INTRODUCTION
Information on the age of individual fish significantly enhances the quality of studies of population characteristics such as growth, recruitment, mortality and reproduction, and it is often a prerequisite for more detailed studies of life history strategies and ecology (Labropoulou & Papaconstantinou, Reference Labropoulou and Papaconstantinou2000). Most of the studies on age and growth of fish involve the determination of age of specimens by counting of growth increments in hard body parts, usually otoliths. The mechanism that regulates the cyclic deposition of these growth increments in otoliths is not well understood (Beckman & Wilson, Reference Beckman, Wilson, Secor, Dean and Campana1995), but it is commonly assumed to be related to seasonal variation in somatic growth, spawning, environmental factors and food availability (Boehlert, Reference Boehlert1985; Campana, Reference Campana1999; Morales-Nin & Panfili, Reference Morales-Nin and Panfili2005).
Interpreting otolith structure and counting annual growth increments underlies the method used for age assignment (e.g. Bagenal, Reference Bagenal1974; Pentilla & Dery, Reference Pentilla and Dery1988; Fowler, Reference Fowler, Secor, Dean and Campana1995). To ensure that the age estimates are accurate, ages assigned to specimens need to be validated (Beamish, Reference Beamish and Hancock1992; Campana, Reference Campana2001), since non-validated ages commonly result in seriously biased age estimates (Beamish & McFarlane, Reference Beamish and McFarlane1983, Reference Beamish, McFarlane, Secor, Dean and Campana1995; Lai & Gunderson, Reference Lai and Gunderson1987). Since growth parameters constitute key input to stock assessment models used to analyse population dynamics in relation to exploitation and management measures (Cailliet et al., Reference Cailliet, Andrews, Burton, Watters, Kline and Ferry-Graham2001; Stewart & Hughes, Reference Stewart and Hughes2007), biased ageing could lead to less effective fishery management policies (Beamish and McFarlane, Reference Beamish and McFarlane1983; Campana, Reference Campana2001; Cailliet & Andrews, Reference Cailliet, Andrews, Tsukamoto, Kawamura, Takeuchi, Beard and Kaiser2008).
Recent analyses suggest that most fished stocks are fully or over-exploited (Botsford et al., Reference Botsford, Castilla and Peterson1997). The most dramatic results of intensive fishing have been the economic collapse of some commercially important stocks such as cod, Gadus morhua Linnaeus, 1758, and herring, Clupea harengus Linnaeus, 1758 (Beverton, Reference Beverton1990; Myers et al., Reference Myers, Hutchings and Barrowman1997). One of the challenges for fisheries scientists is the provision of management advice for developing fisheries and for species for which there are limited biological data.
The forkbeard, Phycis phycis (Linnaeus, 1766) lies in the category of a developing fishery species, but is already one of the most important gadiform species commercially exploited by the Portuguese fleet in southern north-east Atlantic, together with European hake, Merluccius merluccius Linnaeus, 1758, pouting, Trisopterus luscus Linnaeus, 1758 and blue whiting, Micromesistius poutassou Risso, 1827 (INE, 2013). In Portuguese waters, forkbeard is mainly caught by a longline fishery, with trawl, trammel net and trap fisheries contributing a small percentage of the landings. The fisheries range from coastal waters to offshore seamounts off the mainland and also in the Azores and Madeira archipelagos, reaching a total of 800 t landed per year (INE, 2013). Forkbeard has already attained an important acceptance by final consumers, with its commercial price reaching approximately €9/kg. Although this species has a wide distribution, from the Bay of Biscay to Morocco, south to Cape Verde and also in the Mediterranean Sea (Cohen et al., Reference Cohen, Inada, Iwamoto and Scialabba1990), little information exists on its biology. In fact, only data from Azorean and Mediterranean waters are available. With regard to age and growth, data obtained from burned sectioned otoliths from fish caught off the Azores showed forkbeard to be a relatively slow growing, long lived species (maximum age of 18 years for specimens of 71 cm total length (TL)), with no sexual dimorphism in growth (Abecasis et al., Reference Abecasis, Canha, Reis, Pinho and Gil-Pereira2009). Matić-Skoko et al. (Reference Matić-Skoko, Ferri, Škeljo, Bartulović, Glavić and Glamuzina2011), using ground otoliths from fishes collected in the south-eastern Adriatic Sea, estimated maximum ages of 5 years for fish of 45.8 cm TL, and these authors stated that forkbeard showed sexual dimorphism in growth, with males reaching a larger size and growing more slowly than females.
The present study investigated age and growth of forkbeard from Portuguese continental waters, the first study on this subject for this area, where forkbeard is an important commercial species. The main goals were (1) to understand the deposition pattern of growth increments in sectioned otoliths of the forkbeard, (2) to validate the assignment of age and (3) to model the growth of the species.
MATERIAL AND METHODS
Sampling
A total of 687 samples of the forkbeard were collected monthly between May 2011 and December 2012 from commercial vessels operating off mainland Portugal (Peniche), using longline (at depths between 220 and 275 m), trawl (up to 310 m depth), trammel net and traps (at 55–90 m depth). TL (to the nearest 0.1 cm), total weight (TW, to the nearest 0.01 g) and sex of each fish were recorded, and the sagitta otoliths were removed, rinsed with water, air dried and stored in labelled plastic tubes.
Length-weight relationship
Length–weight relationships are important in fisheries science, notably to raise length–frequency samples to total catch, or to estimate biomass from landings length composition (Morato et al., Reference Morato, Afonso, Lourinho, Barreiros, Santos and Nash2001).
The relationship between TL (cm) and TW (g) was calculated using a power function:
The Student's t-test was used to verify the existence of significant differences between sexes and to test the allometry in growth (Zar, Reference Zar1996).
Ageing methodology and validation
Right sagitta otoliths were transversally sectioned (Bedford, Reference Bedford1983; McCurdy, Reference McCurdy1985) with a diamond-tipped saw blade (Labcut 230 Cutting Machine) rotating at 3700 rpm. Slides 0.5 mm thick were mounted in a glass slide with translucent glue, brushed with a 1:1 glycerin-alcohol solution and observed under a stereomicroscope under transmitted light with a 12× magnification. The distances from the nucleus to each successive translucent increment (hereafter referred as nucleus-to-increment distances) and to the otolith edge were measured on the same axis of the ventral face of the sectioned otoliths, using a micrometer eyepiece. The ventral face was chosen since this area showed a larger radius and, thus, a better individualization of the growth increments.
Marginal increment ratio (MIR) (Samamé, Reference Samamé1977) and edge analysis (N = 687) were used to examine the periodicity of growth increment formation and therefore semi-directly validate the frequency of growth increments formation (Panfili and Morales-Nin, Reference Panfili, Morales-Nin, Panfili and Pontual2002). The nucleus-to-increment distances were used to calculate the marginal increment. The edge of the otoliths was classified as opaque (highly calcified, light increments) or translucent (less calcified, dark increments). Mean MIR and standard deviation, and edge type were plotted by month and for both younger (0–4 years) and mature older specimens (5–18 years) to verify periodic trends in growth increment formation. Age at first maturity was used as the criterion for the separation of younger and older specimens (A.R. Vieira, personal observatiom).
Precision
Precision of age assignments (and their reproducibility) within and between readers was evaluated. For this purpose, a random sample of 306 otoliths, covering the length range of the total sample, were read twice, with a lag of four months, by two readers (reader 1, R1, and reader 2, R2).
The average percentage error (APE) (Beamish & Fournier, Reference Beamish and Fournier1981), the coefficient of variation (CV) (Chang, Reference Chang1982) and the index of precision (D) (Chang, Reference Chang1982) were used to compare age readings within and between readers. APE and CV produce similar values (Chang, Reference Chang1982), but the latter is statistically more rigorous and thus is more flexible (Campana et al., Reference Campana, Annand and McMillan1995). Bias evaluation was based on age bias plots (Campana et al., Reference Campana, Annand and McMillan1995) which allows visualizing deviation of the age readings from the 1:1 equivalence line.
The nonparametric Mann–Whitney U-test (since data did not meet the assumption of normality and homogeneity of variance) was used to compare age assignments within and between readers. The Bowker-type test for symmetry (Bowker, Reference Bowker1948) was applied to investigate the existence of systematic differences on the ages assigned between readers (Hoenig et al., Reference Hoenig, Morgan and Brown1995).
Growth model
To model the growth of forkbeard, sex-specific length-at-age data were fitted to the von Bertalanffy growth model (von Bertalanffy, Reference von Bertalanffy1938). Akaike's information criterion (AIC; Shono, Reference Shono2000) was used to evaluate the models’ adequacy to the model assumptions and the goodness-of-fit. The likelihood ratio tests (Kimura, Reference Kimura1980) were used to evaluate the significance of differences on growth parameters between sexes (Venables & Ripley, Reference Venables and Ripley2002). The von Bertalanffy growth model was estimated in R software v.2.15.1.
RESULTS
Length–weight relationship
A total of 687 specimens were sampled: 369 females (53.7%) and 318 males (46.3%). The sex-ratio did not differ significantly from unity (χ 2 = 3.64; df = 1; P = 0.06), and total length and total weight were not significantly different between sexes (paired t-test, TL: t-test = 1.35; df = 685; P = 0.178; TW: t-test = 1.61; df = 685; P = 0.109).
The equations that express the length–weight relationship for the forkbeard were:
and
Both females and males of forkbeard showed positive allometric growth (paired t-test, females: t-test = 6.27; df = 368; P < 0.001; males: t-test = 6.90; df = 317; P < 0.001).
Ageing methodology and validation
In sectioned otoliths of the forkbeard (N = 687, 369 females and 318 males), a regular pattern was visible, with alternate opaque and translucent concentric growth increments deposited around a large opaque nucleus. Inside the nucleus structure, a strongly marked check ring was always evident and appeared, in the ventral face, at 0.41 + 0.03 mm (mean + SD) from the nucleus. The deposition pattern of growth increments varied as the otolith grows: in the region closer to the nucleus, growth increments were wide (typically the first four increments) and afterwards they became thinner and closer together (Figure 1). The first growth increment corresponds to the first well-marked increment visible after the otolith nucleus and appeared, in the ventral face, at 2.00 ±0.02 mm (mean ±SD) from the nucleus. Figure 2 shows the level of overlapping of increment deposition range of the first 14 years at the ventral face of sectioned otoliths. The growth increments corresponding to ages 1, 2 and 3 years were completely separated from each other; thereafter the overlap of growth increments increased with fish age.

Fig. 1. Right sectioned sagitta otolith of a 10 years old forkbeard, Phycis phycis, from the Portuguese continental waters, with 52.8 cm TL. Inside the nucleus, the demersal check ring (DCR) is shown. White dots represent the growth increments. D, dorsal face; V, ventral face. Scale bar = 1 mm.

Fig. 2. Frequency distributions of the distances from the nucleus to growth increments of the ages assigned in ventral face of sectioned otoliths of forkbeard, Phycis phycis, from the Portuguese continental waters.
Marginal increment ratio and proportion of opaque and translucent edges of younger (0–4 years; N = 149) and older specimens (5–18 years; N = 538) are shown in Figure 3. An identical pattern throughout the year could be seen. MIR presented a clear annual pattern of growth increment formation for both younger and older specimens, with the marginal increment showing an increasing trend from January to June and a decreasing trend after June (Figure 3A, B). The highest values of MIR occurred between May and August and the lowest between October and January for both younger and older specimens (Figure 3A, B), an indication that new increments are formed during this period. For both younger and older specimens, opaque edges were more frequent in spring and summer months (April–August), while translucent edges dominated during autumn and winter (October–March) (Figure 3C, D).

Fig. 3. Monthly evolution of marginal increment ratio in sectioned otoliths of (A) younger (0–4 years) and (B) older (5–18 years) specimens of forkbeard, Phycis phycis, from the Portuguese continental waters. Dots are the mean values and whiskers are ± standard deviation. Annual variation pattern of the percentage of opaque and translucent edges in sectioned otoliths of (C) younger (0–4 years) and (D) older (5–18 years) specimens. Black bars are opaque edges and white bars are translucent ones.
Based on the previous results, the assumptions for age assignment of the forkbeard were: (1) an annual growth increment corresponds to the succession of an opaque and a translucent growth zone (validated by MIR and edge type), so age can be assigned by counting translucent zones; and (2) first January is considered to be the birth date since the spawning season of the species occurs in the last quarter of the year (A.R. Vieira, personal observation).
Precision
Both readers aged twice 306 forkbeard otoliths. Indices of precision for age readings within and between readers are presented in Table 1, and the age-bias plots are in Figure 4. For R1, the estimates of APE, CV and D were 1.51%, 1.30% and 0.92%, respectively, reflecting high precision of the age readings. R1 comparative readings showed a total agreement of 81%. Ages assigned by R1 showed non-significant differences between the 1st and 2nd readings (Mann–Whitney U test: U = 234947; P = 0.815). For R2 the APE, CV and D indices were 2.77%, 2.52% and 1.96%, respectively. The comparative readings of this reader showed a total agreement of 86%. Ages assigned by R2 showed non-significant differences between age readings (Mann–Whitney U test: U = 11041; P = 0.781).

Fig. 4. Age bias plots for the readings comparisons within readers: (A) reader 1, (B) reader 2 and (C) between readers, for forkbeard, Phycis phycis, from Portuguese continental waters. The 45° line represents 100% agreement.
Table 1. Indices of precision for age readings of forkbeard, Phycis phycis, from the Portuguese continental waters, within and between readers. R1 = reader 1; R2 = reader 2.
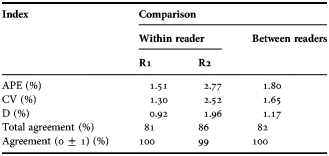
The indices of precision between readers were 1.80%, 1.65% and 1.17% for APE, CV and D, respectively, reflecting a high precision. A total agreement of 82% was allocated. Only one difference between readers higher than ±1 year was assigned (Figure 4C). The Mann–Whitney test proved that there were non-significant differences between ages assigned by R1 and R2 (Mann–Whitney U test: U = 46158.5; P = 0.763). There was also no evidence of systematic disagreement for ages assigned between R1 and R2 (test of symmetry; χ 2 = 24.20, df = 16, P = 0.085).
Ages assigned by R1 were used to fit the von Bertalanffy growth model to the length-at-age data of forkbeard due to the higher precision obtained and previous experience in ageing gadiform species (see Vieira et al., Reference Vieira, Figueiredo, Figueiredo and Menezes2013).
Growth model
For ageing estimate all 687 otoliths were used. Forkbeard females caught off the Portuguese continental coast ranged between 18.8 and 66.6 cm TL and were aged by sectioned otoliths from 1 to 17 years. Males ranged from 15.5 to 67.1 cm TL and were aged from 0 to 18 years. The age–length key is shown in Table 2. The estimated von Bertalanffy growth parameters for forkbeard from the Portuguese continental waters are presented in Table 3. No significant differences between males and females growth parameters were found (Likelihood ratio test: χ 2 = 2.71, df = 3, P = 0.439) and the von Bertalanffy growth curve is presented in Figure 5. It is worthwhile to mention that 50% of the maximum observed total length is attained at age 4 years.

Fig. 5. The von Bertalanffy growth model for the entire sample (n = 687) of forkbeard, Phycis phycis, from the Portuguese continental waters, for the period 2011–2012.
Table 2. Age–length key for the forkbeard, Phycis phycis, from the Portuguese continental waters for the period 2011–2012. n, number of fish; TL, mean, mean total length; SD, standard deviation.

Table 3. Summary of the von Bertalanffy growth parameters estimated for forkbeard, Phycis phycis, from the Portuguese continental waters. Standard error of parameters is given in parentheses. L∞, asymptotic length; k, growth rate; t0, hypothetical age when L = 0.

DISCUSSION
Age estimation in gadiform fish has been known to present some difficulties due to otolith thickness, which in most species requires special processing methods so that growth increments become visible (Morales-Nin et al., Reference Morales-Nin, Torres, Lombarte and Recasens1998; Deree, Reference Deree1999). Sectioning has been considered a suitable technique for dense gadiform otoliths, allowing faster and better otolith analysis (e.g. Morales-Nin et al., Reference Morales-Nin, Torres, Lombarte and Recasens1998; Deree, Reference Deree1999; Casas & Piñeiro, Reference Casas and Piñeiro2000; Vieira et al., Reference Vieira, Figueiredo, Figueiredo and Menezes2013). Regarding the forkbeard, this study shows that sectioned otoliths are a precise method for age estimation, since the values of precision indices obtained both within and between readers were very low; much lower than those adopted in the literature (CV < 7.6% and APE = 5.5%) (Campana, Reference Campana2001). Other studies were published recently on age and growth of forkbeard from Azorean waters (Abecasis et al., Reference Abecasis, Canha, Reis, Pinho and Gil-Pereira2009) and the Adriatic Sea (Matić-Skoko et al., Reference Matić-Skoko, Ferri, Škeljo, Bartulović, Glavić and Glamuzina2011). Burning and sectioning otoliths was considered a precise procedure for age estimation of this species from Azorean waters by Abecasis et al. (Reference Abecasis, Canha, Reis, Pinho and Gil-Pereira2009); however, with this technique, the authors obtained 9.5% of unreadable otoliths. Matić-Skoko et al. (Reference Matić-Skoko, Ferri, Škeljo, Bartulović, Glavić and Glamuzina2011) used both otolith grinding and morphometric otolith parameters (length, width and weight) to estimate age of forkbeard from the Adriatic Sea, and they suggested that the most precise age estimator was the otolith weight linear model, instead of otolith growth increment counting. These otolith weight models are cheaper and quicker than otolith analysis (Cardinale et al., Reference Cardinale, Arrhenius and Johnsson2000). However, they still require ageing techniques to achieve an appropriate regression between age estimated by otolith analysis and otolith weight (to reduce any bias that could modify the estimated age-structure and affect the analysis) (Lai & Gunderson, Reference Lai and Gunderson1987; Bradford, Reference Bradford1991) and a recalibration for each new fish sample (Cardinale et al., Reference Cardinale, Arrhenius and Johnsson2000; Francis & Campana, Reference Francis and Campana2004). For these reasons, these models can be advantageous for ageing species with high commercial interest and with a large sample (thousands) of otoliths for analysis (e.g. Atlantic cod, Gadus morhua, Cardinale et al., Reference Cardinale, Arrhenius and Johnsson2000), but seem to be less effective for analysing a smaller sample (hundreds) of fish.
Age validation is crucial in age and growth studies. It is common to underestimate ages of long-lived species and thus overestimate growth rates and reproductive potential. This may, in turn, lead to too-optimistic stock assessments and harvesting strategies (Beamish & McFarlane, Reference Beamish and McFarlane1983; Campana, Reference Campana2001; Cailliet & Andrews, Reference Cailliet, Andrews, Tsukamoto, Kawamura, Takeuchi, Beard and Kaiser2008). Regarding this issue, MIR and edge analysis support the hypothesis that a single set of translucent and opaque increments is formed every year in the forkbeard off the Portuguese continental coast. Translucent increments are deposited during autumn and winter and opaque increments are laid down in the spring and summer. The same results have been obtained by Abecasis et al. (Reference Abecasis, Canha, Reis, Pinho and Gil-Pereira2009) for forkbeard from Azorean waters, and by Matić-Skoko et al. (Reference Matić-Skoko, Ferri, Škeljo, Bartulović, Glavić and Glamuzina2011) for forkbeard caught in the Adriatic Sea, who found that opaque increments were laid down during spring and summer months.
The von Bertalanffy growth parameters estimated for the forkbeard show that it is a relatively slow growing and long lived species. Similar results were obtained in Azorean waters (Abecasis et al., Reference Abecasis, Canha, Reis, Pinho and Gil-Pereira2009). Although the maximum TL recorded in the present study was 67.1 cm, the historical record of 74 cm TL (Pinho, Reference Pinho2003) is close to the estimated L∞ of 75.1 cm TL. The present study showed no sexual dimorphism in growth, although differences in growth between sexes are a common feature among related gadiforms, such as the greater forkbeard, Phycis blennoides (Casas & Piñeiro, Reference Casas and Piñeiro2000). This study showed that this species can attain a maximum age of 18 years. A strongly marked check ring—that appears in other species from genus Phycis, was always evident in the otolith nucleus (Matarrese et al., Reference Matarrese, D'Onghia, Basanisi and Mastrototaro1998; Casas & Piñeiro, Reference Casas and Piñeiro2000). The maximum age observed in this study is very different from the one reported for the Adriatic Sea, where a maximum age of 5 years was reported. Contrarily to the results obtained for Portugal (mainland and Azores), forkbeard from the Adriatic Sea showed a sexual dimorphism in growth, with males reaching a larger size and growing more slowly than females (Matić-Skoko et al., Reference Matić-Skoko, Ferri, Škeljo, Bartulović, Glavić and Glamuzina2011). These differences in minimum and maximum ages and estimated von Bertalanffy growth parameters are probably related to the sizes of the specimens obtained in the different areas, which could be due to the sampling gear used: trammel nets in the Adriatic, longline in the Azores and longline, trawl and traps in mainland Portugal. Depending on mesh size, trammel nets may not be effective at sampling larger specimens (maximum 45.8 cm TL in the Adriatic study) whilst longlines do not capture smaller individuals (few specimens under 30 cm TL were used in the Azorean study). The use of different fishing gears in the present study allowed sampling of both smaller and larger specimens. Nevertheless, the possibility of different population size structures (or even different fishing pressure) in the north-east Atlantic and in the Mediterranean Sea cannot be discarded. The growth parameters obtained for the forkbeard from Azorean waters (Abecasis et al., Reference Abecasis, Canha, Reis, Pinho and Gil-Pereira2009) and the Adriatic Sea (Matić-Skoko et al., Reference Matić-Skoko, Ferri, Škeljo, Bartulović, Glavić and Glamuzina2011) were not statistically compared with those from mainland Portugal due to the absence of age–length keys.
Improved biological and fishery data for developing fisheries are essential to allow more robust assessments in the future (Hilborn & Waters, Reference Hilborn and Waters1992). In this context, age and growth studies are fundamental, since it is most important to know the relative abundance of specimens of different ages in order to be able to evaluate population age structure in relation to fishing. The results of this study, associated with the improved knowledge of other biological aspects of the forkbeard, will provide important data to allow for improved assessments and management in the future.
ACKNOWLEDGEMENTS
The authors would like to thank Pedro Gomes and the crew of the trawler ‘Sagittarius’ by providing smaller specimens of forkbeard used in this study. We appreciate anonymous referees’ comments on the manuscript.
FINANCIAL SUPPORT
This study was partially support by the project PROMAR 31-03-05-FEP-8, and by Fundação para a Ciência e Tecnologia (FCT), through the grants attributed to Ana Rita Vieira (SFRH/BD/73506/2010), Vera Sequeira (SFRH/BPD/70200/2010) and Rafaela Barros Paiva (SFRH/BD/80268/2011).










