INTRODUCTION
Spatial disorientation is defined as the inability to establish relations between positions, directions, movement of objects, or points in space, resulting in an inaccurate perception of one’s position and surroundings (Guariglia & Nitrini, Reference Guariglia and Nitrini2009; Lester et al., Reference Lester, Moffat, Wiener, Barnes and Wolbers2017). Deficits in spatial orientation are commonly seen in Alzheimer’s disease (AD) and have also been reported in amnestic Mild Cognitive Impairment (MCI) (DeIpolyi et al., Reference DeIpolyi, Rankin, Mucke, Miller and Gorno-Tempini2007; Gazova et al., Reference Gazova, Vlcek, Laczó, Nedelska, Hyncicova, Mokrisova and Hort2012; Lithfous et al., Reference Lithfous, Dufour and Després2013; Serino et al., Reference Serino, Cipresso, Morganti and Riva2014). Individuals with non-amnestic MCI, on the other hand, perform similarly to healthy controls in spatial orientation tasks (Cerman et al., Reference Cerman, Ross, Laczo, Martin, Zuzana, Ivana and Jakub2018; Hort et al., Reference Hort, Laczo, Vyhnalek, Bojar, Bures and Vlcek2007; Laczo et al., Reference Laczo, Vlcek, Vyhnalek, Vajnerova, Ort, Holmerova and Hort2009; Rusconi et al., Reference Rusconi, Suardi, Zanetti and Rozzini2015).
Studies using AD biomarkers have also demonstrated a relation with spatial orientation performance. Spatial orientation tasks have successfully differentiated MCI patients with hippocampal atrophy, abnormal CSF levels of amyloid-β and total tau, and apolipoprotein E ϵ4 allele carriers from those without biomarkers (Howett et al., Reference Howett, Castegnaro, Krzywicka, Hagman, Marchment, Henson and Chan2019; Laczó et al., Reference Laczó, Andel, Vyhnalek, Vlcek, Nedelska, Matoska and Hort2014; Moodley et al., Reference Moodley, Minati, Contarino, Prioni, Wood, Cooper and Chan2015; Nedelska et al., Reference Nedelska, Andel, Laczó, Vlcek, Horinek, Lisy and Hort2012). In addition, AD biomarkers have even been related to poorer spatial orientation performance in preclinical individuals (Allison et al., Reference Allison, Babulal, Stout, Barco, Carr, Fagan and Head2018; Coughlan et al., Reference Coughlan, Coutrot, Khondoker, Minihane, Spiers and Hornberger2019; Konishi et al., Reference Konishi, Joober, Poirier, MacDonald, Chakravarty, Patel and Bohbot2018). The allocentric orientation process depends on the provision of visual cues from the environment, allowing one to estimate one’s position in relation to the distance and direction of topographical landmarks or objects (Lithfous et al., Reference Lithfous, Dufour and Després2013). This process is known to be mediated by medial temporal lobe structures, particularly the hippocampus (Astur et al., Reference Astur, Taylor, Mamelak, Philpott and Sutherland2002; Lester et al., Reference Lester, Moffat, Wiener, Barnes and Wolbers2017). The egocentric orientation process, on the other hand, requires information from the position of one’s body in space, and is independent of topographical landmarks (Lithfous et al., Reference Lithfous, Dufour and Després2013; Morganti et al., Reference Morganti, Marrakchi, Urban, Iannoccari and Riva2009). While egocentric information is processed in the parietal lobes (DeIpolyi et al., Reference DeIpolyi, Rankin, Mucke, Miller and Gorno-Tempini2007; Vlček & Laczó, Reference Vlček and Laczó2014), the retrosplenial cortex is responsible for the integration of both allocentric and egocentric information (Gramann et al., Reference Gramann, Onton, Riccobon, Mueller, Bardins and Makeig2010; Pengas et al., Reference Pengas, Patterson, Arnold, Bird, Burgess and Nestor2010; Vann et al., Reference Vann, Aggleton and Maguire2009).
These regions, along with others that constitute the human navigational network, are vulnerable to the deleterious consequences of aging, as well as to the accumulation of plaques and tangles in AD pathology (Braak & Braak, Reference Braak and Braak1991; Lester et al., Reference Lester, Moffat, Wiener, Barnes and Wolbers2017; Pengas et al., Reference Pengas, Patterson, Arnold, Bird, Burgess and Nestor2010). Tasks that could isolate or focus on distinct spatial orientation processes would allow for the study of the integrity of these particular brain regions. The authors have demonstrated that early stage AD individuals, and MCI subjects, present both allocentric (mainly), and egocentric orientation impairments (Iachini et al., Reference Iachini, Iavarone, Senese, Ruotolo and Ruggiero2009; Laczó et al., Reference Laczó, Andel, Vyhnalek, Vlcek, Magerova, Varjassyova and Hort2010; Ritchie et al., Reference Ritchie, Carrière, Howett, Su, Hornberger, O’Brien, Ritchie and Chan2018; Ruggiero et al., Reference Ruggiero, Ruotolo, Iavarone and Iachini2020).
Results like these highlight the importance of spatial orientation assessment in older adults, as a means of identifying individuals at a higher risk of conversion to AD. However, to date, there is still no gold standard for the assessment of spatial cognition and traditional paper-and-pencil tests lack ecological validity.
In animal research, the Morris Water Maze (MWM) has been extensively employed for the study of spatial learning, spatial memory, and hippocampal damage (Gallagher & Rapp PR Reference Gallagher and Rapp1997). The MWM allows the assessment of egocentric and allocentric processes of spatial cognition (Moghaddam & Bures Reference Moghaddam and Bures1996), and to date, it remains a reliable model of spatial abilities. In recent decades, authors have attempted to reproduce the MWM to study spatial orientation in humans. Both real-world and virtual versions of the task have been developed (Driscol et al., Reference Driscoll, Hamilton, Yeo, Brooks and Sutherland2005; Laczó et al., Reference Laczo, Vlcek, Vyhnalek, Vajnerova, Ort, Holmerova and Hort2009; Moffat & Resnick, Reference Moffat and Resnick2002) and different clinical populations, as well as different contributors to human spatial cognition, have been studied (Laczó et al., Reference Laczó, Andel, Vyhnalek, Vlcek, Magerova, Varjassyova and Hort2012; Fajnerová et al., Reference Fajnerová, Rodriguez, Levčík, Konrádová, Mikoláš, Brom and Horáček2014; Negut et al., Reference Negut, Andrei-Matu, Sava and David2016). The authors have consistently reported the effects of age, sex, hormone levels, exercise, and cognitive decline as some of the moderators for human spatial capacity (Moffat & Resnick, Reference Moffat and Resnick2002; Driscoll et al., Reference Driscoll, Hamilton, Yeo, Brooks and Sutherland2005; Herting & Nagel Reference Herting and Nagel2012; Sneider et al., Reference Sneider, Hamilton, Cohen-Gilbert, Crowley, Rosso and Silveri2015; Laczó et al., Reference Laczó, Andel, Vyhnalek, Vlcek, Magerova, Varjassyova and Hort2010). This vast literature has advanced our understanding of the cognitive processes implicated in spatial learning and memory, as well as the multiple mediators involved in this cognitive process.
Important contributions like these have also highlighted the complexity of human spatial processing, and the challenges involved in reproducing daily activities and human needs. Different authors have suggested that small-scale forms of assessment differ significantly from the large-scale environments in the real world in which our spatial cognition is engaged (Hegarty et al., Reference Hegarty, Montello, Richardson, Ishikawa and Lovelace2006; Morganti & Riva, Reference Morganti and Riva2014). The ability to orient oneself in familiar and unfamiliar surroundings involves the integration of self-perceived position of the body in relation to space, as well as visual estimation of distance and directions of objects or topographical landmarks (Coughlan et al., Reference Coughlan, Laczó, Hort, Minihane and Hornberger2018; Lithfous et al., Reference Lithfous, Dufour and Després2013; Miniaci & De Leonibus, Reference Miniaci and De Leonibus2018). These cognitive processes are seldom detected by traditional cognitive evaluation (Cogné et al., Reference Cogné, Taillade, N’Kaoua, Tarruella, Klinger, Larrue and Sorita2017; Morganti & Riva, Reference Morganti and Riva2014; Peter et al., Reference Peter, Sandkamp, Minkova, Schumacher, Kaller, Abdulkadir and Kloeppel2018).
With the advent of virtual reality technology in recent years, new possibilities for the ecological assessment of a variety of cognitive processes have emerged. Virtual reality enables the reproduction of the real world, enabling exploration of the environment from different points of view, angles, and distances, the engagement in everyday activities, and even manipulation of objects in the surroundings. Furthermore, a sense of presence in the virtual world and the similarity between virtual tasks and real-life demands, contribute to the increased ecological validity of this method (Negut et al., Reference Negut, Andrei-Matu, Sava and David2016; Cogné et al., Reference Cogné, Taillade, N’Kaoua, Tarruella, Klinger, Larrue and Sorita2017). In the case of spatial cognition, the advantages of virtual reality tasks are particularly noticeable. This technology allows patients to be evaluated in dynamic situations, closer to the real world, and large-scale environments make virtual tasks advantageous over traditional small-scale approaches (Cogné et al., Reference Cogné, Taillade, N’Kaoua, Tarruella, Klinger, Larrue and Sorita2017; Howett et al., Reference Howett, Castegnaro, Krzywicka, Hagman, Marchment, Henson and Chan2019; Serino et al., Reference Serino, Morganti, Colombo, Pedroli, Cipresso and Riva2018).
Given the importance of an ecological assessment of spatial orientation deficits in older adults and the growing field of virtual reality tasks for the assessment of complex cognitive processes, this paper proposes two novel immersive virtual reality tasks: Spatial Orientation in Immersive Virtual Environment Test (SOIVET) Maze and SOIVET Route. In the SOIVET Maze task, participants can explore their surroundings from an egocentric reference frame, using a two-dimensional survey map to guide them through the maze. This task explores the “survey to route” spatial ability, without any input from topographical landmarks. On the other hand, in the SOIVET Route task, a sequence of visual cues (landmarks) marks the route to be learned and reproduced by participants, focusing on an allocentric-type orientation. Analysis of concurrent validity with traditional neuropsychological tests and group comparisons between MCI and cognitively healthy older adults were performed. Additionally, Receiver Operating Characteristic (ROC) analysis revealed the accuracy of the proposed tasks for the diagnosis of MCI and optimal cutoff points for each task.
METHODS
We conducted a case–control study to assess the performance of older participants with and without an MCI diagnosis in two new immersive virtual reality tasks (SOIVET Maze and SOIVET Route), and in traditional paper-and-pencil neuropsychological tests. The study was conducted at the University of São Paulo Faculty of Medicine Clinics Hospital and the Polytechnic School of the University of São Paulo, in São Paulo, Brazil. Ethical approval for the study was obtained from the Ethics Committee of the University of São Paulo Faculty of Medicine Clinics Hospital (CAPPesq). This study was performed in accordance with the Helsinki Declaration for human studies.
All participants were volunteers and provided written consent. Control group participants were recruited from programs for the elderly offered by the University of São Paulo to the local community. MCI participants were referred from the Reference Center for Cognitive Disorders (CEREDIC) of the University of São Paulo Faculty of Medicine Clinics Hospital, where an MCI diagnosis was established according to Petersen or Jak & Bondi criteria (Petersen, Reference Petersen2004; Jak et al., Reference Jak, Bondi, Delano-Wood, Wierenga, Corey-Bloom, Salmon and Delis2009), and other conditions, such as major depression, structural abnormalities in brain imaging, or Fazekas scale >1 were screened for and ruled out (Fazekas et al., Reference Fazekas, Chawluk, Alavi, Hurtig and Zimmerman1987).
Eligible subjects were required to be 60 years or older, have normal or corrected eyesight, and no hearing impairment. The control group included subjects without cognitive complaints and with Addenbrooke’s Cognitive Examination – Revised (ACE-R) scores above 82 points (César et al., Reference César, Yassuda, Porto, Brucki and Nitrini2017; Mioshi et al., Reference Mioshi, Dawson, Mitchell, Arnold and Hodges2006). Only amnestic MCI subjects were included in this study. The classification of amnestic MCI was determined for subjects with scores below 1.5 or greater standard deviation in the Rey Auditory Verbal Learning Test (Malloy-Diniz et al., Reference Malloy-Diniz, Lasmar, Gazinelli, Fuentes and Salgado2007).
Participants were invited to come to the University of São Paulo on two different occasions: one to answer questionnaires, provide baseline demographic information, and undergo traditional neuropsychological assessment and another to perform the two virtual tasks. The order of the visits was counterbalanced. We collected baseline characteristics of participants such as age, sex, and educational level. All participants answered the Technology Use Profile Questionnaire (da Costa et al., Reference da Costa, Pompeu, Mello, Moretto, Rodrigues, Dos Santos and Brucki2018) and the Charlson Comorbidity Index (Charlson et al., Reference Charlson, Pompei, Ales and MacKenzie1987).
The virtual tasks were administrated on the same visit, but the order of the tasks (SOIVET Maze or SOIVET Route) was counterbalanced. One study investigator accompanied participants at all times: explaining how participants could interact with the virtual system, monitoring any signs of discomfort, and following their progression on the computer screen. Participants who could not complete the virtual tasks due to tolerability issues were excluded.
Virtual Reality System
The operational characteristics of the Spatial Orientation in Immersive Virtual Environment Test (SOIVET) system and feasibility results with adults have been described elsewhere (da Costa et al., Reference da Costa, Pompeu, de Mello, Moretto, Rodrigues, Dos Santos and Brucki2018). The tasks were administered using the Oculus Rift CV1 kit, which includes a head-mounted display, two touch controllers, and two sensors that track constellations of infrared LEDs to translate the participant’s movement, whether sitting or standing. For this study, participants performed the virtual tasks sitting on an office chair with an armrest. A compatible Microsoft Windows 10 computer was used as the system processor. Both SOIVET Maze and SOIVET Route tasks were developed using the Unity® platform. Participants were able to navigate through the virtual environment using the touch controllers and their body position – turning the chair either right or left.
SOIVET Maze Task
This task was based on the Money Road-Map Test of Direction Sense (MRMT) described in 1967 (Money et al., Reference Money, Alexander and Walker1967) and adapted from the computer version proposed by Morganti et al. (Reference Morganti, Stefanini and Riva2013). In the SOIVET Maze task, participants were required to navigate in a virtual maze using the route depicted on the original MRMT map as a reference. A green point marked the last correct turn on the map, in order to reduce working memory efforts. No topographical landmarks were provided. To navigate in the first-person perspective, participants were required to follow the route depicted on the map, but also update information from their body position at each turn on the maze (Figure 1).

Fig. 1. Participant’s view in practice trial of SOIVET Maze.
Scoring was based on the total number of correct turns. After three incorrect turns, the task ended (“game over”). Different from the task described with young adults (da Costa et al., Reference da Costa, Pompeu, de Mello, Moretto, Rodrigues, Dos Santos and Brucki2018), for this study, the maze was reduced to the first 18 turning points of the original MRMT. This reduction was due to task difficulty – as the task was rarely completed in full – and to lower anxiety for older participants.
SOIVET Route Task
In the SOIVET Route task, participants entered the virtual reconstruction of the lobby of the Central Institute of the University of São Paulo Clinics Hospital. An avatar performed a route consisting of five specific locations inside the hospital lobby and its surroundings. Participants were required to follow the avatar in a first-person perspective (Figure 2). Subsequently, participants were required to repeat the same route alone, and to visit the five locations in the same order (SOIVET Route immediate). After a 20-minute interval, the participants repeated the route one more time (SOIVET Route delayed). The five locations included a reception balcony, a newsstand, a cafeteria, a table, and the entrance to a study center. This task was based on the item “Route” from the Rivermead Behavioral Memory Test (RBMT) (Kurtz, Reference Kurtz2011). Akin to the original RBMT, scoring was based on total locations visited in the correct order, both for immediate and delayed phases.

Fig. 2. Participant’s view of the avatar in instructions phase of SOIVET Route.
Traditional Neuropsychological Assessment
All participants were screened using ACE-R total score, and each cognitive domain of the ACE-R was also scored separately. Additionally, participants performed the Corsi Block Test as a measure of visuospatial working memory (Milner, Reference Milner1971); the Benton’s Judgment of Line OrientationTest (BJLO) (Benton et al., Reference Benton, Varney and Hamsher1978) for visuospatial perception and mental rotation; the Tower of London as a measure of planning (Shallice, Reference Shallice1982); and the original MRMT (Money et al., Reference Money, Alexander and Walker1967). Apart from the MRMT, all other neuropsychological tests and the ACE-R were administered on a separate day to the virtual tasks to reduce cognitive burden. This interval was no longer than 14 days.
Statistical Analysis
The primary endpoint of this study was the correlation between performance in the SOIVET Maze and SOIVET Route tasks and the neuropsychological assessment. Secondary endpoints included a performance in the virtual tasks compared between groups and the accuracy of each virtual task for the MCI diagnosis. Due to non-normal distribution of the data, correlation analysis was performed using the Spearman’s rank correlation. Group comparisons of continuous data were analyzed using the Wilcoxon Mann–Whitney test. The Fisher’s exact rest was used to compare categorical variables between groups. ROC curves were used for the analysis of sensitivity and specificity of the two virtual tasks for the MCI diagnosis. The Youden’s index was used to establish optimal cutoff points (Youden, Reference Youden1950).
RESULTS
Sample Selection Flow
A total of 35 cognitively healthy subjects and 27 MCI subjects were recruited for this study. However, four subjects from the control group (11.43%) and four from the MCI group (14.81%) dropped out due to tolerability issues with the virtual tasks (cybersickness). Dropout rates were not statistically significant between groups (p = 0.719). Additionally, three participants from the control group and five participants from the MCI group did not perform the traditional neuropsychological tests used for the correlation analysis.
Sample Characteristics
Twenty-nine participants aged 61–85, with educational levels ranging from 9 to 16 years were included in the control group. The MCI group included 19 subjects aged 64–92, with educational levels ranging from 9 to 18 years. Sample characteristics, the Charlson Comorbidity Index, scores from the Technology Use Profile Questionnaire, and cognitive screening are described in Table 1. All participants included in the study understood the virtual tasks and how to navigate in the virtual environments. The overall attitude toward the immersive technology was positive.
Table 1. Sample characteristics

CHE, Cognitively Healthy Elderly; ACE-R, Addenbrooke Cognitive Examination – Revised; CCI, Charlson Comorbidity Index.
Traditional Neuropsychological Assessment and Correlations with Virtual Tasks
Correlation between the SOIVET Maze task and other measures
Correlation analysis combined all participants (n=40) without group separation. The SOIVET Maze task revealed a significant correlation with the MRMT (r = .345, p = 0.016), Tower of London (r = .350, p = 0.027), BJLO test (r = .428, p = 0.006), and total ACE-R score (r = .368, p = 0.02). No significant correlation was found between the SOIVET Maze and Corsi Block Test forward or backward scores. Additionally, when analyzing the scores from each ACE-R category, we found a significant correlation between the SOIVET Maze and memory (r = .480, p = 0.002) and visuospatial (r = .317, p = 0.046) scores.
Performance in the SOIVET Maze task did not correlate with age (r = .013, p = 0.932), education (r = .200, p = 0.177), or technology use profile (r = .111, p = 0.500).
Correlation between SOIVET Route tasks and other measures
Correlation analysis of the SOIVET Route task indicated a significant correlation between SOIVET Route immediate and delayed scores (r = .511, p < 0.001). The SOIVET Route immediate also correlated with the MRMT (r = .392, p = 0.006) and the ACE-R total score (r = .396, p = 0.011). In addition, the memory (r = .341, p = 0.031) and visuospatial categories (r = .319, p = 0.045) from the ACE-R correlated with SOIVET Route immediate scores. SOIVET Route delayed scores did not correlate with any neuropsychological test, or with ACE-R total score and its categories.
Performance in the immediate phase of the SOIVET Route correlated with educational level (r = .442, p = 0.002), but not with age or familiarity with technology.
Performance in the virtual reality tasks and group comparison analysis
Measures of central tendency and dispersion of the scores of the SOIVET Maze, MRMT, and immediate and delayed SOIVET Route tasks are reported in Table 2.
Table 2. Performance in the virtual tasks and neuropsychological assessment
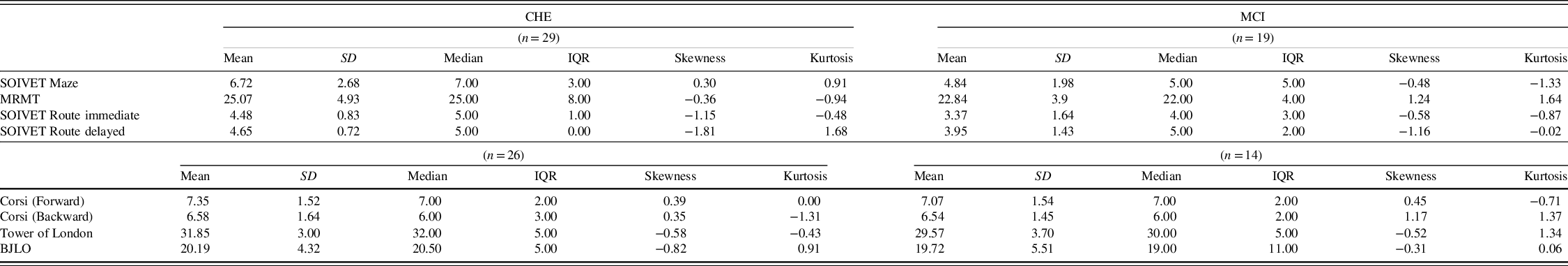
CHE, Cognitively Healthy Elderly; MCI, Mild Cognitive Impairment; MRMT, Money Road-Map Test; BJLO, Benton’s Judgment of Line Orientation test; SD, Standard Deviation; IQR, Interquartile Range.
The SOIVET Maze task was able to significantly differentiate groups (U = 151.500, p = 0.008), while the MRMT showed no statistically significant difference (U = 190.00, p = 0.071) (Figure 3).

Fig. 3. Average SOIVET Maze and MRMT scores by group.
SOIVET Maze maximum correct turns = 18. MRMT maximum correct turns = 32. **p < 0.01.
In the SOIVET Route task, the control group significantly outperformed the MCI group in the immediate phase (U=164.50; p = 0.009) as well as in the delayed phase (U=197.00; p = 0.044) (Figure 4).

Fig. 4. Scores in SOIVET Route immediate and delayed tasks by group.
*p < 0.05 **p < 0.01.
Receiver Operating Characteristic (ROC) analysis and cutoff points
Scores from each task (SOIVET Maze, SOIVET Route immediate, and SOIVET Route delayed) were selected as test variables and the categorical diagnosis of MCI was selected as the state variable. Results indicated a moderate accuracy for the SOIVET Maze and SOIVET Route immediate tasks for the MCI diagnosis (Table 3). We included sensitivity analysis for the traditional MRMT neuropsychological test for the purpose of comparison. Sensitivity and specificity results, as well as optimal cutoff points, are reported in Table 3. Other sensitivity and specificity values for different cutoff points are shown in Table 4.
Table 3. Accuracy results for the SOIVET Maze, SOIVET Route immediate, SOIVET Route delayed, and the MRMT

Data are obtained using ROC curve analysis and the Youden’s index. AUC, Area Under the Curve; CI, Confidence Interval; MRMT, Money Road-Map Test; ACE-R, Addenbrooke’s Cognitive Examination – Revised.
Table 4. Proposed cutoff points for the SOIVET Maze, SOIVET Route immediate and delayed tasks, and the different sensitivity and specificity values

Cutoff points considering the number of correct turns in SOIVET Maze and the number of locations visited in the correct order in SOIVET Route. Sens., sensitivity; Spec., Specificity.
DISCUSSION
Findings from the present study demonstrate that the SOIVET Maze task correlated with measures of visuoperception, mental rotation, and planning, and it was not influenced by age, technology use profile, or educational level. The SOIVET Route immediate task correlated with measures of mental rotation, memory, and visuoconstruction, and was influenced by educational level. The SOIVET Maze and SOIVET Route immediate tasks successfully differentiated cognitively healthy older adults from MCI subjects, demonstrating moderate accuracy for the MCI diagnosis. Additionally, our study demonstrated the feasibility of immersive virtual reality tasks for participants aged 61–92 with and without MCI. Enrolled participants had different levels of education, technology use profiles, and overall cognitive function, which did not seem to interfere with their ability to understand and engage with the virtual system.
The results found in the present study add to a growing body of evidence that the ecological assessments of cognitive functions are important for the detection of early cognitive decline (Bessi et al., Reference Bessi, Mazzeo, Padiglioni, Piccini, Nacmias, Sorbi and Bracco2018; Plancher et al., Reference Plancher, Tirard, Gyselinck, Nicolas and Piolino2012; Weakley & Schmitter-Edgecombe, Reference Weakley and Schmitter-Edgecombe2019). In particular, the ecological assessments of spatial orientation (in both virtual and real environments) has proven useful for differentiating normal from pathological aging (Coughlan et al., Reference Coughlan, Coutrot, Khondoker, Minihane, Spiers and Hornberger2019; Konishi et al., Reference Konishi, Joober, Poirier, MacDonald, Chakravarty, Patel and Bohbot2018), detecting MCI groups at a higher risk of conversion (Howett et al., Reference Howett, Castegnaro, Krzywicka, Hagman, Marchment, Henson and Chan2019; Laczó et al., Reference Laczó, Andel, Vlček, Mat’oška, Vyhnálek, Tolar and Hort2011), and identifying preclinical individuals with AD biomarkers (Allison et al., Reference Allison, Babulal, Stout, Barco, Carr, Fagan and Head2018; Allison et al., Reference Allison, Rodebaugh, Johnston, Fagan, Morris and Head2019; Coughlan et al., Reference Coughlan, Coutrot, Khondoker, Minihane, Spiers and Hornberger2019). Studies have demonstrated that allocentric spatial deficits are particularly common in MCI patients and individuals with early stages of AD, as well as the capacity to switch between allocentric and egocentric reference frames (Ruggiero et al., Reference Ruggiero, Iavarone and Iachini2018; Ruggiero et al., Reference Ruggiero, Ruotolo, Iavarone and Iachini2020; Serino et al., Reference Serino, Cipresso, Morganti and Riva2014; Morganti et al., Reference Morganti, Stefanini and Riva2013). We believe that our allocentric-type task (SOIVET Route) and our “survey to route” spatial switching task (SOIVET Maze) are in agreement with other ecological spatial orientation tasks that evidenced spatial deficits in MCI.
Immersive virtual reality has been increasingly utilized in different contexts related to the health care of older adults, such as postural rehabilitation (Bisson et al., Reference Bisson, Contant, Sveistrup and Lajoie2007; Virk & McConville, Reference Virk and McConville2006), cognitive training (García-Betances et al., Reference García-Betances, Jiménez-Mixco, Arredondo and Cabrera-Umpiérrez2015; Optale et al., Reference Optale, Urgesi, Busato, Marin, Piron, Priftis and Bordin2010), rehabilitation after cerebral ischemia (House et al., Reference House, Burdea, Polistico, Roll, Kim, Grampurohit and Pollack2016; McEwen et al., Reference McEwen, Taillon-Hobson, Bilodeau, Sveistrup and Finestone2014), and even in education against abuse and neglect of older adults (Pickering et al., Reference Pickering, Ridenour, Salaysay, Reyes-Gastelum and Pierce2018). Although there is still some concern about the acceptance of immersive technology by this particular group, we found great acceptance by the participants in testing the virtual system, and a good understanding of how to operate it. Additionally, we found dropout rates of 11.43% and 14.81% due to tolerability issues for the control and MCI groups, respectively, lower rates than the cybersickness rates described in the general population (LaViola, Reference LaViola2000).
Correlation results from the SOIVET Maze task showed a weak but significant correlation with the MRMT (which the task was based on), with planning abilities measured by the Tower of London test and with the ACE-R visuospatial category. The task demonstrated a moderate and significant correlation with visuoperception and mental rotation measured by the BJLO test and the ACE-R memory category. We expected at least a moderate correlation of the SOIVET Maze with the BJLO and the MRMT. In fact, previous results of the SOIVET Maze task with adult participants showed a moderate correlation with the MRMT task (da Costa et al., Reference da Costa, Pompeu, de Mello, Moretto, Rodrigues, Dos Santos and Brucki2018). Among older participants; however, this correlation was weak. Our previous study with adults also demonstrated a moderate correlation between the SOIVET Maze task and several items and total score of the Santa Barbara Sense of Direction Scale – a self-report on spatial abilities (da Costa et al., Reference da Costa, Pompeu, de Mello, Moretto, Rodrigues, Dos Santos and Brucki2018). Differences in correlation results among older adults possibly indicate that other factors influenced their performance in the virtual tasks – such as global cognitive function. Another interesting aspect was that no correlation was found between the SOIVET Maze and the Corsi Block test. In the study from Morganti et.al. (Reference Morganti, Stefanini and Riva2013), a weak but significant correlation was found between the computerized version of the MRMT and the Corsi Block test, but no correlation was found with the BJLO and TOL. An important difference between the two maze tasks is the non-immersion of the task described by Morganti et al. Another difference was the way the path on the map was provided: in the study from 2013, participants held the original MRMT map on paper, outside the computerized environment. In the present study, the map was visible within the virtual environment with the possibility of monitoring the progression of the route through the green point. We believe that this way of depicting the map and the participant’s progression may have contributed to reducing the visual working memory load.
In addition, the transposition from a two-dimensional survey map to an egocentric type of orientation, as well as the integration of one’s body positioning and movement inside the maze may lead to the interpretation that the SOIVET Maze task is a dual task. The ability to perform dual tasks, in turn, is known to be influenced by global cognitive function (Hirsch et al., Reference Hirsch, Nolden and Koch2017) and age (Korotkevich et al., Reference Korotkevich, Trewartha, Penhune and Li2015), and is impaired in AD and MCI (Camicioli et al., Reference Camicioli, Howieson, Lehman and Kaye1997; Montero-Odasso et al., Reference Montero-Odasso, Sarquis-Adamson, Speechley, Borrie, Hachinski, Wells and Muir-Hunter2017). Indeed, it seems difficult to isolate with precision all cognitive domains involved in virtual tasks, which are dependent on overall cognitive abilities and could be interpreted as dual tasks. This complexity, on the other hand, seems to be an advantage, at least with respect to the multiple aspects required for spatial cognition (Coutrot et al., Reference Coutrot, Schmidt, Coutrot, Pittman, Hong, Wiener and Spiers2019; Riva, Reference Riva2018).
The SOIVET Route immediate task demonstrated a weak but significant correlation with the MRMT task, and ACE-R memory and visuospatial categories, and a moderate correlation with the SOIVET Route delayed task. These results indicate the influence of some cognitive domains involved in spatial orientation, such as mental rotation, memory, and visuoconstruction, but not with other domains such as planning and visuoperception. Correlation results favored the SOIVET Route immediate in detriment of the SOIVET Route delayed, which did not correlate with any other cognitive test. This supports the influence of route learning in the SOIVET Route immediate task, instead of visuospatial memory, as proposed in the original “Route” item of the RBMT (Kurtz, Reference Kurtz2011). The original task described in the RBMT aims to investigate spatial memory, and is supposed to be administered inside a clinicians’ office. However, by enlarging the space and route to be traveled, other cognitive processes are recruited, such as allocentric orientation. Different authors have defended the use of hospital environments for route learning tasks as a form of ecological assessment of spatial orientation (Boccia et al., Reference Boccia, Di Vita, Diana, Margiotta, Imbriano, Rendace and Guariglia2019; Irish et al., Reference Irish, Lawlor, Coen and O’Mara2011; Peter et al., Reference Peter, Sandkamp, Minkova, Schumacher, Kaller, Abdulkadir and Kloeppel2018). A growing number of studies aiming to assess spatial orientation have made use of route learning tasks, which have proven to be useful in differentiating pathological from non-pathological aging (Benke et al., Reference Benke, Karner, Petermichl, Prantner and Kemmler2014; Boccia et al., Reference Boccia, Di Vita, Diana, Margiotta, Imbriano, Rendace and Guariglia2019; Mitolo et al., Reference Mitolo, Gardini, Fasano, Crisi and Pelosi2013; Pengas et al., Reference Pengas, Patterson, Arnold, Bird, Burgess and Nestor2010; Peter et al., Reference Peter, Sandkamp, Minkova, Schumacher, Kaller, Abdulkadir and Kloeppel2018).
Once again, it was interesting not to find a correlation between the SOIVET Route immediate task and the Corsi Block test, particularly because in this task participants were required to reproduce a five-location sequence. We believe that a ceiling effect may have influenced this finding since the majority of participants in the control group found the task quite easy. The average number of correct responses in the SOIVET Route immediate task was close to five in this group, and their forward visual-spatial span ranged from four to seven. By increasing the number of locations on the route, it could have been possible to detect a stronger relation to the visual working memory capacity.
A recent review from our group found that spatial orientation tasks show moderate to high accuracy in detecting MCI in cognitively healthy older adults, evidencing the importance of spatial orientation in cognitive assessment (da Costa et.al. Reference da Costa, Pompeu, Viveiro and Brucki2020). In line with these findings, our study found that both SOIVET Maze and SOIVET Route immediate tasks presented moderate accuracy for the MCI diagnosis. It was interesting to note that the traditional MRMT was not able to significantly classify the MCI group. This comparison, however, is mainly for illustration, as the selected test is not routinely used for identifying older adults with a higher risk of conversion to AD.
Besides the results of the correlation with the neuropsychological test and the accuracy analysis, the noninfluence of age, education, and technology use profile are other important findings of the SOIVET Maze task. The SOIVET Route immediate task was influenced only by education. These results support their use by different populations. Virtual reality tasks are also advantageous in terms of the facility to administer, reduced need for specialized personnel, shorter application time, reduced sociocultural and linguistic influence, and even greater engagement from participants (Li et al., Reference Li, Yu, Shi, Shi, Tian, Yang and Jiang2017; Massetti et al., Reference Massetti, da Silva, Crocetta, Guarnieri, de Freitas, Lopes and Monteiro2018).
In the last decade, a rising number of virtual reality tasks for the assessment of spatial orientation have been described in the literature, and creative settings with wide landscapes, public spaces like museums, and mazes were employed to investigate the roles of aging and age-related cognitive decline in the human spatial processing (Howett et al., Reference Howett, Castegnaro, Krzywicka, Hagman, Marchment, Henson and Chan2019; Tarnanas et al., Reference Tarnanas, Laskaris, Tsolaki, Muri, Nef and Mosimann2015; Colombo et al., Reference Colombo, Serino, Tuena, Pedroli, Dakanalis, Cipresso and Riva2017). In line with the current literature, we found that our virtual reality tasks were able to identify deficits in spatial orientation in MCI individuals, and significantly differentiate groups. Interestingly, the original paper-and-pencil task – the MRMT – was not capable of this differentiation, supporting the notion that large-scale ecological environments are preferable over small-scale forms of assessment when it comes to spatial processing. Several tests of different cognitive domains are able to identify the established AD pathology, but few can distinguish groups at a higher risk of conversion. Ecological approaches seem to be helpful for this purpose (Allison et al., Reference Allison, Rodebaugh, Johnston, Fagan, Morris and Head2019).
Some limitations of this study should be acknowledged: First, in the SOIVET Maze task, participants performed the MRMT before the virtual task. This was conducted to facilitate participants’ understanding of the virtual task so that they could experience, outside the immersive system, the mental rotation required by the map of the MRMT. Therefore, this flow was not counterbalanced, and may have influenced performance in the SOIVET Maze task. On the other hand, although a learning effect would have benefited the second task (i.e., the virtual task), participants from both groups demonstrated greater difficulty in performing the SOIVET Maze task than the MRMT. A learning effect and its influence, however, cannot be ruled out in the present study.
Second, even though the diagnosis of MCI was established according to the Petersen or Jak & Bondi criteria prior to virtual task administration, the presence of AD pathology was not confirmed by biomarkers. Although indicative of an underlying pathological process, confirmation of MCI due to AD would need a follow-up. The present study could have benefited from the additional characterization of the clinical sample, including for example, medial temporal lobe atrophy or CSF biomarkers. Future studies with a clearer aMCI sample with minimal confounding factors will contribute to better understanding of the capabilities of the SOIVET system.
Third, the traditional neuropsychological tests chosen a priori to explore the cognitive processes involved in the SOIVET Maze task provided some different conclusions from what was initially expected. The lack of correlation with the Corsi Block test and a weak correlation with the MRMT are some of these intriguing results. Further studies using the SOIVET Maze task and other measures of visual-spatial learning and mental rotation will contribute to confirming the construct behind this virtual task.
Despite being able to differentiate groups significantly, the SOIVET Route delayed task did not correlate with any neuropsychological task, and showed low accuracy for the MCI diagnosis. Although the small sample size may have underpowered statistical differences, these findings limit the implementation of the SOIVET Route delayed task for the cognitive assessment of spatial orientation at this point.
Additional considerations for future studies include longer follow-up, in order to assess the ability of the virtual system in predicting AD conversion; including AD biomarkers; investigating test–retest and inter-rater reliability of the SOIVET system; and including a path integration component in the SOIVET Route immediate task. Path integration is the ability to identify and return to a previously visited location. This process is dependent on the continuous integration of multisensory cues (visual, proprioceptive, and vestibular) that provide current position information and the direction from the previous site (Etienne & Jeffery, Reference Etienne and Jeffery2004). The entorhinal cortex, a key node in the AD pathology (Knopman et al., Reference Knopman, Lundt, Therneau, Vemuri, Lowe, Kantarci and Jack2019), mediates path integration (McNaughton et al., Reference McNaughton, Battaglia, Jensen, Moser and Moser2006; Wolbers et al., Reference Wolbers, Wiener, Mallot and Büchel2007). Deficits in this process have been described in MCI, and were able to differentiate MCI individuals with and without AD biomarkers, better than traditional neuropsychological tests (Howett et al., Reference Howett, Castegnaro, Krzywicka, Hagman, Marchment, Henson and Chan2019; Mokrisova et al., Reference Mokrisova, Laczo, Andel, Gazova, Vyhnalek, Nedelska and Hort2016). The inclusion of path integration in spatial orientation assessment appears promising for the identification of high-risk populations.
In conclusion, immersive virtual environments were shown to be advantageous for the reproduction of complex cognitive processes such as spatial cognition. Traditional neuropsychological assessment has limitations, and virtual reality technology has advanced the field of cognitive evaluation. Spatial orientation deficits are related to the pathological process of AD and should be included in regular cognitive evaluation of older adults. Virtual reality tasks for the assessment of spatial orientation like the SOIVET Maze and SOIVET Route immediate tasks are feasible for the older population and could help identify individuals with a higher risk of developing AD.
FINANCIAL SUPPORT
This work was supported by the São Paulo Research Foundation (Grant number: FAPESP 2016/04984-3). Author Raquel Quimas Molina da Costa received a PhD fellowship from the Brazilian National Research Council (Grant number: CNPq 140534/2018-0).
CONFLICTS OF INTEREST
The authors state no conflicts of interest.
ETHICAL STANDARDS
This research was completed in accordance with the Helsinki Declaration.










