Introduction
Currently, people have been interested in their nutrition and look for selecting healthful foods for their diets. Fishing products, especially fish, are ranked first among these foods, dint of their richness in proteins and fatty acids. Fish oil is of greatest importance in terms of nutrition physiology (Celik, Reference Celik2008) and its importance for human health has been extensively described and proven (Ruxton et al., Reference Ruxton, Reed, Simpson and Millington2007). The polyunsaturated fatty acids (PUFA) contained in fish oil are the main contributors to these properties, more specifically, those belonging to the omega-3 family such as eicosapentaenoic (EPA, C20:5n3) and docosahexaenoic (DHA, C22:6n3) acids.
Omega-3 long-chain fatty acids play an important role in prevention of cardiovascular diseases owing to their anti-thrombotic, anti-arrhythmic and anti-inflammatory properties (Huang et al., Reference Huang, Zandi, Tucker, Fitzpatrick, Kuller, Fried, Burke and Carlson2005; Morales-Medina et al., Reference Morales-Medina, Garc ıa-Moreno, Pérez-Galvez, Munio, Gaudix and Gaudix2016). They also have beneficial effects on high blood pressure, brain development, depression, diabetes and cancer in children (Simopoulos, Reference Simopoulos2002; Tapiero et al., Reference Tapiero, Nguyen Ba, Couvreur and Tew2002), contribute to the improvement of mitochondrial functions and inflammation in obese people (Borja-Magno et al., Reference Borja-Magno, Furuzawa-Carballeda, Guevara-Cruz, Arias, Granados, Bourges, Tovar, Sears, Noriega and Gómez2023) and exert an important neuroprotective effect (Neto et al., Reference Neto, Chaves Filho, Casadevall, de Azevedo, Macêdo and de Vasconcelos2022). Also, DHA is one of the components of the ocular retina, brain and myocardium. It plays an essential role in the development of the eyes and the brain, thus ensuring good cardiovascular health (Ward and Singh, Reference Ward and Singh2005). Moreover, EPA is also useful in anxiety cases and cancer treatment (Fenton et al., Reference Fenton, Hibbeln and Knable2000; Neto et al., Reference Neto, Chaves Filho, Casadevall, de Azevedo, Macêdo and de Vasconcelos2022). People that consume between 0.5 and 0.7 g day−1 of DHA have a lower incidence of heart troubles. However, their deficiency leads to some disorders such as poor eyesight, dermatoses and anaemia (Celik, Reference Celik2008). The general recommendation for daily DHA/EPA intake is 1 g day−1 for adults and 0.5 g for infants (Kris-Etherton et al., Reference Kris-Etherton, Harris and Appel2002). Pelagic fish are considered to be one of the main sources of these fatty acids, and are generally known for their high fat content (Passi et al., Reference Passi, Cataudella, Di Marco, De Simone and Rastrelli2002).
On the eastern Algerian coasts, the genus Trachurus is represented by three species: horse mackerel Trachurus trachurus, Mediterranean horse mackerel Trachurus mediterraneus and blue-jack mackerel Trachurus picturatus (Derbal and Kara, Reference Derbal and Kara2001). These are very important pelagic fish in terms of economic interest and biomass in the Mediterranean and throughout the world. On a local scale, production of combined species of Trachurus genus was estimated at 45% of total pelagic fish production between 2010 and 2020 in Jijel city. Besides that, they are highly appreciated and requested by consumers because of their high organoleptic value.
The geographical location is one of the factors influencing the fatty acid composition of fish (Winston and Di Giulio, Reference Winston and Di Giulio1991). The species of the Trachurus genus are characterized by more or less different geographical distribution areas. Trachurus trachurus is characterized by a wide distribution on the continental shelf and the edge of the slope in the Atlantic Ocean; the coasts of South Africa and the sub-tropical and tropical seas (the Norwegian Sea, the North Sea, the Mediterranean Sea, the Sea of Marmara and the Black Sea). Trachurus mediterraneus is present in the North-East Atlantic, from the Bay of Biscay to Mauritania, in the Black Sea and especially in the Mediterranean. However, it is absent in the Levantine basin. Trachurus picturatus is a cosmopolitan, common in the Adriatic, in the eastern and western Mediterranean, and in the eastern Atlantic, from the Bay of Biscay to Mauritania (Bauchot, Reference Bauchot, Fischer, Bauchot and Schneider1987). Also, according to Winston and Di Giulio (Reference Winston and Di Giulio1991), the fatty acid profile of fish generally, and of PUFA especially, is related to dietary. The horse mackerel feeds mainly on small crustaceans, but also on small fish such as sardines and anchovies (Rahmani et al., Reference Rahmani, Koudache and Bennabi2020). However, the Mediterranean horse mackerel is mostly piscivorous, it feeds on sardines and anchovies, but also sometimes on small crustaceans. The young individuals target only crustaceans (Bensalem, Reference Bensalem1988). Blue-jack mackerel feeds mainly on crustaceans such as copepods and zooplankton and mesopelagic fish (Bauchot and Paras, Reference Bauchot and Paras1980; Bauchot, Reference Bauchot, Fischer, Bauchot and Schneider1987; Battaglia et al., Reference Battaglia, Pagano, Consoli, Esposito, Granata, Guglielmo, Pedà, Romeo, Zagami, Vicchio, Guglielmo and Andaloro2020).
The fatty acid profile of fish species has been the subject of several studies over the last two decades. On the northern coasts of Mediterranean, T. trachurus fatty acid profile was studied in the Alborán Sea by Morales-Medina et al. (Reference Morales-Medina, Garc ıa-Moreno, Pérez-Galvez, Munio, Gaudix and Gaudix2016), and on the Italian coasts by Passi et al. (Reference Passi, Cataudella, Di Marco, De Simone and Rastrelli2002), Orban et al. (Reference Orban, Di Lena, Nevigato, Masci, Casini and Caproni2011) and Giandomenico et al. (Reference Giandomenico, Nigro, Parlapiano, Spada, Grattagliano, Prato and Biandolino2023). Fernandez-Jover et al. (Reference Fernandez-Jover, Lopez Jimenez, Sanchez-Jerez, Bayle-Sempere, Gimenez Casalduero, Martinez Lopez and Dempster2007) was interested in T. mediterraneus on the North-West Mediterranean coasts. Turan et al. (Reference Turan, Gürlek and Yaglioglu2007) were also interested by the same species in the Black Sea. Furthermore, the fatty acid composition of T. picturatus in Mediterranean was targeted only by Zlatanos and Sugredos (Reference Zlatanos and Sugredos1993) on the Greece coast. However, on the South-West coasts of the Mediterranean, these three species have never been studied from the point of view of their biochemical composition despite their great economic interest and their importance for human health.
This study aims to provide original data on lipid content, fatty acid profile and nutritional quality of three pelagic species: T. trachurus, T. mediterraneus and T. picturatus, in the coast of Jijel, eastern of Algeria. The results to be obtained will make it possible to determine the biochemical status of these three species in the region studied. They will also help guide consumer choice.
Materials and Methods
Sampling
A total of 90 specimens of three species from genus Trachurus, caught mainly by pelagic trawls and having commercial and similar sizes (N T. trachurus = 30, TL T. trachurus = 19 ± 0.6 cm, TW = 51.35 ± 7.66 g; N T. mediterraneus = 30, TL T. mediterraneus = 19 ± 0.5 cm, TW = 55.85 ± x 7.81; N T. picturatus = 30, TL T. picturatus = 19 ± 0.6 cm, TW = 54.17 ± 8.74 g), were collected during October of 2022 from Jijel Bay on the South-western Mediterranean of Algeria (36°49′39.1″N 5°45′58.8″E), via wholesalers and fishmongers (Figure 1). (N) means the sample size of each species, the total length (TL) was measured to the nearest mm, and the total weight (TW) was recorded to the nearest g.
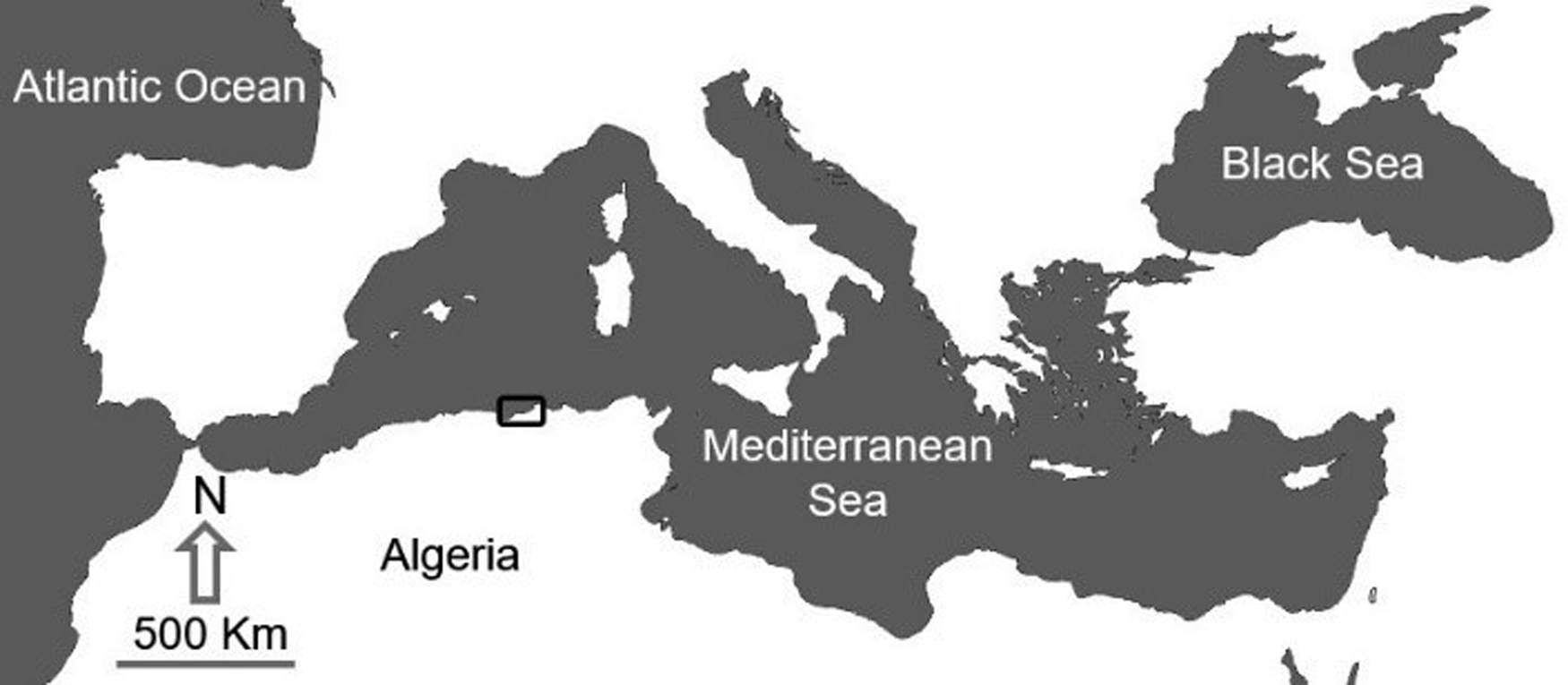
Figure 1. Map showing study area in Eastern Algerian coast.
Sample preparation
After capture, the samples were kept in ice and transported to the laboratory. Only the fillets were taken after removing the skin and the head of the eviscerated fish. Afterwards, the fillets of every ten individuals for each species were independently crushed as a paste (the total weight of the meat obtained by species: 1109.4 g for T. trachurus; 1225.85 g for T. mediterraneus and 1189.57 g for T. picturatus), using a warring blender with a variable transformer (VWR Scientific, Norwalk, CA, USA). The chemical analysis was therefore carried out in three biological replicates per species (n = 3), separately.
Lipid extraction
For each subsample of the three species, an amount of 20 g of crushed fillets were homogenized with 100 ml of methanol (grade AA), using the waring blender. Next, 40 ml of double distilled water and 50 ml of chloroform (CHCl3) were added, and the obtained mixture was homogenized again for 2 min using always the warring blender. Then, 50 ml of double distilled water and 50 ml of chloroform were added, and homogenization was applied again for 30 s. After that, 100 ml of the mixture were centrifuged in glass tubes (3300 × g, 10°C). The decanted liquids were collected by filtration through Whatman No. 1 filter paper in glass funnels, and kept separately. The resulting solids were newly extracted by adding 20 ml of 1:1 (v/v) chloroform: methanol; both resulting extracts were combined and transferred into a separate funnel, which is a device facilitating the separation of two liquids. The chloroform layer was passed through a layer of 2.5 cm of anhydrous sodium sulphate using Whatman No. 1 filter paper, in a funnel. The last step consists of removing the solvents from the tared flask by using a rotary evaporator under vacuum (Heidolph model VV2000), at 40°C (Folch et al., Reference Folch, Lees and Sloane Stanley1957). To avoid the risk of contamination, the glassware was washed carefully after each use, with hot water and detergent, then rinsed with acetone.
Estimation of lipid content
The content of lipids in the sample of each species was calculated according to the following formula (Guil-Guerrero et al., Reference Guil-Guerrero, Venegas-Venegas, Rincón-Cervera and Suárez2011):
Where the weight of extracted lipids was calculated as follows:
Transesterification
The main objective of this step is to extract fatty acid methyl esters (FAMEs) and increase their volatility in the extracts to be analysed. For that, 20 mg of each oil sample were mixed with 20 ml of a solution of methanol and acetyl chloride (20:1, v/v) and 20 ml of hexane, according to Rodríguez-Ruiz et al. (Reference Rodríguez-Ruiz, Belarbi, Sanchez, Garcia and Lopez Alonso1998). The mixture was heated under stirring at 100°C during 30 min, and cooled afterwards in room temperature. Afterward, 20 ml of water were added and the FAMEs were extracted in a layer of hexane. Three extractions with hexane were made on the same extract to ensure maximal recovery of FAMEs (Guil-Guerrero et al., Reference Guil-Guerrero, Venegas-Venegas, Rincón-Cervera and Suárez2011).
Fatty acid analysis
FAMEs were analysed using an Agilent gas chromatography system (model GC-2030). An Rtx-5MS capillary column (28 m long, with an inner diameter of 0.25 mm and a film thickness of 0.25 μm) was employed for the separation of fatty acids. The column temperature was initially set to 70°C for 1 min after injection, then increased at a rate of 7°C min−1 to reach 170°C, which was maintained for 55 min. Subsequently, the temperature was raised at a rate of 10°C min−1 to 230°C and held at this final temperature for 33 min. Helium served as the carrier gas, set at a split ratio of 1:20. The injector and detector temperatures were maintained at 250 and 350°C, respectively, with an injection volume of 1 μl.
FAME chromatogram peaks were identified by comparing their retention times with recognized FAME standards. Each fatty acid's concentration was determined by calculating its peak area relative to the total peak area, and the results were reported as a percentage of each fatty acid in the total lipids.
Nutritional quality of fatty acids
Nutritional quality of studied species fillets was verified by calculating the values of the atherogenic index (AI), thrombogenic index (TI), hypocholesterolemic-to-hypercholesterolemic ratio (HH) and n6-to-n3 ratio, as following (Ulbricht and Southgate, Reference Ulbricht and Southgate1991; Santos-Silva et al., Reference Santos-Silva, Bessa and Santos-Silva2002):
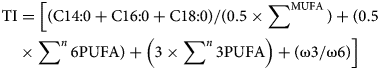
For each species, the calculation of the indices was carried out in three biological repetitions, separately. AI assesses the possibility of a food or substance to reduce blood fat content, and TI evaluates the capacity of a food or substance to inhibit platelet activity, contributing to blood clot formation (Iwamoto et al., Reference Iwamoto, Crepaldi, Boaventura, Teixeira, Teixeira and Luz2019). Generally, low values of these indices reflect a protective effect against cardiovascular diseases, while high ones may reflect the opposite (Turan et al., Reference Turan, Gürlek and Yaglioglu2007; Grigorakis et al., Reference Grigorakis, Fountoulaki, Vasilaki, Mittakos and Nathanailides2011). These indices are based on the fatty acid composition of foods and are useful to choose the good diet for heart health (Grigorakis, Reference Grigorakis2007). Moreover, HH is strongly dependent on cholesterol metabolism, and high HH values are considered to be beneficial for human health (Ramos Filho et al., Reference Ramos Filho, Lima Ramos, Hiane and Talá de Souza2010). Also, the high n6-to-n3 ratio is an index of the lipid quality and is considered as an element of nutritional relevance (Orban et al., Reference Orban, Di Lena, Nevigato, Masci, Casini and Caproni2011).
Statistical analysis
The SPSS 23 software package was used for statistical analysis, and significant differences were considered at ρ < 0.05. The statistical comparison of the lipid content between the studied species was made by the ANOVA test. An overall comparison of the composition of saturated fatty acids (SFA), monounsaturated fatty acids (MUFA) and PUFA in the three species was carried out by the MANOVA test. The ANOVA test was also used to compare the SFA, MUFA and PUFA contents, as well as to compare each of the AI, TI, HH indices and the n6-to-n3 ratio separately between species.
Results
Lipid content
The obtained results show that the mean values of lipid content in T. trachurus, T. mediterraneus and T. picturatus are 5.06% (SD = 0.16), 2.87% (SD = 0.18) and 2.82% (SD = 0.15), respectively, where SD represents the standard deviation. Statistical comparison of these means, using ANOVA test, indicates significant differences between species (F = 176.84, ρ < 0.05) (Table 1).
Table 1. Lipid contents (g\100 g muscle of fish) of Trachurus trachurus, Trachurus mediterraneus and Trachurus picturatus
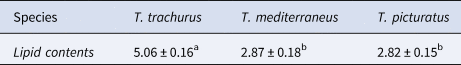
Data are means of triplicate determinations ± standard deviation: the different letters indicate significant differences between species; error bars, standard deviations.
Fatty acid profile
The study of fatty acid profile in horse mackerel, Mediterranean horse mackerel and blue-jack mackerel caught from the study area, reveals the presence of each of the SFA (35.1–53.23%), MUFA (29.24–37.65%) and PUFA (9.11–33.24%) in the muscle of all species (Table 2). Overall statistical comparison, using MANOVA test, shows significant differences (F = 2631.31, ρ < 0.05), in the composition of the main FAMEs groups in the three species studied.
Table 2. Fatty acids composition in Trachurus trachurus, Trachurus mediterraneus and Trachurus picturatus (% of total fatty acids)
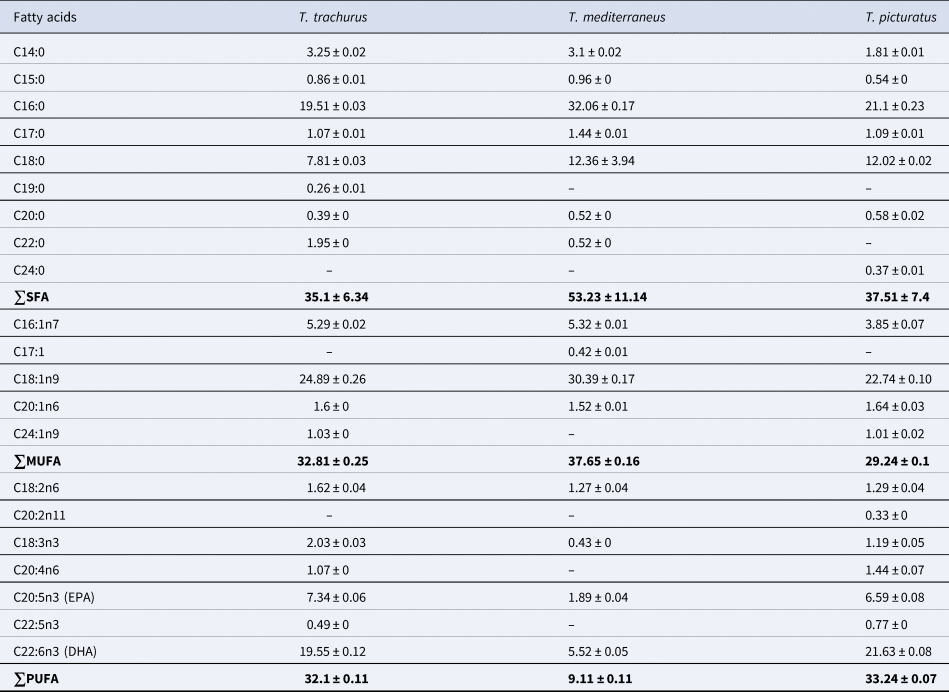
∑SFA, total saturated fatty acid; ∑MUFA, total monounsaturated fatty acid; ∑PUFA, total polyunsaturated fatty acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid. Data are means of triplicate determinations ± standard deviation.
Fatty acid profile of T. trachurus
Trachurus trachurus is composed mainly of SFA (35.1%), of which the most abundant fatty acids are palmitic acid (19.51%), stearic acid (7.81%) and myristic acid (3.25%). MUFA constitutes 32.81% of total fatty acids, mainly split into oleic acid (24.89%) and palmitoleic acid (5.29%). Moreover, PUFA represent 32.1% of fatty acids, with 19.55% of DHA and 7.34% of EPA. Statistical comparison between SFA, MUFA and PUFA fractions using the ANOVA test showed significant differences (F = 21.50, ρ < 0.05) in the same species.
Fatty acid profile of T. mediterraneus
The mean fatty acid composition of T. mediterraneus was found to be 53.23% of SFA, 37.65% of MUFA and 9.11% of PUFA (ANOVA: F = 249.98, ρ < 0.05). The majority fatty acids in the SFA class are palmitic acid, stearic acid and myristic acid, corresponding to 32.06, 12.36 and 3.1%, respectively. In the MUFA class, the most abundant acids are oleic acid and palmitoleic acid, corresponding to 30.39 and 5.32%, respectively. PUFA is the class least represented by this species, with only 9.11%, distributed essentially over 5.52% of DHA and 1.89% of EPA.
Fatty acid profile of T. picturatus
Trachurus picturatus is composed of 37.51% SFA, 29.24% MUFA and 33.24% PUFA (ANOVA: F = 60.20, ρ < 0.05). A large proportion of SFA is represented by palmitic acid (21.10%) and stearic acid (12.02%). Oleic acid constitutes 22.74% and palmitoleic acid accounts for 3.85% of total MUFA. Also, DHA is the major component of PUFA, followed by EPA, with proportions corresponding respectively to 21.63 and 6.59% (Figure 2A).
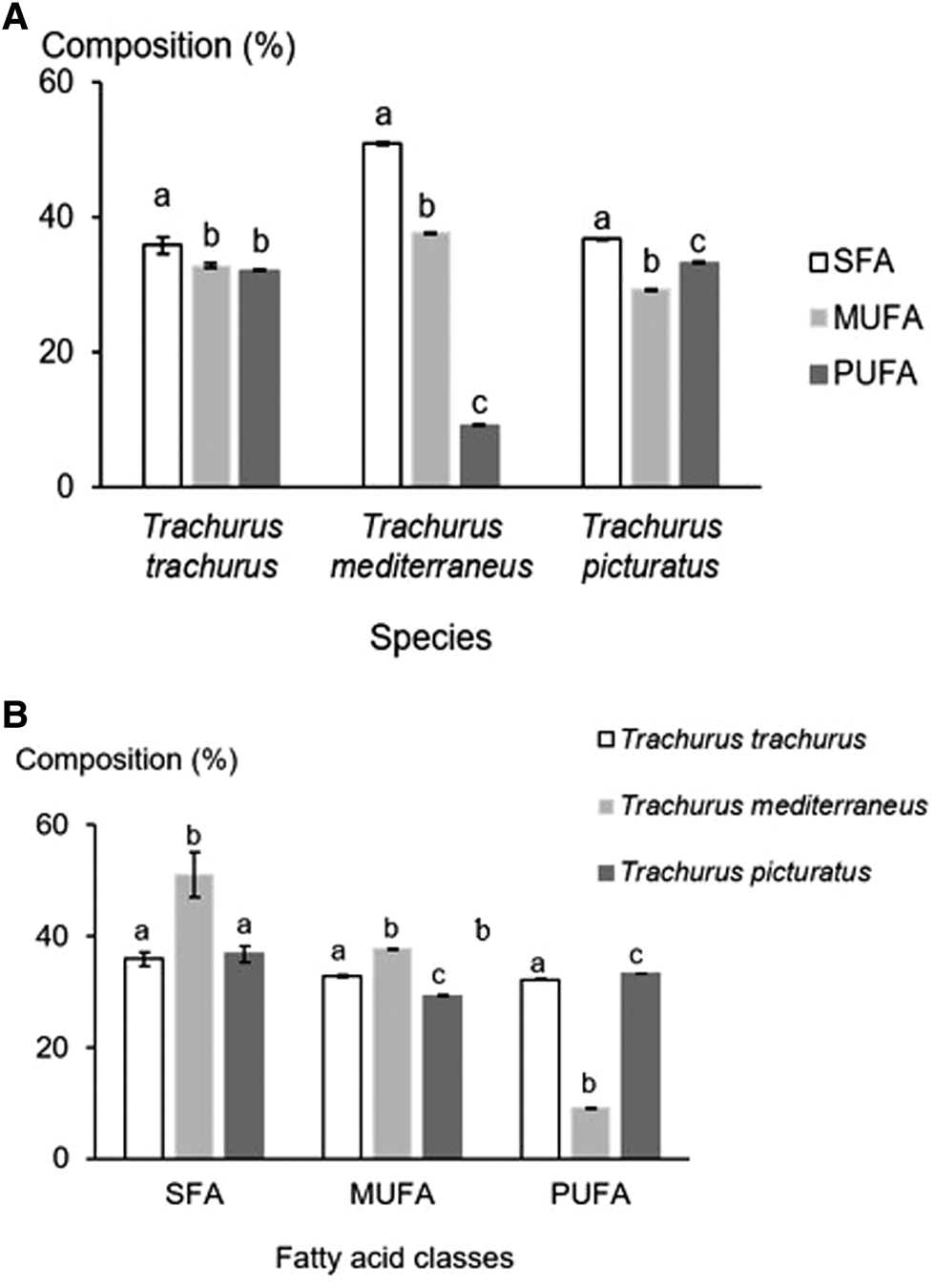
Figure 2. Intra (A) and interspecific (B) comparison of principal fatty acid classes in Trachurus trachurus, Trachurus mediterraneus and Trachurus picturatus: the different letters indicate significant differences between species, considering each species separately (A); and between fatty acid classes considering each class separately (B); SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid and error bars, standard deviations.
The three studied species are rich in SFA, MUFA and PUFA, with percentages varying from one species to another. Statistical comparison of the contents of these main fatty acid classes according to species reveals significant differences (ANOVA: F SFA = 32.17, ρSFA < 0.05, F MUFA = 1602.69, ρMUFA < 0.05 and F PUFA = 56547.28, ρPUFA < 0.05) (Figure 2B).
Nutritional quality of fatty acids
The average values of the AI, TI, HH and n6-to-n3 ratio are 0.50, 0.26, 2.50 and 0.15 in T. trachurus; 0.95, 0.97, 1.12 and 0.36 in T. mediterraneus and 0.45, 0.30, 2.43 and 0.14 in T. picturatus, respectively (Table 3). These values varied significantly among species (ANOVA: F AI = 26746.06, ρAI < 0.05; F TI = 222, ρTI < 0.05; F HH = 26746.06, ρHH < 0.05 and F n6-to-n3 = 19832.85, ρn6-to-n3 < 0.05).
Table 3. Nutritional quality assessment of Trachurus trachurus, Trachurus mediterraneus and Trachurus picturatus
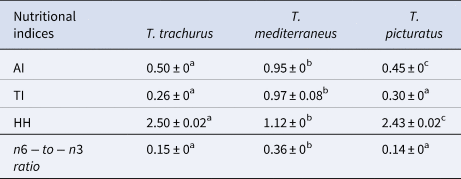
AI, atherogenic index; TI, thrombogenicity index; HH, hypocholesterolemic-to-hypercholesterolemic ratio and $n6 \hbox{-}{\rm to}\hbox{-} n3\;{\rm ratio}$![]() .
.
Data are means of triplicate determinations ± standard deviation: the different letters indicate significant differences between indices for each species.
Discussion
Lipid content
The highest lipid content value was recorded in T. trachurus (5.06%), while the lowest one was estimated in T. picturatus (2.82%). Our results differ from those obtained in other regions, which can be explained by many factors, such as: feeding habits (Lee and Lip, Reference Lee and Lip2003), species (Mohanty et al., Reference Mohanty, Mahanty, Ganguly, Mitra, Karunakaran and Anandan2019), stage of development (Costa et al., Reference Costa, Iannotta, Maggio, Giovanni, Barone and Giovanni2018), geographic origin, environmental factors, fishery period, reproductive season, etc. (Winston and Di Giulio, Reference Winston and Di Giulio1991). Thus, the extraction method can influence the yield of lipid content: Morales-Medina et al. (Reference Morales-Medina, Garc ıa-Moreno, Pérez-Galvez, Munio, Gaudix and Gaudix2016), Nougueira et al. (Reference Nogueira, Cordeiro and Aveiro2013) and Turan et al. (Reference Turan, Gürlek and Yaglioglu2007) used the AOAC official methods (1980, 1990, 2006) respectively. Fernandez et al. (Reference Fernandez-Jover, Lopez Jimenez, Sanchez-Jerez, Bayle-Sempere, Gimenez Casalduero, Martinez Lopez and Dempster2007) carried out the extraction of fatty acids by ethyl ether, using a laboratory extractor. Orban et al. (Reference Orban, Di Lena, Nevigato, Masci, Casini and Caproni2011) followed the method of Bligh and Dyer (Reference Bligh and Dyer1959) and Zlatanos and Sugredos (Reference Zlatanos and Sugredos1993) carried out the extraction by anhydrous sodium sulphate and n-hexane using Soxhlet. The chloroform-based Folch approach that we used achieves very high extraction efficiency compared to several other methods (Pilecky et al., Reference Pilecky, Kainz and Wassenaar2024).
The spawning period extends from January to May for T. trachurus (Ciccone et al., Reference Ciccone, Scicchitano, Gesualdo, Zito, Carbonara, Ricci, Cortese and Giordano2013; Gherram et al., Reference Gherram, Bensahla Talet, Dalouche and Abi Ayad2018), from May to August for T. mediterraneus (Viette et al., Reference Viette, Giulianini and Ferrero1997; Demirel and Yüksek, Reference Demirel and Yüksek2013) and from January to March for T. picturatus (Neves et al., Reference Neves, Sequeira, Vieira, Silva, Silva, Duarte, Mendes, Ganhão, Assis, Rebelo, Magalhães, Manuel Gil and Gordo2022). The sampling period of the three species coincides with the gonadal rest stage, which minimizes the effect of the reproductive season on the obtained results. Comparing with previous studies, lipid content in T. trachurus ranges from 2.10% (Orban et al., Reference Orban, Di Lena, Nevigato, Masci, Casini and Caproni2011) to 4.90% (Morales-Medina et al., Reference Morales-Medina, Garc ıa-Moreno, Pérez-Galvez, Munio, Gaudix and Gaudix2016) in the north-western Mediterranean where it is 2.36% in T. mediterraneus (Fernandez-Jover et al., Reference Fernandez-Jover, Lopez Jimenez, Sanchez-Jerez, Bayle-Sempere, Gimenez Casalduero, Martinez Lopez and Dempster2007), while it is 1.13% in the Black Sea (Turan et al., Reference Turan, Gürlek and Yaglioglu2007). In T. picturatus, it is 1.70% in the Aegean Sea (Zlatanos and Sugredos, Reference Zlatanos and Sugredos1993) and 1.87% in the north-east Atlantic (Nogueira et al., Reference Nogueira, Cordeiro and Aveiro2013). The aforementioned values are all lower than the obtained results on the south-western Mediterranean coast, confirming the effect of geographical location on lipid content in fish (Winston and Di Giulio, Reference Winston and Di Giulio1991).
Additionally, fish are classified by Haard (Reference Haard1992) as following: high-fat (lipid content ≥8%), medium-fat (4% ≤lipid content <8%), low-fat (2% ≤lipid content <4%) and lean (lipid content <2%). Based on these intervals, our results indicate that T. trachurus is medium-fat fish, contrary to T. mediterraneus and T. picturatus which are considered as low-fat fish. Fatty acids are key organic components of fish, serving as important energy sources for growth, reproduction and locomotion (Tocher, Reference Tocher2003). They are also essential to animal and human nutrition due to their role in essential physiological processes.
Fatty acid profile
The results obtained for the main FAMEs groups confirm that the fatty acid profile differs from one species to another. Trachurus trachurus is considerably richer in SFA than in both MUFA and PUFA. These results vary in comparison with those obtained in Alboran Sea (SFA: 32.8%, MUFA: 29.6% and PUFA: 36.2% or DHA and EPA present together 27.9%) by Morales-Medina et al. (Reference Morales-Medina, Garc ıa-Moreno, Pérez-Galvez, Munio, Gaudix and Gaudix2016) and on the Italian coast (SFA: 37.55%, MUFA: 24.49% and PUFA: 32.69%, where DHA and EPA represent 20.85 and 5.29% respectively) by Orban et al. (Reference Orban, Di Lena, Nevigato, Masci, Casini and Caproni2011). Results concerning the composition of FAMEs in T. mediterraneus differ from those found by Turan et al. (Reference Turan, Gürlek and Yaglioglu2007) in the Black Sea (SFA: 25.9%, MUFA: 28.2% and PUFA: 27.9% of which 21.7% correspond to DHA and EPA as one), and Fernandez-Jover et al. (Reference Fernandez-Jover, Lopez Jimenez, Sanchez-Jerez, Bayle-Sempere, Gimenez Casalduero, Martinez Lopez and Dempster2007) (SFA: 36.04–36.10%, MUFA: 14.62–17.39% and PUFA: 45.98–49.28% of which 32.56–36.61% correspond to EPA and 5.83–6.18% correspond to DHA) in two localities in the North-West Mediterranean. In the case of T. picturatus, Nogueira et al. (Reference Nogueira, Cordeiro and Aveiro2013) recorded in the North-East Atlantic, 33.67% SFA, 26.11% MUFA and 40.22 PUFA with 4.10% EPA and 28.46% DHA. Thus, Zlatanos and Sugredos (Reference Zlatanos and Sugredos1993) found, in the same species, 5.9% EPA and 9.5% DHA in the Aegean Sea (Table 4).
Table 4. Comparison of lipid content (g\100 g muscle of fish) and fatty acid profile (g\100 g fatty acid) of T. trachurus, T. mediterraneus and T. picturatus in different areas
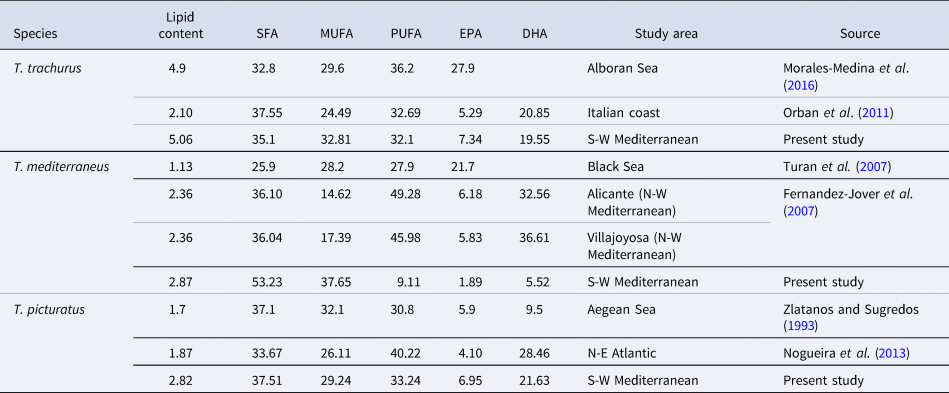
SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; DHA, docosahexaenoic acid and EPA, eicosapentaenoic acid.
The highest SFA and MUFA values were recorded in T. mediterraneus. These are mainly palmitic and oleic acids. Palmitic acid is a long-chain SFA often found in many kinds of fats and dietary oils. Although excessive consumption of SFA is generally associated with negative effects on cardiovascular health, it is important to note that the total fatty acid composition, including unsaturated fatty acids, is also important in assessing the overall impact of fish oil on health (Agostoni et al., Reference Agostoni, Moreno and Shamir2015). However, oleic acid or omega-6 is known for its various beneficial impacts on humans, including the modulation of inflammatory markers, gastrointestinal functions, insulin sensitivity, blood pressure and even various cancers (Van der Merwe et al., Reference Van Der Merwe, Labuschagne, Herselman and Hugo2013). Nevertheless, T. trachurus and T. picturatus are richer in PUFA than T. mediterraneus. DHA and EPA are omega-3 fatty acids that are associated with multiple human health benefits. These results illustrate the specificity of these two species and the crucial role they could play in a healthy diet to prevent various pathologies (Kugo et al., Reference Kugo, Zaima, Mouri, Tanaka, Yanagimoto, Urano, Unno and Moriyama2016).
The correlation coefficients obtained from analysing the lipid content of the studied species in relation to their SFA, MUFA and PUFA reveal weak linear relationships among the pairs of variables (r (lipid content, SFA) = −0587; r (lipid content, MUFA) = −0067 and r (lipid content, PUFA) = 0446). This suggests that the fatty acid profile of each species is independent of its lipid content.
The differences recorded in fatty acid profile, in T. trachurus, T. mediterraneus and T. picturatus, compared to previous studies, can be explained by differences in geographical location and environmental factors, notably the availability and quality of ingested prey. Celik et al. (Reference Celik, Diler and Küçükgülmez2005) reported that the fatty acid composition of a freshwater species caught from lakes of different water temperatures was different. PUFA amounts of the same fish species caught from the lakes of lower temperatures were much higher than those of higher temperatures. According to Winston and Di Giulio (Reference Winston and Di Giulio1991), the fatty acid profile of fish generally, and of PUFA especially, is species-specific and is related to many factors, such as environmental and dietary factors. Due to the differences in extraction methods and solvents used in previous studies, we can primarily attribute the variations observed in fatty acid profiles to these technical factors.
Nutritional quality of fatty acids
Ulbricht and Southgate (Reference Ulbricht and Southgate1991) developed AI and TI to evaluate the fatty acid profile of a food or dietary product and its impact on human cardiovascular health, especially concerning the prevention or promotion of coronary heart disease (Valfré et al., Reference Valfré, Caprino and Turchini2003). In this study, the lowest AI value was recorded in T. picturatus (0.45), while the lowest TI ones were found in T. trachurus (0.26) and T. picturatus (0.30). These values indicate that these two species have a significant protective effect against cardiovascular diseases compared to T. mediterraneus of the Eastern Algeria. Compared to the other study areas, the values of AI and TI obtained in this study in T. trachurus (0.5 and 0.26, respectively) and T. picturatus (0.45 and 0.30, respectively) are lower. Concerning T. trachurus, AI and TI were estimated at 0.87 and 0.51, respectively, in central Mediterranean of (Giandomenico et al., Reference Giandomenico, Nigro, Parlapiano, Spada, Grattagliano, Prato and Biandolino2023) and at 0.33 and 1.83 in the southern Adriatic coast of Italy (Orban et al., Reference Orban, Di Lena, Nevigato, Masci, Casini and Caproni2011). Also, Nogueira et al. (Reference Nogueira, Cordeiro and Aveiro2013) estimated these indices for T. picturatus in Northeastern Atlantic at 0.57 for AI and 1.07 for TI. However, the same index values obtained in this study are higher (AI = 0.95 and TI = 0.97) in T mediterraneus compared to those recorded in Alboran Sea (AI = 0.61 and TI = 0.22) (Morales-Medina et al., Reference Morales-Medina, Garc ıa-Moreno, Pérez-Galvez, Munio, Gaudix and Gaudix2016), and in two localities of southern Spain (Villajoyosa: AI = 0.47, TI = 0.21; Alicante: AI = 0.52, TI = 0.23) (Fernandez et al., Reference Fernandez-Jover, Lopez Jimenez, Sanchez-Jerez, Bayle-Sempere, Gimenez Casalduero, Martinez Lopez and Dempster2007). The lower the values of the AI and TI indices, the healthier the food and richer in fatty acids with high nutritional properties (Giandomenico et al., Reference Giandomenico, Nigro, Parlapiano, Spada, Grattagliano, Prato and Biandolino2023).
Moreover, T. trachurus represents the highest HH value (2.50), followed by T. picturatus (2.43) and T. mediterraneus (1.12). That indicates the high nutritional importance of T. trachurus compared with the other studied species, concerning cholesterol metabolism. The value obtained in T. trachurus is higher than those estimated in southeastern Adriatic coast of Italy (1.83) by Orban et al. (Reference Orban, Di Lena, Nevigato, Masci, Casini and Caproni2011) and in central Mediterranean of Italy (1.44) by Giandomenico et al. (Reference Giandomenico, Nigro, Parlapiano, Spada, Grattagliano, Prato and Biandolino2023). Thus, Nogueira et al. (Reference Nogueira, Cordeiro and Aveiro2013) found a lower value of the same index in T. picturatus (1.38) in northeastern Atlantic. Nevertheless, T. mediterraneus represents a lower value compared to those estimated in Alboran Sea (1.74) by Morales-Medina et al. (Reference Morales-Medina, Garc ıa-Moreno, Pérez-Galvez, Munio, Gaudix and Gaudix2016), and in two localities of southern Spain (Villajoyosa: 2.40, Alicante: 2.16) (Fernandez et al., Reference Fernandez-Jover, Lopez Jimenez, Sanchez-Jerez, Bayle-Sempere, Gimenez Casalduero, Martinez Lopez and Dempster2007).
The n6-to-n3 ratio values indicate that T. trachurus and T. picturatus represent significantly lower values (0.15 and 0.14, respectively) compared to T. mediterraneus (0.36). Compared to previous studies, T. trachurus represents a more or less different value of this ratio compared to what Orban et al. (Reference Orban, Di Lena, Nevigato, Masci, Casini and Caproni2011) and Giandomenico et al. (Reference Giandomenico, Nigro, Parlapiano, Spada, Grattagliano, Prato and Biandolino2023) found on the Italian coasts (0.13 and 0.25, respectively). Concerning T. mediterraneus and T. picturatus, the values found in this study are lower than those estimated on the northwestern coast of Mediterranean by Morales-Medina et al. (Reference Morales-Medina, Garc ıa-Moreno, Pérez-Galvez, Munio, Gaudix and Gaudix2016) (0.04) and Fernandez et al. (Reference Fernandez-Jover, Lopez Jimenez, Sanchez-Jerez, Bayle-Sempere, Gimenez Casalduero, Martinez Lopez and Dempster2007) (0.12–0.13) for T. mediterraneus, and by Nogueira et al. (Reference Nogueira, Cordeiro and Aveiro2013) on the northeastern Atlantic (0.08) for T. picturatus. A ratio n6-to-n3 of 3:1 to 4:1 might prevent many diseases characteristic of the diet based on animal proteins (Anses, 2011). Likewise, according to Simopoulos (Reference Simopoulos2010), a n6-to-n3 ratio of 1:1–2:1 appears to be consistent with research on evolutionary aspects of diet, neurodevelopment and genetics.
The obtained values of different indices show that the studied species have not the same importance as a healthy food. Trachurus trachurus is the best for cholesterol metabolism, T. picturatus is advisable for the prevention of cardiovascular diseases, and T. mediterraneus is ranked the best in terms of its omega-3 and 6 content, which makes it beneficial for nutrition and neurodevelopment. These differences between species are highly related to the differences in their fatty acid profiles. Despite these recorded differences, they are all essential for human health and are suggested to be included in human diet on a regular basis.
Data
The data that support the findings of this study are available from the corresponding author, R. Mohdeb, upon reasonable request.
Acknowledgements
Our thanks go to Maayache B., Professor of Biology and Belghobsi M., Professor of Chemistry at the University of Jijel, Algeria, for their cooperation throughout the period of this study.
Author contributions
R. M. designed the study, R. M., A. R. and S. D. R. did the sampling and identified species, R. M., N. B., L. M. and M. A. did the experimentation, N. B. analysed data, R. M. and M. H. K. did the statistical comparisons as well as the manuscript writing and revision.
Financial Support
This research received any specific funding.
Competing interest
None.








