Introduction
Traumatic brain injury (TBI) is one of the leading causes of morbidity and mortality in children (Schneier, Shields, Hostetler, Xiang, & Smith, Reference Schneier, Shields, Hostetler, Xiang and Smith2006). Approximately 700,000 children ages 0 to 19 sustain a TBI each year, resulting in at least 6000 deaths (Faul, Xu, Wald, & Coronado, Reference Faul, Xu, Wald and Coronado2010). Nearly 15% of all cases can be classified as moderate or severe injuries, with another 15% of cases involving mild injuries complicated by neurological findings (Kraus, Reference Kraus1995). These higher severity TBIs account for the majority of the morbidity and mortality associated with pediatric TBI.
Previous research has established links between TBI severity and neuropsychological impairments, with some evidence for a dose-response relationship (Anderson et al., Reference Anderson, Catroppa, Haritou, Morse, Pentland, Rosenfeld and Stargatt2001; Jaffe, Polissar, Fay, & Liao, Reference Jaffe, Polissar, Fay and Liao1995; Taylor et al., Reference Taylor, Yeates, Wade, Drotar, Klein and Stancin1999; Yeates et al., Reference Yeates, Taylor, Wade, Drotar, Stancin and Minich2002). According to a meta-analytic review, many children who suffer moderate-to-severe TBI (msTBI) evidence long-term impairments in intellectual ability, verbal memory, processing speed, attention, working memory, and other executive functions (Babikian & Asarnow, Reference Babikian and Asarnow2009). Compared to non–head-injured and healthy youth, as a group children with msTBI perform approximately 0.5 to 1.0 standard deviations below their peers in both the post-acute and chronic phases after injury.
There is significant recovery of cognitive function in the first year or 2 post-injury (Anderson, Catroppa, Haritou, Morse, & Rosenfeld, Reference Anderson, Catroppa, Haritou, Morse and Rosenfeld2005; Babikian & Asarnow, Reference Babikian and Asarnow2009; Jaffe et al., Reference Jaffe, Polissar, Fay and Liao1995; Taylor et al., Reference Taylor, Yeates, Wade, Drotar, Klein and Stancin1999; Yeates et al., Reference Yeates, Taylor, Wade, Drotar, Stancin and Minich2002), although as a group, children with msTBI fail to “catch up” to their peers. However, when using an individual rather than a group-difference/variable-centered approach, there is evidence for significant within-group variation. One study indicated that, among those children with msTBI able to complete cognitive assessments, the majority do not exhibit long-term cognitive deficits; instead, only a subset show deterioration or persistent cognitive deficits over time (Fay et al., Reference Fay, Yeates, Wade, Drotar, Stancin and Taylor2009).
In other words, only a relatively small subgroup of children with msTBI and moderate disability or better demonstrate long-term cognitive impairments. Injury severity, as indicated by Glasgow Coma Scale scores or duration of unconsciousness, have been linked to cognitive outcomes (Donders & Nesbit-Greene, Reference Donders and Nesbit-Greene2004; Fay et al., Reference Fay, Yeates, Wade, Drotar, Stancin and Taylor2009). No prior studies have determined whether detailed descriptions of acute injury severity derived from brain imaging studies and medical records (e.g., post-TBI seizures, increased intracranial pressure) in children with msTBI improve the prediction of long-term cognitive outcomes following pediatric msTBI.
Several demographic and psychosocial factors predict post-TBI outcomes, and may account for variations in recovery. Early age at time of injury (i.e., children injured as infants or preschoolers) is associated with poorer cognitive outcomes than when the TBI occurs in late childhood or adolescence (Anderson, Catroppa, Morse, Haritou, & Rosenfeld, Reference Anderson, Catroppa, Morse, Haritou and Rosenfeld2000; Ewing-Cobbs et al., Reference Ewing-Cobbs, Fletcher, Levin, Francis, Davidson and Miner1997, Reference Ewing-Cobbs, Barnes, Fletcher, Levin, Swank and Song2004). Family factors, such as burden and parental/home stress, have also received additional attention, although they relate more strongly to behavioral and adaptive than cognitive outcomes (Anderson et al., Reference Anderson, Catroppa, Haritou, Morse, Pentland, Rosenfeld and Stargatt2001; Anderson, Godfrey, Rosenfeld, & Catroppa, Reference Anderson, Godfrey, Rosenfeld and Catroppa2012; Yeates et al., Reference Yeates, Taylor, Drotar, Wade, Klein, Stancin and Schatschneider1997; Yeates, Taylor, Walz, Stancin, & Wade, Reference Yeates, Taylor, Walz, Stancin and Wade2010).
As risk for TBI, and particularly higher severity incidents such as motor vehicle accidents, is higher in those from lower socioeconomic (SES) backgrounds and of minority status (Brown, Reference Brown2010; Howard, Joseph, & Natale, Reference Howard, Joseph and Natale2005; Langlois, Rutland-Brown, & Thomas, Reference Langlois, Rutland-Brown and Thomas2005; McKinlay et al., Reference McKinlay, Kyonka, Grace, Horwood, Fergusson and MacFarlane2010), it is important to account for these factors. In the acute and chronic phases following injury, both ethnicity and SES, as assessed by parental occupation, parental education, and family income, predict deficits in cognitive performance above and beyond those attributable to TBI (Donders & Nesbit-Greene, Reference Donders and Nesbit-Greene2004; Hoofien, Vakil, Gilboa, Donovick, & Barak, Reference Hoofien, Vakil, Gilboa, Donovick and Barak2002; Max et al., Reference Max, Roberts, Koele, Lindgren, Robin, Arndt and Sato1999, Reference Max, Schachar, Levin, Ewing-Cobbs, Chapman, Dennis and Landis2005; Ryan et al., Reference Ryan, Anderson, Godfrey, Beauchamp, Coleman, Eren and Catroppa2014). Finally, although many premorbid risk factors have been identified, very few prior studies have determined the extent to which pre-injury cognitive functioning predicts long-term post-TBI cognitive outcomes.
Although prior studies have examined acute injury severity and psychosocial predictors of cognitive functioning following pediatric TBI, we still are not able to accurately identify the subset of youth who are at risk for exhibiting long-term cognitive deficits following msTBI. In this study, we sought to develop a model for detecting the subset of youth with msTBI who perform below expectations on neuropsychological evaluation. The aim of this study was to determine the relative contribution of acute injury, demographic, and psychosocial factors in predicting both post-acute (1–5 months) and long-term (11–20 months) cognitive outcomes after pediatric msTBI.
Methods
Participants
Participants included in this study were 46 youth with moderate-to-severe traumatic brain injury (msTBI) and 53 uninjured healthy control (HC) participants, all between the ages of 8 and 19 at the time of enrollment. Participants with msTBI were recruited from six pediatric intensive care units and two rehabilitation centers located across a major U.S. West Coast metropolitan area. A study representative provided all potential participants with the opportunity to “opt in” to be contacted by study staff for a phone screening. Medical records were reviewed with consent to verify study eligibility and to collect data regarding acute medical variables (e.g., seizures, CT scans). HC participants were recruited from the community through flyers, magazines, and school postings.
Inclusion criteria applying to both groups included normal visual acuity or normal vision once corrected with eyeglasses or contact lenses and a proficiency in English language. An inclusion criterion for the msTBI group was a Glasgow Coma Scale score below 13, or greater than 13 with documented abnormalities on acute brain imaging. Exclusion criteria applied to both the msTBI and HC groups were: injuries or a motor condition that would interfere with neuropsychological testing (e.g., paralysis); presence of metal implants or devices that would preclude an MRI scan; previous diagnosed head injury or other neurological illness; history of developmental or psychiatric disorder (e.g., learning disability, attention-deficit/hyperactivity disorder); or a history of substance abuse.
This study was part of a larger multi-modal neuroimaging protocol, the specifics of which are published in multiple other manuscripts, which is why the MRI criteria were used for inclusion. Children with a history of mild uncomplicated TBI or concussion (i.e., absence of positive brain imaging findings) were not excluded, and there were no instances were an eligible subject presented with a history of a severe TBI. Furthermore, youth with mild anxiety or depression were not excluded; only more severe psychiatric disorders requiring pharmacological treatment (e.g., ADHD or OCD) were excluded. Youth with msTBI with more severe psychiatric conditions who were receiving pharmacological treatment were excluded because these pre-TBI psychiatric disorders frequently have cognitive impairments, including impaired performance on the cognitive tests used in this study. These preexisting conditions would be confounds in analyses that assess the effect of msTBI on cognitive functioning.
Using the above guidelines, a total of 123 subjects were approached because they were hospitalized in one of our recruitment centers for a msTBI. Of those, 50 enrolled in the overall study and we include data on 46 of the 50 due to some missing information in variables included specifically in this study. The remainder of the subjects enrolled were lost to contact (n=27, e.g., never returned phone calls), did not meet criteria (n=21, e.g., were too severe to participate in the measures or could not undergo an MRI for safety reasons), or were not interested (n=25).
Participants with msTBI were on average 14.54 years old (SD=2.72) at the first visit, 77% male, 91% right-handed, 25% white/non-Hispanic, 60% of Hispanic/Latino ethnicity, and their parents’ highest level of education was 13.38 years (SD=4.00). The HC sample was on average 14.97 years old (SD=2.79) at the first visit, 60% male, 93% right-handed, 31% white/non-Hispanic, 48% of Hispanic/Latino ethnicity, and their parents’ highest level of education was 15.64 years (SD=3.16). The groups differed significantly in gender and parental education (more females and greater parental education in the HC cohort), but on no other demographic variables.
Procedure and Measures
Institutional review board approval and informed parental consent and child assent were obtained before participation. An initial post-acute assessment typically occurred between one and five months after injury (M=12.96 weeks; SD=4.96) for the msTBI group. Following the initial visit, both groups returned approximately 12 months later for follow-up (range: 48.57–82.71 weeks). This study includes data from 46 youth with msTBI and 53 HC patients at the post-acute visit, and 33 participants with msTBI and 36 HC youth at the follow-up.
Longitudinal data were only available for 26 youth with msTBI due to variations in the administered battery, attrition, and the ongoing status of the study. Of the remaining 21, 17 only underwent their initial evaluation (between 1 and 5 months post-injury). The remaining four only had a chronic evaluation (between 16 and 19 months post-injury). Of note, we evaluated all of our variables (demographic, predictor, and outcome variables) in a subgroup of the participants for whom we had longitudinal data for. We compared the subsample of msTBI and HC youth for whom we had longitudinal data to all the youth we included in cross sectional analyses at Time 1 and Time 2. There were no significant differences in demographic, acute injury severity, or cognitive outcomes at Time 1 or Time 2 between the youth included in longitudinal analyses and the youth included in cross sectional analyses at Time 1 and Time 2.
Prior reviews (Babikian & Asarnow, Reference Babikian and Asarnow2009) found that msTBI frequently results in deficits in processing speed, memory, and executive functions. Participants therefore completed several standardized tests designed to assess their neuropsychological functioning in those domains at both time points. Processing speed (PSI) and working memory (WMI) performance was obtained via intellectual assessment (Wechsler Intelligence Scale for Children, 4th edition; Wechsler Adult Intelligence Scale, 3rd edition). Verbal learning and memory was assessed using the California Verbal Learning Test (CVLT Children’s version or 2nd edition), specifically the standardized score for Trials 1–5. Finally, a measure of inhibition and set-switching was collected using the Trail Making Test Condition 4 subtest from the Delis-Kaplan Executive System (D-KEFS).
Predictors of outcome were grouped in the following categories: (1) descriptors of the acute injury such as relevant findings from CT scan and acute medical variables; (2) demographic variables—age, gender, race/ethnicity, and parental education; (3) premorbid functioning, specifically the Total Problems and School Competence scales from the Child Behavior Checklist (CBCL); and (4) post-injury cognitive performance at the first visit. The premorbid and post-acute CBCLs were completed at the post-acute visit by a parent or legal guardian. There were no missing data from subjects for demographic information, CBCL data, and neuropsychological test scores (in cases where not all measures were administered, a description of how the missing data was handled is presented in the Data Reduction section below).
The injury severity variables were extracted from patients’ medical records using a form created specifically to quantify injury related characteristics by a pediatric critical care intensivist who was very familiar with the format of the patients’ records. The variables extracted included: GCS (lowest in history), presence of seizures (dichotomous yes/no), and several findings from CT scans (dichotomous yes/no for intracranial pressure, epidural hematoma, intracerebral hematoma, subarachnoid hemorrhage, and subdural hematoma).
Statistical Methods
Performance on neuropsychological tests of processing speed, memory, and executive functions is often highly correlated (McCabe, Roediger, McDaniel, Balota, & Hambrick, Reference McCabe, Roediger, McDaniel, Balota and Hambrick2010). Principal components analysis was conducted on the neurocognitive variables at each time point to determine whether the cognitive scores could be combined into a single index to minimize the number of analyses and thereby reduce experiment-wide Type I error. Analyses were conducted within and across groups to confirm similar structure. Receiver operating characteristic (ROC) curves were then generated for both time points to determine the efficiency of the index in differentiating youth with msTBI from HC participants. Cut scores were selected to identify low-performing youth with msTBI. Finally, analyses (i.e., correlation, independent-samples t test, chi-square) were conducted to determine which predictors were associated with the cognitive performance index. All individually significant predictors were then included in a series of hierarchical regressions to identify the best predictors of the cognitive performance index at each of the two time points.
Results
Data Reduction
The four individual scores used in the PCA for each group are presented in Table 1. All of the analyses of variance (ANOVAs) for both T1 and T2 were statistically significant (p<.001). At T1, post hoc analyses showed that in all four cases, the lower performing group was significantly different than the healthy control group and the normal performing TBI group (all p<.001). Similarly, at T2, post hoc analyses showed that, in all four cases, the lower performing group was significantly different than the healthy control group and the normal performing group. In addition, for PSI and CVLT only, the normal performing group was also different than the healthy control group at T2, but not at T1. Effect sizes for the various group differences are presented in Table 1.
Table 1 Group differences on key variables

Note: Groups split by Time 1 CPI into low or typical performers. Data displayed as M (SD) unless otherwise indicated.
CPI=cognitive performance index; CBCL=Child Behavior Checklist; GCS=Glasgow Coma Scale; PSI=Processing Speed Index; WMI=Working Memory Index.
Separate principal components analysis (PCA) were initially conducted on data from each time point for both the msTBI and control groups. Only components with eigenvalues ≥1.00 were retained. If solutions were consistent across all three groups, then the groups were combined. At both time points, similar single component solutions were derived for both the msTBI and control groups combined. Ultimately, the groups were combined but there were two separate time points, resulting in two final analyses. Combining all participants, post-acute (Time 1) data yielded a single component solution accounting for 62% of the variance (component loadings: PSI, 0.87; WMI, 0.75; CVLT, 0.66; D-KEFS Trails 4, 0.84). Similarly, follow-up (Time 2) data produced a single component solution accounting for 75% of the variance (component loadings: PSI, 0.88; WMI, 0.84; CVLT, 0.84; D-KEFS Trails 4, 0.90).
An unweighted composite index was subsequently generated for each participant at each time point. Norm-based scores for each of the tests included in the cognitive performance index (CPI) were transformed into a common metric (standard scores; M=100; SD=15). Scores for PSI, WMI, CVLT, and D-KEFS Trails 4 were then averaged together to create the CPI, such that a score of 100 is equivalent to the population average based on the standardization samples of the tasks included in the index. An index was not generated for participants who were missing either PSI or WMI, as these scores already reflect a composite of two other tests and therefore, any imputation method would add further noise/error. If a participant was missing either the CVLT or D-KEFS Trails 4, the composite was mean imputed using the other three scores (n=2).
Diagnostic Accuracy
The neuropsychological measures included in the CPI were selected because they are sensitive to the effects of msTBI. Consistent with prior research, youth with msTBI performed more poorly than the HC group on the index (Time 1; msTBI: M=93.18; SD=13.04; HC: M=103.51; SD=9.81; p<0.02). ROC curves were subsequently computed to test the ability of the CPI to discriminate between the msTBI and HC groups (Figure 1). Both Time 1 and Time 2 performance indices produced statistically significant models (area under curve; ps>.001), indicating significantly better than chance rates of correct classification. Cutoff scores were selected to maximize the ratio between sensitivity and false positives whilst also minimizing the false positive rate (<5%). Given those restrictions, sensitivity of the Time 1 CPI was maximized at a cut score of 87.69 (37% sensitivity; 4% false positives). A cut score of 91.81 was used for the Time 2 data (39% sensitivity; 0% false positives). The area under the curve for the Time 1 analyses was 0.739 (p<.001), and the area under the curve for the Time 2 analyses was 0.835 (p<.001).

Fig. 1 Receiver operating characteristic curves of efficiency of different cut scores in discriminating between youth with msTBI and HC children using the CPI generated at Time 1 (a) and Time 2 (b). The dotted line indicates chance levels of discrimination between the groups. Values above the line exceed chance levels of discrimination. The arrows mark the cut scores selected to maximize discrimination.
Predicting Neurocognitive Outcomes
Cutoff scores were used to divide the msTBI group into subgroups with low and typical cognitive performers. Table 1 presents the characteristics of the two msTBI subgroups defined by the Time 1 CPI and the HC group. The three groups differed on parental education and premorbid school competence using either Time 1 or Time 2 cutoff score groupings, with post hoc analyses indicating that only the low performing msTBI subgroup differed from the HC group on the above named variables. Furthermore, among those with longitudinal data, there was a strong relationship between performance at Time 1 and 2 (r=0.90; p<.001). Not surprisingly, the mean CPI for the msTBI subjects who had longitudinal data did not change from T1 to T2 (92.1, SD 14.0 at T1 to 94.5; SD 14.3 at T2). Furthermore, with just a few exceptions, the classification of low versus normal performers did not change over time. Specifically, only 1 of the 15 normal performers at T1 was classified as low performing at T2 and only 2 of the 11 low performers at T1 were classified as normal performers at T2.
Next, we conducted hierarchical linear regressions to determine the relative contribution of each factor to the Time 1 and Time 2 CPI (Table 2). To predict the Time 1 CPI, the first step included only group (msTBI vs. HC), and the model accounted for a significant amount of the variance in cognitive performance, R 2=0.20, F(1,88)=21.71, p<.001. In the next two steps, the addition of parental education and premorbid school competence both resulted in a significant increase in the proportion of Time 1 performance explained (ΔR 2=0.07 and 0.09, respectively), a 16% increase altogether. When predicting the Time 2 CPI, Time 1 performance was entered in the first step given the strong correlation between the scores. All other factors, added in the second step (namely, group, parental education, and CBCL School Competence) did not significantly increase the amount of explained variance in Time 2 cognitive performance. Of note, there was no significant interaction between parent education and premorbid school competence in the msTBI group (F=1.567; p=.242); however, the interaction between these two variables was significant in the control group (F=3.347; p=.008).
Table 2 Hierarchical regressions predicting CPI
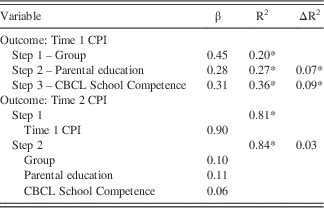
* p<.005.
CPI=cognitive performance index.
Discussion
Within a sample of youth with msTBI with a mean lowest GCS around 7 recruited from trauma centers, there was no relationship between detailed medical acute injury severity descriptors and cognitive performance at either 4 months or 16 months post-injury when GCS is used as a marker of injury severity. It is helpful to underscore that in this study, the average lowest GCS within the first 24 hrs for children with TBIs was around 7. This level of GCS falls by convention in the severe range, although there were a few children whose GCS within the first 24 hr post-TBI fell in the moderate range.
Consistent with the results of our 2009 meta-analysis (Babikian & Asarnow, Reference Babikian and Asarnow2009), the TBI group had significantly lower scores on cognitive tests than healthy controls. In our 2009 meta-analysis, the differences in effect sizes for cognitive outcomes between studies of patient with moderate versus severe TBIs were not significant, at least within the post-acute period. Dichotomizing the TBI patients in this study into severe versus moderate categories based on a GCS cutoff would not be helpful as most of the participants were at the cusp (e.g., scores of 3 and 7 would be grouped together, but a 7 and 8, which are closer together, would be in separate groups). Therefore, our conclusion is based on a single GCS based continuum of injury severity, with the caveat that the range of GCS was limited based on our patient clinical presentation as described above.
The only acute injury variable that predicted cognitive outcome was the occurrence of a msTBI (i.e., participants with msTBI performed more poorly than their uninjured peers). The occurrence of a msTBI accounted for approximately 20% of the variance in cognitive outcomes in the post-acute phase. While the absence of an effect of acute injury severity appears to conflict with previous research (c.f., Donders & Nesbit-Greene, Reference Donders and Nesbit-Greene2004), there was less variance in acute injury severity in this sample than some other studies as we did not include individuals with mild, concussive-type injuries. This is not to say that there was no variability within our sample of youth with msTBI: 20 to 40% of the sample were positive for each measure of acute injury severity. In this study, there was no dose-response relationship between detailed descriptors of injury severity (derived from medical records and brain imaging reports) and cognitive functioning after pediatric msTBI.
Of the demographic and premorbid factors assessed, only parent ratings of parental education and premorbid academic/intellectual abilities predicted cognitive performance. After accounting for injury status, these two pre-injury variables accounted for an additional 16% of the variance in cognitive outcome above that attributable to the occurrence of a msTBI. In other words, following pediatric msTBI, parental education (SES) and pre-injury ability account for nearly the same amount of variance in post-injury cognitive functioning as the presence of a msTBI.
In addition, post-acute performance on neuropsychological tests was highly correlated with cognitive functioning 1 year later. Approximately 90% of the sample showed consistency in their classification as a low versus typical performer: of those with longitudinal data, 35% were classified as low performers at both time points, while 54% are typical performers at both time points. There are important clinical implications of these findings, namely that a brief (approximately 40 min) cognitive assessment administered within 4 to 5 months of injury can be used to identify which patients are likely to be at risk for long-term cognitive difficulties and prompt medical providers to initiate appropriate services.
It must be noted that the results highlighted here apply only to a subset of youth with TBI, specifically older children (ages 8 to 19) with msTBI. This study was part of a larger study including multi-modal neuroimaging (the results of which are published in other papers). Because of our interest in brain imaging data, our sample included children who had fairly severe injuries (GCS scores on the lower end of the possible range) but who were capable of completing the assessment and undergoing MRI by 5 months post-TBI. As a result, our sample does not include individuals with the most severe TBIs. Our sample also excluded youth with a significant history of psychiatric issues (i.e., requiring pharmacological intervention). Although we acknowledge that premorbid psychiatric histories are relatively common in pediatric TBI populations and we have skewed our sample somewhat by excluding them, we believed it would be important to do so in order for us to more confidently attribute deficits in cognitive functioning to the index injury, rather than confounding our findings with premorbid functioning.
Findings are also limited with regard to the medical variables used to characterize acute injury severity: we had to rely on available medical records to extract data on markers of injury severity. In cases where limited records (e.g., discharge summary only) were received, our data may underreport the occurrence of injury factors. In addition, no data regarding family functioning were included as possible predictors of outcome. Family functioning may contribute to whether or not a child with msTBI performs below expectations on cognitive assessment.
The relatively small sample size also limited the ability to detect significant effects, so the influence of other variables should not be discounted. The study findings may also have been limited by the chosen research battery, although careful attention was paid to choosing measures of cognitive skills that in our experience and per the literature show sensitivity following a brain injury (e.g., attention, memory, processing speed). Also, true longitudinal analyses were limited by some attrition and as a consequence, a smaller sample size. Therefore, findings based on longitudinal studies should be interpreted with caution. Finally, only GCS was used to characterize injury severity instead of other markers of severity or recovery (e.g., duration of coma or post-traumatic amnesia), as this was the only measure available to us consistently across our recruitment sites. It is possible that our analyses would have yielded different results had a different, more sensitive, measure been used to characterize severity.
Overall, this study indicates that only a subgroup of youth with msTBI exhibit cognitive deficits following injury. While detailed descriptors of acute injury do not appear to predict cognitive outcome, parental education and premorbid academic ability together explain a significant portion of the variance in cognitive outcome, consistent with other published work that has highlighted the importance of non-clinical predictors of outcome, including genetic, environmental, family, premorbid function, interpersonal, and community related factors (Babikian, Merkley, Savage, Giza, & Levin, Reference Babikian, Merkley, Savage, Giza and Levin2015; Taylor et al., Reference Taylor, Yeates, Wade, Drotar, Klein and Stancin1999; Yeates et al., Reference Yeates, Taylor, Drotar, Wade, Klein, Stancin and Schatschneider1997). Future studies should further examine whether other pre-injury factors play a role in recovery following pediatric msTBI. Exploring post-acute physiological factors, such as markers of inflammation and neuronal integrity/repair, may help to further identify which youth are at risk for cognitive difficulties after msTBI.
Acknowledgments
No competing financial interests exist. The authors declare no conflict of interest. This work was supported by grant R01 HD061504 from the National Institute of Child Health and Human Development to Robert Asarnow, Ph.D.





