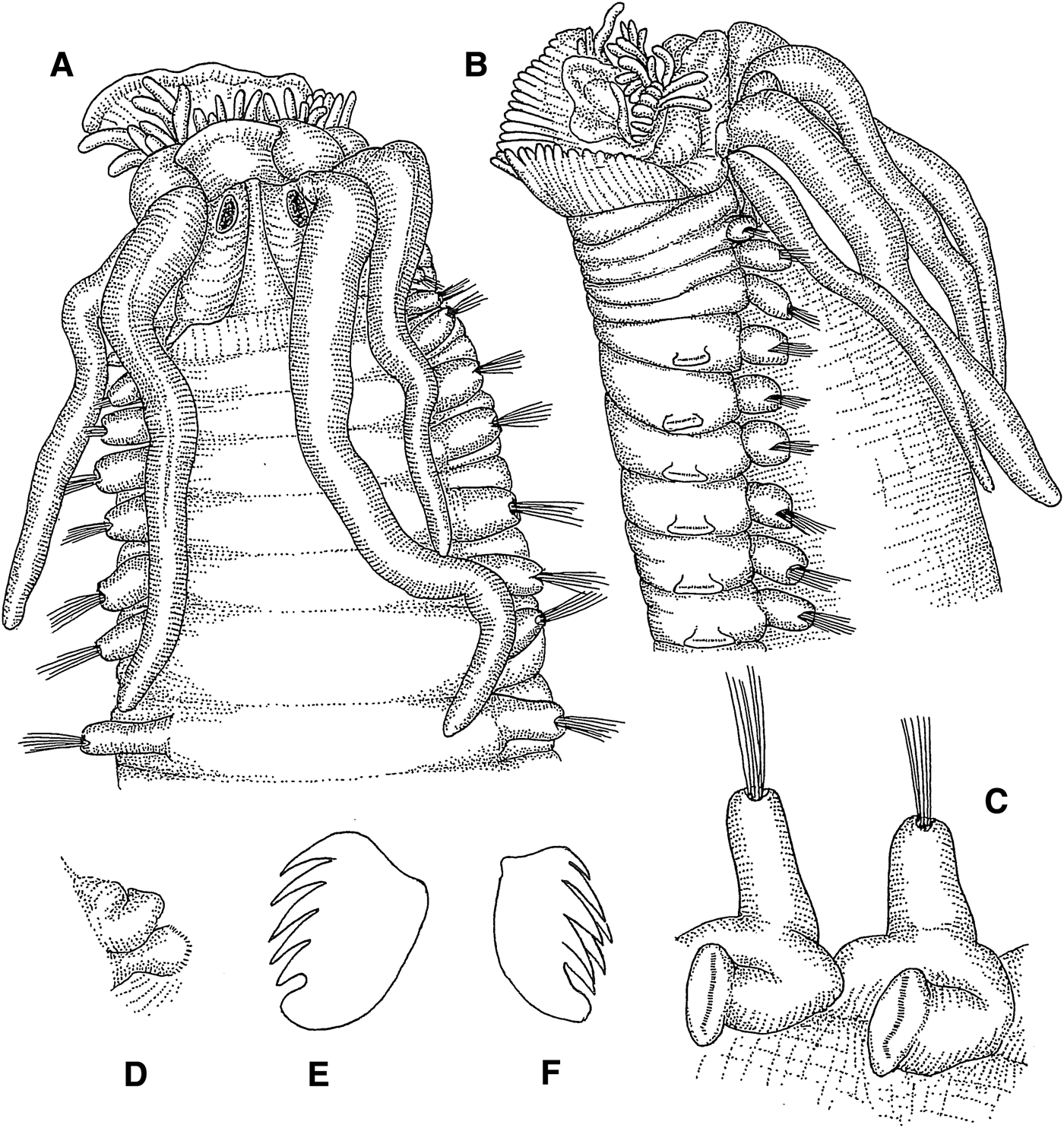
(Figures 1A–H; 13A)

Fig. 1. Amage cf. adspersa: (A) prostomium, dorsal view, 38×; (B) anterior end, lateral view, 27×; (C) thoracic parapodium, anterior view, 48×; (D) abdominal parapodium, anterior view, 67×; (E) posterior end, lateral view, 54×; (F) thoracic uncinus, frontal view, 935×; (G) same, lateral view, 900×; (H) abdominal uncinus, lateral view, 957×.
Sabellides adspersa Grube, Reference Grube1863: 57–58, plate VI, figure 2.
Amage adspersa: Hessle, Reference Hessle1917: 121–122. – Fauvel, Reference Fauvel1927: 234–236, figure 82a–f.
SPECIMENS EXAMINED
Sagami Bay, 35°07.8′N 139°34.0′E – 35°07.3′N 139°33.9′E, 100–111 m, Rinkai-maru, Station 1, 10.2006 (1 cs).
DESCRIPTION
Length 8 mm, width 1.2 mm. Prostomium with middle lobe delimited by incision from inflated surrounding lobe, lacking glandular ridges; anterior and lateral edges of prostomial middle lobe concave, corners convex; two groups of eye spots in posterior prostomial middle lobe (Figure 1A). Buccal tentacles smooth (Figure 1A, B). Four pairs of cirriform branchiae emerging from dermal fold, arranged in two transversal rows in segments II and III, separated by median gap of one branchial width; innermost branchiae of anterior transverse row originating from segment II, outermost branchiae of anterior transverse row originating from segment III, outermost branchiae of posterior transverse row originating from segment IV, innermost branchiae of posterior transverse row originating from segment V (Figure 13A). Segment II without chaetae. Notopodia with capillary chaetae and minute ventral papilla (Figure 1B, C) from segment III, present in 17 chaetigers; anterior notopodia small, increasing in size from first to fourth pair. Neuropodial tori with uncini (Figure 1C) from segment VI, present in 14 thoracic uncinigers; tori without cirri. Continuous ventral shields conspicuous to thoracic unciniger 12, faint to thoracic unciniger 14. Elevated or modified notopodia absent. Intermediate uncinigers absent. Fourteen abdominal uncinigers with tuberculate rudimentary notopodia, emerging from glandular pads (Figure 1D). Pinnules with minute tuberculate dorsal cirrus. Pygidium with crenulated terminal anus and one pair of cirriform, ventrolateral anal cirri (Figure 1E). Thoracic and abdominal uncini with 3 teeth in one vertical row over basal prow and rostral tooth; some uncini with minute fourth tooth on top (Figure 1F–H).
REMARKS
Only two species of Amage with 14 thoracic uncinigers have been described. A. septemdecima, which, we suspect, may belong to a different genus (see discussion in remarks of the genus), differs from our species by the lack of abdominal rudimentary notopodia, ventral cirri in thoracic notopodia and dorsal cirri in abdominal neuropodia, and the branchial arrangement. Our specimen fits the description of A. adspersa, even though Grube (Reference Grube1863) did not specifically mention the dorsal fold from which the branchiae emerge or the tiny ventral papillae in thoracic notopodia. Grube described the species with only three pairs of branchiae. However, Hessle (Reference Hessle1917) and Fauvel (Reference Fauvel1927) both mention the peculiar variability in the number of branchiae in A. adspersa. Both authors conclude that most specimens have 4 pairs of branchiae. Furthermore, Grube (Reference Grube1863) described 15 abdominal uncinigers, whereas our specimen had only 14. Fauvel (Reference Fauvel1927) noted that the number of abdominal uncinigers may vary between 11 and 15, but is most commonly 13. The shape of the uncini is the same as in Fauvel's (Reference Fauvel1927) specimens. Because of the slight differences observed in our specimen, the taxonomic uncertainties, and the discrepancy to the known geographic distribution (see below), we tentatively identified it as Amage cf. adspersa.
DISTRIBUTION
The species was originally described from the Adriatic Sea and has been found in other parts of the Mediterranean Sea (Fauvel, Reference Fauvel1927) and in the Atlantic Ocean from Iceland (Spärck, Reference Spärck1937), Scotland (Clark, Reference Clark1952), and Madeira (Langerhans, Reference Langerhans1884). If our specimen from the Sagami Bay does belong to Amage adspersa, it would be a new record from the Pacific Ocean.
Amage ehlersi sp. nov.
(Figures 2A–H; 13B)

Fig. 2. Amage ehlersi sp. nov.: (A) prostomium, dorsal view, 18×; (B) anterior end, lateral view, arrow pointing at lower lip, 14×; (C) anterior end, dorsal view, 11×; (D) thoracic parapodium, anterior view, 32×; (E) abdominal parapodium, anterior view, 20×; (F) posterior end, ventral view, 35×; (G) thoracic uncinus, lateral view, 670×; (H) abdominal uncinus, frontolateral view, 670×.
SPECIMENS EXAMINED
Holotype: NSMT-Pol. H580, off Kashima-nada, 36°33.5′N 141°01.3′E – 36°34.2′N 141°02.1′E, 197–206 m, KT-79-13, Station KB-12, 8.1979 (1 cs). Paratypes: SMF 23955, same locality as holotype (1 cs). NSMT-Pol. P593, Suruga Bay, 35°03.6′N 138°47.2′E – 35°03.2′N 138°46.2′E, 245–315 m, KT-76-16, Station B, 9.1976 (2 cs). NSMT-Pol. P581, Kashima-nada, 36°05.4′N 140°44.6′E – 36°06.3′N 140°44.4′E, 29–36 m, KT-79-13, Station KB-1, 8.1979 (1 cs).
ADDITIONAL SPECIMENS EXAMINED
Sagami Sea, 35°09.4′N 139°23.3′E, 480 m, KT 66–23, Station 10, 10.1966 (3 cs). Off Tsushima Islands, 34°45.9′N 129°35.9′E, 64 m, RV ‘Genkai’, Station 25, 8.1968 (1 cs). Suruga Bay, 35°03.9′N 138°47.3′E – 35°06.6′N 138°46.6′E, 290–320 m, KT-74-14, Station B-10, 9.1974 (3 cs); 35°03.6′N 138°47.2′E – 35°03.2′N 138°46.2′E, 245–315 m, KT-76-16, Station B, 9.1976 (2 cs); 34°47.0′N 138°30.4′E – 34°47.0′N 138°30.3′E, 435–590 m, KT-78-2, Station Z-11, 2.1978 (1 cs). Off Sanriku, 39°12.8′N 142°16.0′E – 39°13.9′N 142°16.4′E, 562–563 m, KT-85-11, Station SR 15, 8.1985 (2 af).
DESCRIPTION
Length 13 mm, width 2.2 mm. Prostomium with middle lobe bearing anterolateral frontal horns, delimited by incision from inflated surrounding lobe (Figure 2A); nuchal organs in posterior part of prostomial middle lobe, arranged in transverse line, separated by small median gap; prostomium without glandular ridges or eyes. Lower lip cushion like (Figure 2B). Buccal tentacles smooth. Four pairs of cirriform branchiae in L-shaped arrangement in segments II–IV, separated by wide median gap (Figure 2B, C); innermost branchiae of anterior transverse row originating from segment II, outermost branchiae of anterior transverse row originating from segment III, median branchiae of longitudinal row originating from segment IV, posterior branchiae of longitudinal row originating from segment V (Figure 13B). Segment II without chaetae. Notopodia with capillary chaetae from segment III, present in 14 chaetigers (Figure 2D); anterior notopodia small, increasing in size from first to fourth pair; notopodial cirri absent. Neuropodial tori with uncini from segment VI, present in 11 thoracic uncinigers (Figure 2D); tori without cirri. Continuous ventral shields conspicuous to thoracic unciniger 11, faint in abdominal unciniger 1. Elevated or modified notopodia absent. Intermediate uncinigers absent. Ten abdominal uncinigers with digitiform rudimentary notopodia (Figure 2E). Pinnules with stout tuberculate dorsal cirrus. Rudimentary notopodia and pinnules connected by glandular fold. Pygidium with crenulated terminal anus and one pair of short, ovoid, ventrolateral anal cirri (Figure 2F). Thoracic uncini with 4 teeth in 1 row over basal prow and rostral tooth (Figure 2G). Abdominal uncini with about 7 teeth in 2 staggered rows over basal prow and rostral tooth (Figure 2H).
REMARKS
In some paratypes the ventral shields are conspicuous to thoracic unciniger 10 and faint in thoracic unciniger 11.
The most similar species of the genus that shares number of branchiae, number of thoracic unciniger and absence of thoracic notopodial cirri is Amage tumida Ehlers, Reference Ehlers1887. The new species can be distinguished from A. tumida by the absence of longitudinal prostomial grooves, the much more prominent dorsal cirri in abdominal pinnules, the number of abdominal uncinigers (10 vs. 9), and the shape of abdominal uncini, which have 3–4 vertical rows of teeth in A. tumida.
ETYMOLOGY
The species is named after Ernst Heinrich Ehlers (1835–1925), renowned German polychaetologist and describer of the sister species A. tumida.
DISTRIBUTION
Off Sanriku, Kashima-nada, Sagami Sea and Suruga Bay along the Pacific coast of Honshu, off Tsushima Islands in the Sea of Japan, in 30–590 m.
Amage longitorus sp. nov.
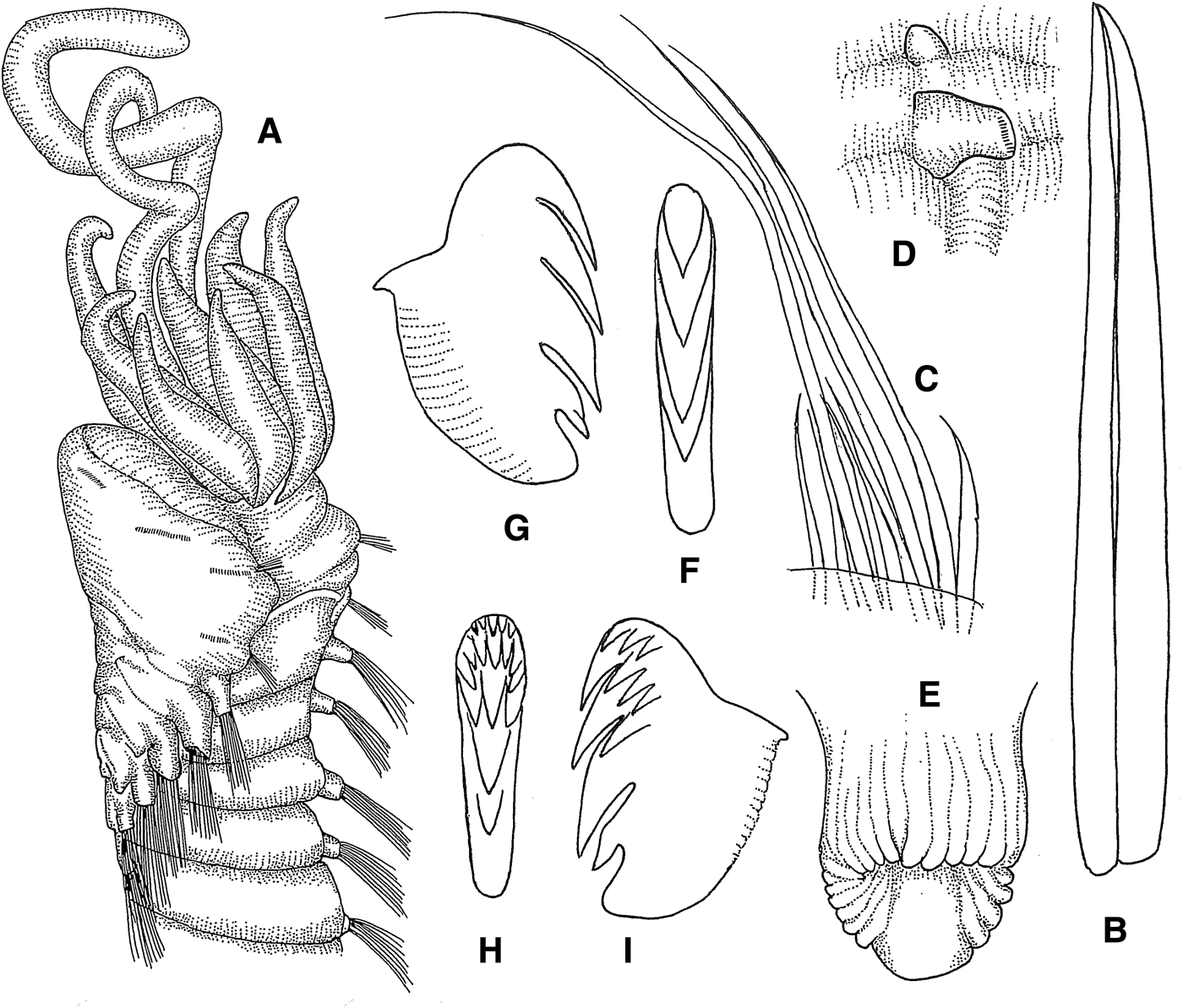
(Figures 3A–G; 13C)

Fig. 3. Amage longitorus sp. nov.: (A) prostomium, dorsal view, 16×; (B) anterior end, ventral view, 16×; (C) same, lateral view, 16×; (D) same, dorsal view, 13×; (E) abdominal parapodium, anterior view, 54×; (F) posterior end, ventral view, 54×; (G) thoracic uncinus, frontolateral view, 750×.
SPECIMENS EXAMINED
Holotype: NSMT-Pol. H582, off Shimokita Peninsula, 41°05.0′N 143°49.9′E – 41°05.4′N 143°51.5′E, 2879–3016 m, KT-08-27, Station S-3, 10.2008 (1 cs). Paratype: NSMT-Pol. P594, same locality as holotype (2 af). SMF 23956, off Shimokita Peninsula, 41°11.8′N 143°59.0′E – 41°11.9′N 144°00.4′E, 2889–2995 m, KT-08-27, Station S-4, 10.2008 (1 cs).
ADDITIONAL SPECIMENS EXAMINED
Sagami Sea, 35°00.9′N 139°35.7′E – 35°00.7′N 139°36.0′E, 1060–990 m, KT-66-12, Station 7, 7.1966 (4 cs, 1 af).
DESCRIPTION
Length 18 mm, width 1.5 mm. Prostomium with middle lobe encircled by inflated lobe (Figure 3A); prostomium without glandular ridges or eyespots. Lower lip as long as following four segments, with several anterior longitudinal and one median transverse furrow (Figure 3B). Buccal tentacles smooth (Figure 3B, C). Three pairs of cirriform branchiae in triangular arrangement in segments II and III (Figure 3D); third pair of left group missing; large median gap between groups of branchiae; innermost branchiae originating from segment II, outermost branchiae originating from segment III, posterior branchiae originating from segment IV (Figure 13C). Segment II without chaetae. Notopodia with capillary chaetae from segment III, present in 15 chaetigers; anterior notopodia small, increasing in size from first to third pair; notopodia without cirri. Neuropodial tori with uncini from segment VI, present in 12 thoracic uncinigers; first pair of tori very long, extending far onto ventral side and nearly meeting mid-ventrally (Figure 3B); second pair of tori slightly shorter than first pair; third pair of tori about half as long as second pair; tori of thoracic uncinigers 4–12 of same length, slightly shorter than tori of unciniger 3; tori without cirri. Continuous ventral shields conspicuous to thoracic unciniger 12. Elevated or modified notopodia absent. Intermediate uncinigers absent. Thirteen abdominal uncinigers with rudimentary notopodia, emerging from glandular pads. Pinnules with minute conical dorsal cirrus (Figure 3E). Pygidium with terminal crenulated anus and one pair of lateral cirriform anal cirri (Figure 3F). Thoracic and abdominal uncini with numerous teeth in several vertical rows, over rostral tooth and basal prow (Figure 3G).
REMARKS
Some specimens have only 11 abdominal uncinigers.
Amage longitorus sp. nov. is only the second species of the genus with three pairs of branchiae. The other species, A. gallasii Marion, Reference Marion1875, differs from the new species by the presence of 11 thoracic and 9 abdominal uncinigers. Furthermore, A. gallasii has tuberculate ventral cirri in thoracic notopodia, whereas they are lacking in A. longitorus. The other species that is rarely found with three pairs of branchiae is A. adspersa (see Fauvel, Reference Fauvel1927). This species differs from A. longitorus sp. nov. by the presence of 14 thoracic uncinigers, and the presence of tuberculate ventral cirri in thoracic notopodia. Furthermore, the new species differs from A. adspersa and A. gallasii by the presence of the very long tori in anterior chaetigers. Amage benhami Reuscher et al., Reference Reuscher, Fiege and Wehe2009 is the only other species in the genus with 12 thoracic uncinigers. It also has long tori in anterior chaetigers. However, in A. benhami the length of the tori decreases more gradually. Furthermore, A. benhami differs from the new species by the presence of four pairs of branchiae and tuberculate ventral cirri in thoracic notopodia.
ETYMOLOGY
The new species is named after the characteristically elongated tori in anterior thoracic uncinigers.
DISTRIBUTION
Northern Honshu and Sagami Sea of central Honshu, in ~1000–3000 m.
Amphisamytha Hessle, Reference Hessle1917
TYPE SPECIES
Amphisamytha japonica Hessle, Reference Hessle1917
SYNONYM
Mooresamytha Williams, Reference Williams1987: 256–257.
Amathys Desbruyères & Laubier, Reference Desbruyères and Laubier1996: 248–249.
GENERIC DIAGNOSIS
Prostomium with middle lobe encircled by surrounding lobe, lacking glandular ridges. Buccal tentacles smooth. Four pairs of cirriform branchiae. Segment II without chaetae. Thorax with 17–20 chaetigers and 14–17 uncinigers. No modified or intermediate segments. Glandular pads above abdominal neuropodia.
REMARKS
We disagree with Jirkov's (Reference Jirkov2011) suggestion to synonymize Amphisamytha with Phyllocomus Grube, Reference Grube1978b, based on the shape of the prostomium and the neuropodia. In contrast to Jirkov (Reference Jirkov2011), who described the prostomium of Amphisamytha as simple and not divided into lobes, we have observed that it consists of a middle lobe and a surrounding lobe. We also disagree with Jirkov's statement that thoracic and abdominal neuropodia are identical in Amphisamytha. Furthermore, the synonymy is rejected here because Phyllocomus has strongly modified branchiae and a very large number of abdominal uncinigers, which makes this genus quite unique among Ampharetidae.
Amphisamytha japonica Hessle, Reference Hessle1917
(Figures 4A–H; 13D)

Fig. 4. Amphisamytha japonica: (A) anterior end, dorsal view, 10×; (B) same from another individual, lateral view, 18×; (C) thoracic parapodium, lateral view, 20×; (D) limbate capillary chaeta, 135×; (E) abdominal parapodia, lateral view, 20×; (F) posterior end, ventral view, 20×; (G) thoracic uncinus, frontolateral view, 703×; (H) abdominal uncinus, lateral view, 782×.
Amphisamytha japonica Hessle, Reference Hessle1917: 114–115, text figure 20, plate 1, figures 7–8. – Imajima, Reference Imajima and Fujita2009: 155–157, figure 56
SPECIMENS EXAMINED
Otsuchi Bay, 39°21.7′N 141°59.8′E – 39°21.5′N 141°59.6′E, 79–74 m, Station 13, 8.1979 (3 cs). Off Sanriku, 36°30.9′N 140°59.6′E – 36°31.1′N 140°59.8′E, 248–248 m, RV ‘Wakataka-maru’, Station WA06-H250D, 11.2006 (1 cs); 38°26.7′N 142°23.8′E, 1005–1004 m, Station WA05-E1000D, 10.2005 (3 cs). Tokyo Bay, 35°20.2′N 139°41.6′E, 25 m, Station 5, 12.1978 (4 cs). Sagami Bay, 35°08.0′N 139°35.4′E – 35°07.8′N 139°35.3′E, Rinkai-maru, Station 2, 3.2003 (3 cs); 35°07.6′N 139°34.8′E – 35°07.8′N 139°34.7′E, 91–91 m, Rinkai-maru, Station 5, 3.2002 (2 cs). SMF 23957, Sagami Bay, 35°07.4′N 139°33.6′E – 35°07.8′N 139°33.2′E, 121–156 m, Rinkai-maru, Station 2, 6.2002 (5 cs); 35°08.3′N 139°32.9′E – 35°08.3′N 139°32.7′E, 177–148 m, RV ‘Shin'yo-maru’, Station 2, 10.2002 (1 cs). Sagami Sea, 35°06.7′N 139°34.7′E – 35°06.8′N, 139°34.1′E, 310–381 m, Rinkai-maru, Station 2, 3.2002 (3 cs). Kushimoto, 33°27.7′N 135°44.8′E – 33°27.5′N 135°44.4′E, 44–44 m, Station 5, 7.1978 (5 cs). Toyama Bay, 36°58.6′N 137°06.0′E, 105–115 m, KT-75–6, Station 30, 6.1975 (2 cs). Off Okinoshima Isle, 32°42.2′N 132°29.7′E – 32°42.3′N 132°29.5′E, 231–232 m, KT-99-18, Station DG-4, 12.1999 (3 cs). Sasebo Bay, 33°06.1′N 129°40.9′E, 53 m, Station 4, 5.1972 (1 cs). Ariake Sea, 32°55.4′N 130°20.2′E, 22 m, Station 14, 9.1958 (2 cs). Kagoshima Bay, 31°40.0′N 130°43.1′E, 130 m, Station 42, 8.1975 (1 cs); 31°39.6′N 130°46.9′E, 183 m, Station 60, 8.1975 (2 af). Off Tsushima Islands, 33°49.6′N 129°29.0′E, 100 m, RV ‘Genkai’, Station 8, 7.1968 (3 cs).
DESCRIPTION
Length 14–19 mm, width 2–3.5 mm. Prostomium with middle lobe delimited by incision from surrounding lobe (Figure 4A); prostomium without glandular ridges or eyes. Buccal tentacles unknown. Four pairs of cirriform branchiae, arranged in two transverse rows in segments II and III, separated by small median gap (Figure 4A, B); innermost branchiae of anterior transverse row originating from segment II, outermost branchiae of anterior transverse row originating from segment III, innermost branchiae of posterior transverse row originating from segment IV, outermost branchiae of posterior transverse row originating from segment V (Figure 13D). Segment II without chaetae. Notopodia with capillary chaetae from segment III, present in 17 chaetigers (Figure 4C, D); anterior notopodia small, increasing in size from first to third pair; notopodia without cirri. Neuropodial tori with uncini from segment VI, present in 14 thoracic uncinigers (Figure 4C); tori without cirri. Last segment of continuous ventral shields indeterminable. Elevated or modified notopodia absent. Intermediate uncinigers absent. Twelve abdominal uncinigers with glandular pads above pinnules (Figure 4E). Pinnules without dorsal cirrus. Pygidium with terminal anus and one pair of short, conical, ventrolateral anal cirri (Figure 4F). Thoracic and abdominal uncini with about 7 teeth in 2 staggered rows over basal prow and rostral tooth (Figure 4G, H).
REMARKS
Our specimens have 12 abdominal uncinigers, whereas Hessle (Reference Hessle1917) described 14 and Imajima (Reference Imajima and Fujita2009) described 13 abdominal uncinigers. Whereas the number of abdominal uncinigers is considered constant in species of most genera, variable numbers have been described in most species of Amphisamytha (Reuscher et al., Reference Reuscher, Fiege and Wehe2009; Stiller et al., Reference Stiller, Rousset, Pleijel, Chevaldonné, Vrijenhoek and Rouse2013).
DISTRIBUTION
Japanese Pacific coast from Otsuchi Bay to Kushimato, near the entrance to Kagoshima Bay, off Toyama Bay, Okinoshima, and off Tsushima Islands in the Sea of Japan, in 22–1005 m. This species is only known from Japanese waters. It has previously been found off the southern and eastern coast of Honshu (Hessle, Reference Hessle1917; Imajima & Hartman, Reference Imajima and Hartman1964; Imajima, Reference Imajima and Fujita2009).
Glyphanostomum Levinsen, Reference Levinsen1884
TYPE SPECIES
Samytha pallescens Théel, Reference Théel1879
GENERIC DIAGNOSIS
Prostomium without glandular ridges. Buccal tentacles smooth. Three pairs of cirriform branchiae. Segment II without chaetae. Thorax with 14 chaetigers and 11 uncinigers. No modified segments. Two intermediate uncinigers. Abdominal rudimentary notopodia absent.
REMARKS
The generic diagnosis of Jirkov (Reference Jirkov2011) includes ‘lateral, whitish glandular fields’ in the prostomium. However, we did not observe this structure in any of our examined specimens and neither was it described in any of the original descriptions of the other five valid species.
Glyphanostomum hesslei sp. nov.
(Figures 5A–G; 13E)

Fig. 5. Glyphanostomum hesslei sp. nov.: (A) anterior end, dorsal view, arrow pointing at lateral lappets, arrowhead pointing at cushion-like peristomial lobe, 28×; (B) same, lateral view, arrowhead pointing at cushion-like peristomial lobe, 28×; (C) thoracic parapodium, anterolateral view, 48×; (D) notochaeta, 490×; (E) abdominal neuropodium, lateral view, 55×; (F) posterior end, ventral view, 27×; (G) thoracic uncinus, lateral view, 627×.
SPECIMENS EXAMINED
Holotype: NSMT-Pol. H583, off Kushiro, Hokkaido, 42°35.0′N 144°48.0′E – 42°34.7′N 144°49.9′E, 1028–1015 m, KT-07-29, Station K-1, 11.2007 (1 cs). Paratypes: NSMT-Pol. P584, same locality as holotype (7 cs, 9 af). SMF 23958, same locality as holotype (8 cs, 4 af).
DESCRIPTION
Length 16 mm, width 1.2 mm. Prostomium with divided middle lobe, delimited by incision from surrounding lobe (Figure 5A); prostomium without glandular ridges or eyes. Buccal tentacles smooth (Figure 5A). Peristomium with cushion-like lobe, encircling ventral half of body collar like (Figure 5A, B). Segments II and III fused dorsally and laterally, forming lateral lappets (Figure 5A). Three pairs of cirriform branchiae in fused segments II and III, separated by wide median gap (Figure 5A); two pairs arranged in transverse row, third pair located close behind, near segmental border to segment IV; innermost branchiae of anterior transverse row originating from segment II, outermost branchiae of anterior transverse row originating from segment III, posterior branchiae originating from segment IV (Figure 13E). Fused segments II and III with one pair of notochaetal fascicles; notopodia with capillary chaetae present in 14 chaetigers (Figure 5C, D); anterior notopodia small, increasing in size from first to third pair; notopodia without cirri. Neuropodial tori with uncini from segment VI, present in 11 thoracic uncinigers (Figure 5C); tori without cirri. Continuous ventral shields present to thoracic unciniger 9. Elevated or modified notopodia absent. Two intermediate uncinigers. Nine abdominal uncinigers without rudimentary notopodia. Pinnules without dorsal cirrus (Figure 5E). Pygidium with crenulated terminal anus and one pair of long, filiform, lateral anal cirri (Figure 5F). Uncini in thoracic and intermediate uncinigers with 5 teeth in one vertical row over rostral tooth and basal prow (Figure 5G). Abdominal uncini with numerous teeth in several vertical rows.
REMARKS
The incisions in the prostomium are obvious in some specimens, and inconspicuous or even invisible in other specimens.
Among the five described species of the genus Glyphanostomum, G. moreirai Aguirrezabalaga & Parapar, Reference Aguirrezabalaga and Parapar2014 is most similar to G. hesslei sp. nov. Both species have the same branchial arrangement and lateral lappets in segment II. However, the large conical lobes in segment I of G. moreirai are missing in our new species. Furthermore, the peristomial cushion-like lobe, found in G. hesslei sp. nov., is absent in G. moreirai.
ETYMOLOGY
The species is named after the Swedish zoologist Christian Waldemar Hessle (1890–1980), whose comprehensive publication ‘Zur Kenntnis der terebellomorphen Polychaeten’ is among the most important contributions on terebellomorph polychaetes.
DISTRIBUTION
Off Kushiro, Pacific coast of Hokkaido, in ~1070 m.
Glyphanostomum pallescens (Théel, Reference Théel1879)
(Figures 6A–J; 13F)

Fig. 6. Glyphanostomum pallescens: (A) prostomium, dorsal view, 50×; (B) anterior end, ventral view, 50×; (C) same, lateral view, 50×; (D) same, dorsal view, 42×; (E) thoracic parapodium, anterolateral view, 80×; (F) notochaeta, 565×; (G) abdominal neuropodium, lateral view, 80×; (H) posterior end, dorsal view, 74×; (I) thoracic uncinus, lateral view, 836×; (J) tube, 25×.
Samytha pallescens Théel, Reference Théel1879: 61–62, plate IV, figures 60–62.
Glyphanostomum pallescens: Levinsen, Reference Langerhans1884: 163. – Hessle, Reference Hessle1917: 105. – Holthe, Reference Holthe1986b: 71–73, figure 29. Hilbig, Reference Hilbig, Blake, Hilbig and Scott2000: 204, figure 8.15.
SPECIMENS EXAMINED
Moroiso Bay, 35°09.4′N 139°36.8′E, 30 m, Station 2, 3.1979 (1 cs). Sagami Bay, 34°45.4′N 139°08.0′E – 34°46.2′N 139°09.0′E, 590 m, KT-65-34, Station 19, 11.1965 (5 cs, 14 af); 35°09.1′N 139°24.2′E, 519 m, KT-76-3, Station BS-1, 3.1976 (2 af); 35°11.5′N 139°28.4′E – 35°11.6′N 139°28.1′E, 491–580 m, Rinkai-maru, Station 2, 3.2001 (3 af); Kushimoto, 33°29.1′N 135°51.0′E – 33°29.2′N 135°51.2′E, 34–34 m, Station 9, 7.1972 (1 af). Off Usa, Tosa Bay, 33°21.4′N 133°35.4′E, 90 m, 4.1970 (3 af).
DESCRIPTION
Length 12–18 mm, width 0.3–0.43 mm. Prostomium with faintly delimited middle lobe, without glandular ridges or eyes (Figure 6A). Lower lip with median longitudinal incision (Figure 6B). Buccal tentacles smooth (Figure 6C). Three pairs of cirriform branchiae arranged in one transverse row in segment III, separated by wide median gap (Figure 6D); second outermost branchiae of transverse row originating from segment II, outermost branchiae of transverse row originating from segment III, innermost branchiae of transverse row originating from segment IV (Figure 13F). Segment II without chaetae. Notopodia with capillary chaetae from segment III, present in 14 chaetigers (Figure 6E, F); anterior notopodia small, increasing in size from first to third pair; notopodia without cirri. Neuropodial tori with uncini from segment VI, present in 11 thoracic uncinigers (Figure 6E); tori without cirri. Continuous ventral shields present to thoracic unciniger 9. Elevated or modified notopodia absent. Two intermediate uncinigers. Twenty-one to 23 abdominal uncinigers without rudimentary notopodia. Pinnules without dorsal cirrus (Figure 6G). Pygidium with terminal anus, encircled by about 10 long conical papillae and one pair of lateral cirriform anal cirri (Figure 6H). Uncini in thoracic and intermediate uncinigers with 5–6 teeth in one vertical row over rostral tooth and basal prow (Figure 6I). Abdominal uncini with numerous teeth in several vertical rows. Tube with concentric annulations (Figure 6J).
REMARKS
The Japanese specimens have 21–23 abdominal segments, whereas the type specimens from the Kara Sea were described with 24–25 abdominal segments. However, Théel (Reference Théel1879) included all posterior segments without notopodia, i.e. intermediate and abdominal uncinigers. Following the suggestion of Imajima et al. (Reference Imajima, Reuscher and Fiege2012), we distinguish segments that lack notopodia but have neuropodia shaped as in thoracic uncinigers as intermediate uncinigers. Thus, we exclude them from the count of abdominal uncinigers. Assuming that Theel's specimens had also two intermediate uncinigers, their number of abdominal uncinigers is 22–23. All other morphological characters are also in accordance with the original description.
DISTRIBUTION
This species seems to have a holarctic distribution as it has been recorded from the Kara Sea, the Barents Sea, the Norwegian Sea, the North Sea, the North-east and North-west Atlantic, the North-east Pacific and the Sea of Okhotsk (Holthe, Reference Holthe1986b). It is newly recorded from Japan, including Sagami Bay and Kushimoto on the Pacific coast of central Honshu and Tosa Bay of Shikoku, in 30–590 m.
Lysippe Malmgren, Reference Malmgren1866
TYPE SPECIES
Lysippe labiata Malmgren, Reference Malmgren1866
SYNONYMS
Lysippides Hessle, Reference Hessle1917
Pterolysippe Augener, Reference Augener1918
Paralysippe Williams, Reference Williams1987
Pseudampharete Hilbig, Reference Hilbig, Blake, Hilbig and Scott2000
GENERIC DIAGNOSIS
Prostomium without glandular ridges, with middle lobe encircled by surrounding lobe. Buccal tentacles smooth. Four pairs of branchiae. Segment II with chaetae, resembling capillary notochaetae. Thorax with 17–18 chaetigers and 13–14 uncinigers. Modified segments absent. One intermediate segment present in species with 13 thoracic uncinigers, absent in species with 14 uncinigers.
REMARKS
We accept the synonymies of Lysippides, Paralysippe, Pseudampharete and Pterolysippe that were suggested, but not discussed in detail by Jirkov (Reference Jirkov2011).
These genera have 14 uncinigers with neuropodial tori. In Lysippides all of these uncinigers carry notopodia as well, which makes them thoracic uncinigers. In Lysippe the last pair of notopodia has been lost, resulting in 13 thoracic uncinigers and one intermediate unciniger. We agree with Jirkov (Reference Jirkov2011) that the loss of one pair of notopodia may not justify the erection of a separate genus. It should be noted that Hessle (Reference Hessle1917) based the genus also on a difference in the number of nephridia between the type species of both genera. However, the value of this character for the generic diagnosis has not been examined any further in other species of Lysippe or Lysippides.
Paralysippe is identical with Lysippides, and therefore considered a synonym of Lysippe. Williams (Reference Williams1987) seemingly did not know of the existence of the genus Lysippides because she erected Paralysippe for the presence of 14 thoracic uncinigers as only major difference from Lysippe. However, Hessle (Reference Hessle1917) had erected Lysippides for the same reason. Additionally, Williams (Reference Williams1987) did not mention the genus Lysippides in her justification to erect Paralysippe.
Pterolysippe is only distinguished from Lysippe by the presence of one pair of pinnate branchiae. We follow Day (Reference Day1964) and Jirkov (Reference Jirkov2011) in their opinion that this single character is not sufficient for the erection of a separate genus.
Pseudampharete was erected by Hilbig (Reference Hilbig, Blake, Hilbig and Scott2000) for the species Lysippe mexicana Fauchald, Reference Fauchald1972. According to Hilbig (Reference Hilbig, Blake, Hilbig and Scott2000) this species was identical to unidentified specimens ‘Ampharetidae gen. A sp. A’ from California (Lissner et al., Reference Lissner, Phillips, Cadien, Smith, Bernstein, Cimberg, Kauwling and Anikouchine1986). Hilbig (Reference Hilbig, Blake, Hilbig and Scott2000) noted that L. mexicana had, in contrast to Fauchald's original description, only 12 thoracic uncinigers and therefore needed to be removed from Lysippe. However, a careful re-examination of the type specimen of L. mexicana revealed that the species has 13 thoracic uncinigers (Southern California Association of Marine Invertebrate Taxonomists, 2001), making the erection of Pseudampharete obsolete. Furthermore, the careful examination of Lissner et al.'s (Reference Lissner, Phillips, Cadien, Smith, Bernstein, Cimberg, Kauwling and Anikouchine1986) material revealed that Ampharetidae gen. A sp. A contained a mixture of specimens with 13 uncinigers, belonging to Lysippe, and specimens with 12 uncinigers, belonging to a new species (Southern California Association of Marine Invertebrate Taxonomists, 2001).
We do not agree with Jirkov's (Reference Jirkov2011) suggested synonymy of Lysippe and Samytha Malmgren, Reference Malmgren1866. We acknowledge that both genera are closely related as they share the shape of their prostomia, the development of their lower lips, and the presence of glandular pads above their abdominal uncinigers. However, Samytha has only three pairs of branchiae. Lysippe species have filiform anal cirri, whereas Samytha species have only short and stout anal cirri.
Lysippe fragilis (Wollebaek, Reference Wollebaek1912)
(Figures 7A–J; 14A)

Fig. 7. Lysippe fragilis: (A) prostomium, dorsal view, 23×; (B) anterior end, ventral view, 19×; (C) same, lateral view, 24×; (D) same, dorsal view, 24×; (E) distal end of branchia, lateral view, 154×; (F) thoracic parapodium, lateral view, 28×; (G) posterior end, ventral view, 28×; (H) thoracic uncinus, lateral view, 825×; (I) same, frontal view, 825×; (J) abdominal uncinus, frontolateral view, 858×.
Amphicteis fragilis Wollebaek, Reference Wollebaek1912: 57–58, plate VIII, figures 1–7.
Lysippides fragilis: Hessle, Reference Hessle1917: 111. – Holthe, Reference Holthe1986b: 55–56, figure 20.
SPECIMENS EXAMINED
Sagami Sea, 34°56.2′N 139°15.0′E – 34°56.9′N 139°15.2′E, 1350 m, KT-65–34, Station 18, 11.1965 (1 cs); 35°03.1′N 138°50.6′E – 35°02.2′N 138°50.9′E, 100–99 m, KT-73-15, Station A, 10.1973 (1 cs). Off Cape Ashizuri, 32°41.2′N 132°38.6′E –32°41.3′N 132°38.2′E, 208–209 m, KT-99-18, Station DG-10, 12.1999 (6 cs). SMF 23959, Bungo Channel, 32°41.9′N 132°15.9′E – 32°41.9′N 132°25.4′E, 260–261 m, KT-99-18, Station DG-5, 12.1999 (7 cs). Off Tsushima Islands, 33°49.6′N 129°29.0′E, 100 m, RV ‘Genkai’, Station 8, 7.1968 (1 cs). Off Okinoshima Isle, 34°15.1′N 130°15.0′E – 34°15.0′N 130°14.9′E, 100–100 m, RV ‘Soyo-maru’, Station SO 08-D5, 7.2008 (2 cs).
DESCRIPTION
Length 10–12 mm, width 0.9–1 mm. Prostomium with middle lobe encircled by surrounding lobe (Figure 7A); prostomium without glandular ridges or eyespots; anterior portion of lower lip crenulated (Figure 7B). Buccal tentacles smooth (Figure 7B, C). Four pairs of cirriform, slightly annulated branchiae (Figure 7D) with transverse rows of cilia ventrally (Figure 7E); three pairs of branchiae in one transverse row between segments II and III, separated by small median gap (Figure 7D); last pair of branchiae located next to notopodia of segments IV; second outermost branchiae of transverse row originating from segment II, outermost branchiae of transverse row originating from segment III, innermost branchiae of transverse row originating from segment IV, posterior branchiae originating from segment V (Figure 14A). Segment II with chaetae, resembling capillary notochaetae of following thoracic chaetigers, but slightly longer (Figure 7C). Notopodia with capillary chaetae from segment III, present in 17 chaetigers (Figure 7F); anterior notopodia small, increasing in size from first to fourth pair; notopodia without cirri. Neuropodial tori with uncini from segment VI, present in 14 thoracic uncinigers (Figure 7F); tori without cirri. Continuous ventral shields conspicuous to thoracic unciniger 8. Elevated or modified notopodia absent. Intermediate uncinigers absent. Eight abdominal uncinigers without rudimentary notopodia, but with glandular pads above pinnules. Pinnules without dorsal cirrus (Figure 7G). Pygidium with terminal anus crenulated and one pair of lateral cirriform anal cirri (Figure 7G). Thoracic and abdominal uncini with numerous teeth in several vertical rows over rostral tooth and basal prow (Figure 7H–J).
REMARKS
Our specimens have only a small median gap between the groups of branchiae, whereas Wollebaek (Reference Wollebaek1912) described it with a larger median gap. We think that this character may vary intraspecifically and change with the maturity of the specimens.
DISTRIBUTION
Sagami Sea of central Honshu, off Cape Ashizuri in the Pacific coast of Shikoku, and off Okinoshima Isle in the Sea of Japan, 100–1350 m.
This species seems to have a holarctic distribution. It has been recorded off the coasts off Norway (Wollebaek, Reference Wollebaek1912) and Japan (Kyushu) (Hessle, Reference Hessle1917).
Lysippe nipponica sp. nov.
(Figures 8A–J; 14B)

Fig. 8. Lysippe nipponica sp. nov.: (A) prostomium, dorsal view, 56×; (B) anterior end, dorsal view, 40×; (C) same, lateral view, 54×; (D) outermost branchia, lateral view, 76×; (E) innermost branchia, dorsal view, 76×; (F) chaeta of segment II, 506×; (G) notochaeta, 255×; (H) abdominal neuropodium, anterolateral view, 84×; (I) thoracic uncinus, frontal view, 935×; (J) thoracic uncinus, lateral view, 935×.
SPECIMENS EXAMINED
Holotype: NSMT-Pol. H585, Nagasaki Bay, 32°42.5′N 129°47.9′E, 50 m, Station 21, 3.1971 (1 af).
DESCRIPTION
Length 6 mm, lacking posterior end. Anterior region ruptured ventrally. Prostomium with middle lobe encircled by surrounding lobe (Figure 8A); prostomium without glandular ridges or eyespots. Lower lip crenulated (Figure 8A). Buccal tentacles unknown. Four pairs of cirriform branchiae; three pairs in one transverse row in segment III, not separated by median gap (Figure 8B); fourth pair of branchiae missing, only represented by visible scars, located behind outermost branchiae of transverse row; branchiae with conspicuous annulations and covered by ventral tufts of cilia (Figure 8B, C, E); outermost and second outermost branchiae of transverse row with smooth tapering tips (Figure 8D); innermost branchiae of transverse row with blunt annulated tips (Figure 8E); second outermost branchiae of transverse row originating from segment II, outermost branchiae of transverse row originating from segment III, innermost branchiae of transverse row originating from segment IV, posterior branchiae originating from segment V (Figure 14B). Segment II with brittle chaetae, resembling capillary notochaetae of following thoracic chaetigers (Figure 8F). Notopodia with capillary chaetae from segment III, present in 16 chaetigers (Figure 8G); anterior notopodia well developed, slightly increasing in size from first to third pair; notopodia without cirri. Neuropodial tori with uncini from segment VI, present in 13 thoracic uncinigers; tori without cirri. Continuous ventral shields conspicuous to thoracic unciniger 8, faint to thoracic unciniger 10. Elevated or modified notopodia absent. One intermediate unciniger. Intermediate unciniger with glandular pads above tori. At least five abdominal uncinigers without rudimentary notopodia or glandular pads (Figure 8H). Pinnules without dorsal cirrus (Figure 8H). Pygidium unknown. Uncini of thoracic, intermediate, and abdominal uncinigers with about seven teeth in two alternating vertical rows over rostral tooth and basal prow (Figure 8I, J).
REMARKS
The scars of the posterior branchiae are difficult to see and may be easily overlooked.
Only two species in the genus Lysippe have been described with cirriform branchiae, 17 thoracic chaetigers (including chaetae of segment II), and 13 thoracic uncinigers, the type species L. labiata and L. mexicana. Both resemble the new species in the shape of the prostomium, the lower lip and the chaetae of segment II. Furthermore, L. mexicana resembles the new species by the possession of annulated branchiae. However, Fauchald describes that ‘the outer and inner pairs’ (the third and fourth pair) are smooth, and only ‘the two median pairs’ (the first and second pair) are annulated. In L. nipponica sp. nov. the first three pairs are annulated, the fourth pair is unknown. Additionally, the two outermost pairs of the transverse row (the first and second pair) have smooth tapering tips, which are lacking in L. mexicana.
ETYMOLOGY
The species is named after its collecting location, Japan (Nippon in Japanese).
DISTRIBUTION
Nagasaki Bay, Kyushu, in 50 m.
Orochi gen. nov.
TYPE SPECIES
Orochi palacephalus sp. nov.
GENERIC DIAGNOSIS
Prostomium spade-like, without glandular ridges or lobes. Buccal tentacles smooth. Four pairs of basally fused, flattened branchiae. Segment II without chaetae. Thorax with 15 chaetigers and 12 uncinigers. Modified segments absent. Neuropodia of last thoracic unciniger shaped as pinnules. No intermediate uncinigers. Abdomen with glandular pads above pinnules.
REMARKS
The new genus is related to Phyllocomus Grube, Reference Grube1878b. Both genera share the peculiar prostomial shape, number of branchiae, thoracic chaetigers, absence of chaetae in segment II, absence of modified segments, presence of abdominal notopodial rudiments and branchiae that are connected by a dermal fold. Phyllocomus differs from the new genus by the presence of strongly modified branchiae. Species of that genus possess either very broad and leaf-like branchiae with undulating margins or uni- and bipinnate branchiae. The new genus has branchiae that are flattened, but still resemble the cirriform branchiae shape, found in most ampharetids. Furthermore, the uncinigerous neuropodia of the last thoracic unciniger are of the same shape as the abdominal pinnules. This is a unique character within the Ampharetinae.
ETYMOLOGY
The new genus is named after the Orochi, the eight-headed dragon of the Japanese Shinto. The eight branchiae resemble the eight long-necked heads of Orochi.
Orochi palacephalus sp. nov.
(Figures 9A–H; 14C)

Fig. 9. Orochi palacephalus gen. et sp. nov.: (A) anterior end, lateral view, 18×; (B) prostomium, dorsal view, 13×; (C) anterior end, dorsal view, 18×; (D) thoracic parapodium, anterior view, 37×; (E) notochaeta, 180×; (F) abdominal parapodium, anterolateral view, 50×; (G) thoracic uncinus, lateral view, 704×; (H) abdominal uncinus, lateral view, 732×.
SPECIMENS EXAMINED
Holotype: NSMT-Pol. H586, off Cape Ashizuri, 32°41.2′N 132°38.6′E – 32°41.3′N 132°38.2′E, 208–209 m, KT-99-18, Station DG-10, 12.1999 (1 af). Paratype: NSMT-Pol. P587, same locality as holotype (4 af).
ADDITIONAL MATERIAL EXAMINED
Phyllocomus crocea Grube, Reference Grube1878b
Holotype: ZMB 858. Sub-Antarctic, between the Crozet Islands and the Kerguelen Islands. 47°15.5′S 70°47′E. Kerguelen Expedition Gazelle, collector Grube. 1 cs + tube.
DESCRIPTION
Length 12 mm, width 2 mm. Prostomium anteriorly elongated into rectangular spatulate lobe (Figure 9A), without glandular ridges or eyespots, but with inconspicuously delimited middle lobe (Figure 9B). Buccal tentacles not visible in holotype. Four pairs of flat cirriform branchiae (Figure 9C); three pairs of branchiae arranged in one transverse row without median gap in segment II; fourth pair of branchiae behind innermost branchiae of transverse row; branchiae connected by high membrane (Figure 9B, C); branchial membrane deeply notched between outermost and second outermost branchiae of transverse row; innermost branchiae of transverse row originating from segment II, outermost branchiae of transverse row originating from segment III, second outermost branchiae of transverse row originating from segment IV, posterior branchiae originating from segment V (Figure 14C). Segment II without chaetae. Notopodia with capillary chaetae from segment III, present in 15 chaetigers (Figure 9D, E); anterior notopodia small, increasing in size from first to fifth pair; notopodia without cirri. Neuropodial tori with uncini from segment VI, present in 12 thoracic uncinigers (Figure 9D); length of tori gradually decreasing between first and seventh thoracic unciniger; tori without cirri. Last thoracic neuropodia developed as pinnules. Body swollen between segments II and VI due to strongly developed ventral shields (Figure 9A); ventral shields conspicuous to thoracic unciniger 9, faint to thoracic unciniger 11. Elevated or modified notopodia absent. Intermediate uncinigers absent. Holotype incomplete, with 15 abdominal uncinigers with glandular pads with terminal globular papilla (Figure 9F). Pinnules without dorsal cirrus (Figure 9F). Pygidium unknown. Thoracic and abdominal uncini with five teeth in one vertical row over rostral tooth, subrostral process and basal prow (Figure 9G, H).
REMARKS
Several smooth buccal tentacles are visible in one of the paratypes.
ETYMOLOGY
The species name palacephalus refers to the unusual shape of the prostomium, resembling a spade or shovel (pala = spade in Latin; cephalus derived from kephale = head in Greek).
DISTRIBUTION
Off Cape Ashizuri, Pacific coast of Shikoku, in 209 m.
Samytha Malmgren, Reference Malmgren1866
TYPE SPECIES
Sabellides sexcirrata Sars, Reference Sars1856
GENERIC DIAGNOSIS
Prostomium without glandular ridges, with middle lobe encircled by surrounding lobe. Buccal tentacles smooth. Three pairs of cirriform branchiae. Segment II without chaetae. Thorax with 17 chaetigers and 14 uncinigers. Modified or intermediate segments absent. Abdomen with glandular pads above pinnules.
REMARKS
For reasons discussed in the remarks of the genus Lysippe above, we disagree with Jirkov's (Reference Jirkov2011) suggestion to synonymize Lysippe and Samytha.
Samytha annenkovae sp. nov.
(Figures 10A–F; 14D)

Fig. 10. Samytha annenkovae sp. nov.: (A) anterior end, dorsal view, 16×; (B) same, lateral view, 16×; (C) thoracic parapodia, lateral view, 18×; (D) abdominal parapodium, anterior view, 18×; (E) thoracic uncinus, lateral view, 770×; (F) abdominal uncinus, frontolateral view, 770×.
SPECIMENS EXAMINED
Holotype: NSMT-Pol. H588, Sagami Bay, 35°09.2′N 139°30.4′E – 35°08.9′N 139°29.5′E, 590–590 m, KT-66-12, Station 1, 6.1966 (1 af). Paratypes: NSMT-Pol. P589, same locality as holotype (2 cs). SMF 23960, Sagami Bay, 35°10.0′N 139°34.9′E – 35°10.3′N 139°34.8′E, 84 m, Rinkai-maru, Station 7, 9.1979 (2 cs, 1 af).
ADDITIONAL SPECIMENS EXAMINED
Yamada Bay, 39°37.9′N 141°59.9′E, 20 m, Station 13, 6.1967 (2 af). Otsuchi Bay, 39°20.7′N 141°57.5′E – 39°20.6′N 141°57.6′E, 45 m, Station 5, 10.1978 (2 cs). Off Sanriku, 36°41.06′N 141°22.04′E, 512–508 m, RV ‘Wakataka-maru’, Station GH510D, 11.2005 (3 cs). Sagami Sea, 35°05.3′N 139°30.0′E – 35°03.4′N 139°29.2′E, 850 m, KT-65-34, Station 1, 11.1965 (2 af); 35°00.9′N 139°35.7′E – 35°00.7′N 139°36.0′E, 1060–990 m, KT-66-12, Station 7, 7.1966 (4 af). Kagoshima Bay, 31°40.1′N 130°45.2′E, 162 m, Station 55, 8.1975 (3 cs).
DESCRIPTION
Length 13 mm, width 2.5 mm. Prostomium with middle lobe encircled by surrounding lobe (Figure 10A); prostomium without glandular ridges or eyespots. Lower lip crenulated (Figure 10B). Tentacle membrane visible; buccal tentacles smooth (Figure 10B). Three pairs of cirriform branchiae, arranged in one transverse row in segment II, separated by small median gap (Figure 10A); second outermost branchiae of transverse row originating from segment II, outermost branchiae of transverse row originating from segment III, innermost branchiae of transverse row originating from segment IV (Figure 14D). Segment II without chaetae. Notopodia with capillary chaetae from segment III, present in 17 chaetigers (Figure 10C); anterior notopodia well developed, slightly increasing in size from first to third pair; first three notopodia slightly elevated; notopodia without cirri. Neuropodial tori with uncini (Figure 10C) from segment VI, present in 14 thoracic uncinigers; tori without cirri. Continuous ventral shields conspicuous to thoracic unciniger 8, faint in thoracic unciniger 9. Elevated or modified notopodia absent. Intermediate uncinigers absent. Posterior end of holotype broken after 12 abdominal uncinigers; with glandular pads above pinnules, but without rudimentary notopodia (Figure 10D). Pinnules without dorsal cirrus (Figure 10D). Thoracic uncini with 4 teeth above rostral tooth and basal prow (Figure 10E). Abdominal uncini with 6 teeth in 2 alternating vertical rows over rostral tooth and basal prow (Figure 10F).
REMARKS
The complete paratypes have 15 abdominal uncinigers. The pygidium has thick but short lateral anal cirri. Tips of buccal tentacles are visible, but the tentacle membrane is not. The lower lip of some paratypes is less conspicuously crenulated. Perhaps the crenulations are more pronounced when the tentacle membrane, carrying the buccal tentacles is more extended.
The new species can be distinguished from its congeners by the number of abdominal uncinigers: S. californiensis Hartman Reference Hartman1969 (16–20), S. gurjanovae Uschakov, Reference Uschakov1950 (19) and S. hesslei Caullery, Reference Caullery1944 (25–26) have a higher, S. sexcirrata (Sars, Reference Sars1856) (13), S. speculatrix Ehlers, Reference Ehlers and von Drygalski1913 (12), and S. storchi Reuscher and Wehe in Wehe, Reference Wehe2009 (11) a lower number of abdominal uncinigers. S. californiensis, the species with the most similar count of abdominal uncinigers, also differs from S. annenkovae sp. nov. by the shape of thoracic and abdominal uncini. Samytha oculata Grube, Reference Grube1878a and S. heterobranchia Caullery, Reference Caullery1944 belong most likely to different genera.
ETYMOLOGY
The new species is named in honour of the renowned Russian polychaetologist Nadezhda Annenkova-Khlopina (1887–1950).
DISTRIBUTION
Pacific coast from Yamada Bay to Kagoshima Bay, in 20–1000 m.
Melinninae Chamberlin, Reference Chamberlin1919
Melinnopsis McIntosh, Reference McIntosh1885
TYPE SPECIES
Melinnopsis atlantica McIntosh, Reference McIntosh1885
SYNONYMS
Amelinna Hartman, Reference Hartman1969
Melinnexis Annenkova, Reference Annenkova1931
Melinnides Wesenberg-Lund, Reference Wesenberg-Lund1950
GENERIC DIAGNOSIS (EMENDED)
Large buccal tentacles occurring along with smaller ones. Four pairs of branchiae. Postbranchial hooks absent. Postbranchial dorsal crest usually present. Brittle acicular neurochaetae in segments II–IV or II–V. Twelve to 14 thoracic uncinigers. Uncini with subrostral process.
REMARKS
The emendation of the generic diagnosis is necessary to include our new interpretation of the uncini morphology. A close examination of the uncini leads us to the conclusion that the lowermost tooth is homologous to the subrostral process and the larger tooth above the subrostral process is homologous to the rostral tooth (according to the nomenclature of Holthe Reference Holthe1986a). Therefore, we recommend that Melinnopsis uncini with 4 teeth over the basal prow (e.g. in Melinnopsis tetradentata Imajima, Reference Imajima, Fujita, Saito and Takeda2001) be described as ‘2 teeth over rostral tooth, subrostral process, and basal prow’. Other genera of the Melinninae also seem to have uncini with a subrostral process beneath their rostral tooth, according to the illustrations in their respective original descriptions.
Melinnopsis augeneri sp. nov.
(Figures 11A–J; 14E)

Fig. 11. Melinnopsis augeneri sp. nov.: (A) prostomium, dorsal view, 42×; (B) anterior end, lateral view, 24×; (C) distal end of innermost branchia, lateral view, 39×; (D) anterior end, dorsal view, 24×; (E) thoracic parapodium, lateral view, 66×; (F) abdominal parapodium, anterior view, 66×; (G) posterior end, ventral view, 54×; (H) thoracic uncinus, lateral view, 913×; (I) abdominal uncinus, frontal view, 924×; (J) same, lateral view, 890×.
SPECIMENS EXAMINED
Holotype: NSMT-Pol. H590, Goto-Kasayama Bank, 32°20.2′N 128°46.4′E – 32°20.3′N 128°46.4′E, 185–185 m, RV ‘Soyo-maru’, Station 08-D7, 8.2008 (1 cs).
DESCRIPTION
Length 14 mm, width 0.8 mm. Prostomium subtriangular, with orange pigment spots on posterior margin (Figure 11A). Lower lip formed as trapezoid cushion. Buccal tentacles of two kinds: four tentacles long, thick and annulated, with deep ventral groove, in dorsal position; three tips of thinner buccal tentacles visible in ventral position (Figure 11A, B). Four pairs of cirriform branchiae, arranged in arch; innermost branchiae covered with transverse rows of cilia (Figure 11C); gap between groups of branchiae absent (Figure 11D); branchiae basally fused (Figure 14E); segmental origin of branchiae indeterminable due to convexly arching dorsal integument behind branchiae in segment II. Postbranchial dorsal membrane in segment V very low and inconspicuous, without serration. Postbranchial hooks absent. Lateral wings of anterior body between peristomium and segment V cushion-like and slightly arched. Segments II–V with brittle acicular neurochaetae. Capillary notochaetae present in 15 thoracic chaetigers from segment IV; segment IV with few brittle capillary notochaetae emerging from body with no notopodia present; segment V with larger number of brittle capillary notochaetae emerging in the same way; notopodia commencing in segment VI (Figure 11E), growing in size to approximately segment VIII; notopodia without cirri. Neuropodial tori with uncini (Figure 11E) from segment VI, present in 13 thoracic uncinigers; neuropodia without cirri. Shape of neuropodia gradually changing between thorax and abdomen, with last thoracic neuropodia resembling abdominal ones. Continuous ventral shields in all thoracic uncinigers. Elevated or modified notopodia absent. Intermediate uncinigers absent. Twenty-six abdominal uncinigers with glandular pads and minute papillae dorsally of pinnules (Figure 11F). Posterior abdomen damaged with 8 posteriormost uncinigers and pygidium only slightly attached to specimen. First four abdominal neuropodia with minute dorsal papilla, lacking in subsequent uncinigers. Pygidium with terminal crenulated anus, lacking anal cirri (Figure 11G). Uncini of thoracic uncinigers 1–12 with 2 teeth in one vertical row over rostral tooth, subrostral process and basal prow (Figure 11H). Uncini of last thoracic unciniger and abdominal uncinigers with numerous teeth over rostral tooth, subrostral process and basal prow (Figure 11I, J).
REMARKS
The majority of Melinnopsis species differ from M. augeneri sp. nov. by the presence of only 12 thoracic uncinigers. Five other species within the genus with 13 thoracic uncinigers have been described. M. angolensis Hilbig, Reference Hilbig2005, M. moorei (Hartman, Reference Hartman1960), and M. tetradentata differ from M. augeneri sp. nov. by the presence of a well-developed crenulated postbranchial dorsal membrane. M. armipotens (Moore, Reference Moore1923) and M. mcintoshi sp. nov. are both lacking the crenulations, but their postbranchial dorsal membrane is well-developed, whereas it is vestigial in M. augeneri sp. nov. The branchiae in M. augeneri sp. nov. are basally all fused. In contrast, both groups are widely separated in M. armipotens; in M. mcintoshi sp. nov. both groups of branchiae are in close proximity, but they are not fused. M. armipotens has uncini with 3, rather than 2 teeth above the rostral tooth. The lateral wings in M. augeneri sp. nov. are relatively low and long, with the acicular neurochaetae arranged in line. In contrast, M. mcintoshi sp. nov. has shorter arched lateral wings, with the first 3 rows of acicular neurochaetae almost being arranged vertically. Furthermore, the presence of uncini of the abdominal type in the last thoracic neuropodia is quite unusual and has not been observed in any other species of the genus.
ETYMOLOGY
The new species is named after the German zoologist Hermann Augener (1872–1938), honouring his valuable contributions to polychaete taxonomy.
DISTRIBUTION
Goto-Kasayama Bank, west off Kyushu, in 185 m.
Melinnopsis mcintoshi sp. nov.
(Figures 12A–I; 14F)

Fig. 12. Melinnopsis mcintoshi sp. nov.: (A) anterior end, dorsolateral view, 13×; (B) acicular neurochaetae of segment 4, 308×; (C) capillary chaetae of third notopodium, 150×; (D) abdominal parapodium, lateral view, 33×; (E) pygidium, ventral view, 62×; (F) thoracic uncinus, frontal view, 935×; (G) same, lateral view, 935×; (H) abdominal uncinus, frontal view, 902×; (I) same, lateral view, 913×.

Fig. 13. ID cards of recorded ampharetid species. (A) Amage cf. adspersa; (B) Amage ehlersi sp. nov.; (C) Amage longitorus sp. nov.; (D) Amphisamytha japonica; (E) Glyphanostomum hesslei sp. nov.; (F) Glyphanostomum pallescens.

Fig. 14. ID cards of recorded ampharetid species. (A) Lysippe fragilis; (B) Lysippe nipponica sp. nov.; (C) Orochi palacephalus gen. et sp. nov.; (D) Samytha annenkovae sp. nov.; (E) Melinnopsis augeneri sp. nov.; (F) Melinnopsis mcintoshi sp. nov.
Melinnopsis atlantica: Imajima, Reference Imajima and Fujita2009: 164, figures 62–63.
SPECIMENS EXAMINED
Holotype: NSMT-Pol. H591, off Otsuchi Bay, 38°42.0′N 143°01.6′E – 38°40.6′N 143°00.2′E, 1642–1659 m, KT 85-11, Station SR17, 8.1985 (1 cs). Paratypes: NSMT-Pol. P592, same locality as holotype (3 cs). SMF 23961, same locality as holotype (2 cs).
ADDITIONAL SPECIMENS EXAMINED
Chishima Trench, 41°25.4′N 146°23.5′E – 41°26.6′N 146°22.7′E, 5566–5613 m, RV ‘Hakuho-maru’, KH01-02, Station XR-10, 9.2001 (4 cs). Bungo Channel, 32°42.2′N 132°29.7′E – 32°42.3′N 132°29.5′E, 231–232 m, KT-99-18, Station DG-4, 12.1999 (2 af). Hyuga Basin, 32°26.5′N 132°14.6′E – 32°27.3′N 132°16.5′E, 1291–1298 m, KT-99-18, Station BT-3, 12.1999 (7 cs, 2 af). Off Cape Toi, 31°18.8′N 131°28.0′E, 164 m, RV ‘Toyoshio-maru’, Station TY-03-03, 5.2003 (7 cs, 15 af); 31°14.5′N 131°32.2′E – 31°15.0′N 131°31.5′E, 317–265 m, Station TY06-12, 5.2003 (1 cs). Off Okinawa Sima, 26°32.5′N 127°44.5′E, 391–394 m, RV ‘Toyoshio-maru’, Station TY-03-10, 5.2003 (2 cs).
DESCRIPTION
Length 44 mm, width 2 mm. Prostomium formed as simple lobe without incisions, ridges or eyes. Buccal tentacles of two kinds: 3 tentacles long, thick and annulated, with deep ventral groove, in dorsal position (Figure 12A); 4 thinner buccal tentacles in ventral position, barely emerging buccal cavity. Four pairs of cirriform branchiae arranged in 2 staggered transverse rows (Figure 12A); 2 pairs of anterior row not separated by median gap; posterior transverse row with median gap of about one branchial width; all four branchiae of each group fused basally (Figure 14F); innermost branchiae of anterior row thicker than other branchiae and with more conspicuous annulations (Figure 12A); segmental origin of branchiae indeterminable. Postbranchial dorsal membrane in segment V conspicuous, without serration (Figure 12A). Postbranchial hooks absent. Lateral wings of anterior body between peristomium and segment V thick and highly arched. Segments II–V with brittle acicular neurochaetae (Figure 12A, B); acicular neurochaetae of segments II–IV almost vertically arranged due to high arch. Capillary notochaetae present in 15 thoracic chaetigers from segment IV (Figure 12C); segment IV with few brittle capillary notochaetae emerging from body with no notopodia present; segment V with larger number of brittle capillary notochaetae emerging in the same way; notopodia commencing in segment VI, growing in size to approximately segment VIII; notopodia without cirri. Neuropodia with uncini from segment VI, present in 13 thoracic uncinigers; neuropodia without cirri. Continuous ventral shields in all thoracic uncinigers. Elevated or modified notopodia absent. Intermediate uncinigers absent. Approximately 45 abdominal uncinigers with glandular pads and minute papillae dorsally of neuropodia (Figure 12D). Change of shape between thoracic and abdominal neuropodia gradual, with last thoracic neuropodia resembling abdominal neuropodia; first 4 abdominal neuropodia with minute dorsal papilla, lacking in subsequent uncinigers. Pygidium long, with longitudinal rifts and terminal crenulated anus, lacking anal cirri (Figure 12E). Thoracic uncini with 2 teeth in 1 vertical row over rostral tooth, subrostral process and basal prow (Figure 12F, G). Abdominal uncini with numerous teeth over rostral tooth, subrostral process and basal prow (Figure 12H, I).
REMARKS
The number of abdominal uncinigers changes with size of the individual. The number in our specimens varied from 24 to 45.
This species has been reported by Imajima (Reference Imajima and Fujita2009) as M. atlantica McIntosh, Reference McIntosh1885. However, the latter has, according to McIntosh's original description (1885), only 14 segments with capillary chaetae and presumably 12 thoracic uncinigers. In contrast, Melinnopsis mcintoshi sp. nov. has 15 segments with capillary chaetae and 13 thoracic uncinigers.
Among the Melinnopsis species with 13 uncinigers (see also discussion of Melinnopsis augeneri sp. nov.), M. angolensis, M. moorei and M. tetradentata all have a crenulated, rather than a smooth postbranchial dorsal membrane. M. armipotens differs from M. mcintoshi sp. nov. by the presence of 1 very large, rather than 3 moderately enlarged, buccal tentacle, the large gap between branchial groups, and the shape of thoracic uncini that have 3, rather than 2 teeth above the rostral tooth. The differences between both new Melinnopsis species described herein are discussed in the remarks of M. augeneri sp. nov. above.
ETYMOLOGY
The new species is named after the Scottish naturalist William Carmichael McIntosh (1838–1931), describer of the genus Melinnopsis.
DISTRIBUTION
Chishima Trench off northern Japan, off Otsuchi Bay in the Pacific, Bungo Channel and Hyuga Basin in the east of Kyushu, off Okinawa in the Pacific Ocean, in 164–5600 m.
CONCLUSION
This paper concludes our taxonomic series about polychaetes of the family Ampharetidae from Japan. We described 35 species, of which 33 belonged to Ampharetinae and two to Melinninae. Twenty-one of these species were new to science. This means that 60% of the species in our samples were undescribed. We also described three new genera, Tanseimaruana Imajima, Reuscher & Fiege, Reference Imajima, Reuscher and Fiege2012, Watatsumi Reuscher, Fiege & Imajima, Reference Reuscher, Fiege and Imajima2014, and Orochi gen. nov.
We compiled a list of all ampharetid species that have been recorded from Japan (Table 1). With the new species and new records from our four papers the number of species known to occur in Japanese waters has more than doubled. Prior to our study, 28 species of Ampharetidae were known from Japan. Our series of publications adds 30 species that are either new to science or new records.
We discussed the taxonomic value and nomenclature of morphological characters of ampharetid polychaetes. Segmental origins of the branchiae and the ventral shields were introduced as valuable taxonomic characters. We commented on the suggested changes to ampharetid taxonomy by Jirkov (Reference Jirkov2011) and provided several alternative suggestions.
Furthermore, we introduced the ‘ID cards’ that facilitate the illustration of important taxonomic characters in the anterior end of the ampharetid body. We also introduced the term ‘intermediate uncinigers’ to describe segments that have neuropodia of the thoracic type but are devoid of notopodia.
ACKNOWLEDGEMENTS
We thank Birger Neuhaus (Museum für Naturkunde Berlin) for the loan of a type specimen from the ZMB.