Introduction
Prospective memory (PM) is the ability to remember to perform a future action. For example, PM includes the ability to remember to call a person at 5:00 pm or pay the electric bill before its due date (Ellis & Kvavilashvili, Reference Ellis and Kvavilashvili2000). According to the Einstein and McDaniel's model (1990), there are two types of PM tasks, event-based and time-based. In conditions requiring event-based PM, a person performs an action when a specific event occurs; while in situations requiring time-based PM, a person forms a self-generated intention to perform an action at a specific time in the future (Einstein & McDaniel, Reference Einstein and McDaniel1990; Kliegel, McDaniel, & Einstein, Reference Kliegel, McDaniel and Einstein2008). Event-based PM tasks are considered to be less cognitively demanding than time-based PM tasks because they require less self-initiated retrieval and there are available external cues to help recall the task to be performed (McDaniel & Einstein, Reference McDaniel and Einstein1993; McDaniel, Guynn, Glisky, Rubin, & Routhieaux, Reference McDaniel, Guynn, Glisky, Rubin and Routhieaux1999; Shum, Valentine, & Cutmore, Reference Shum, Valentine and Cutmore1999). Time-based PM task, relative to event-based, require higher cognitive demands, particularly initiation/inhibition control and updating abilities (Glickson & Myslobodsky, Reference Glickson and Myslobodsky2006; McDaniel et al., Reference McDaniel, Guynn, Glisky, Rubin and Routhieaux1999; McFarland & Glisky, Reference McFarland and Glisky2009). In time-based PM tasks, participants have to constantly monitor the elapsed time to ensure successful initiation of the task (Groot, Wilson, Evans, & Watson, Reference Groot, Wilson, Evans and Watson2002; Kinch & McDonald, Reference Kinch and McDonald2001; McDaniel et al., Reference McDaniel, Guynn, Glisky, Rubin and Routhieaux1999). According to Einstein, McDaniel, Richardson, Guynn, and Cufer (Reference Einstein, McDaniel, Richardson, Guynn and Cufer1995), performance of time-based PM can be evaluated with task accuracy and analysis of the monitoring behavior (Einstein et al., Reference Einstein, McDaniel, Richardson, Guynn and Cufer1995). Efficient monitoring requires a strategic scheduling of actions (i.e., when and how to monitor) and a balance between the cost of monitoring versus the cost of having inaccurate information about the environment (Mäntylä & Carelli, Reference Mäntylä and Carelli2006). Studies of time-based PM suggested that successful performance required that the rate of monitoring increase as the time deadline approaches (Einstein et al., Reference Einstein, McDaniel, Richardson, Guynn and Cufer1995; Mäntylä & Carelli, Reference Mäntylä and Carelli2006; Mäntylä, Carelli, & Forman, Reference Mäntylä, Carelli and Forman2007).
Prior investigations have found that PM is impaired following traumatic brain injury (TBI). These failures have the potential to limit the independence of TBI patients, causing them to rely on a caregiver for prompting the intended actions. Moreover, these failures may impede their ability to return to independent living (Fleming, Shum, Strong, & Lightbody, Reference Fleming, Shum, Strong and Lightbody2005). Studies have found that patients with TBI, relative to healthy controls, performed significantly worse on many PM tasks (Groot et al., Reference Groot, Wilson, Evans and Watson2002; Kinch & McDonald, Reference Kinch and McDonald2001; Mathias & Mansfield, Reference Mathias and Mansfield2005; Shum et al., Reference Shum, Valentine and Cutmore1999; for a review see Shum, Levin, & Chan, Reference Shum, Levin and Chan2011). Furthermore, while both controls and TBI patients are more likely to exhibit failures on time-based tasks than event-based tasks, this discrepancy is often greater for TBI patients than for controls (Henry et al., Reference Henry, Phillips, Crawford, Kliegel, Theodorou and Summers2007; Kinch & McDonald, Reference Kinch and McDonald2001; Shum et al., Reference Shum, Valentine and Cutmore1999). However, there has been limited research regarding the association between TBI and time-based PM, in particular taking into account the contribution of monitoring behavior on time-based PM performance (Carlesimo, Formisano, Bivona, Barba, & Caltagirone, Reference Carlesimo, Formisano, Bivona, Barba and Caltagirone2004; Shum et al., Reference Shum, Valentine and Cutmore1999, Reference Shum, Levin and Chan2011).
Central to time-based PM is the ability to perform the future action at a precise time in the future, and, therefore, time perception may be a further critical component (Glickson & Myslobodsky, Reference Glickson and Myslobodsky2006). In particular, temporal contributions to time-based PM would be related with monitoring frequency rather than PM accuracy. PM accuracy has been reported to be mainly associated with the memory component of PM tasks (Kopp & Thöne-Otto, Reference Kopp and Thöne-Otto2003; Labelle, Graf, Grondin, & Gragné-Roy, Reference Labelle, Graf, Grondin and Gragné-Roy2009).
To our knowledge, only three studies have investigated the time perception component in TBI patients (Meyers & Levin, Reference Meyers and Levin1992; Perbal, Couillet, Azouvi, & Pouthas, Reference Perbal, Couillet, Azouvi and Pouthas2003; Schmitter-Edgecombe & Rueda, Reference Schmitter-Edgecombe and Rueda2008), which were conducted within a prospective time estimation framework (e.g., participants are told in advance that they will be asked to estimate the duration of a stimulus in the future) (Zakay, Reference Zakay1993). Two studies were conducted with time reproduction tasks, where participants were instructed to reproduce the duration of a previously presented stimulus (Meyers & Levin, Reference Meyers and Levin1992; Perbal et al., Reference Perbal, Couillet, Azouvi and Pouthas2003), and one used a time estimation task, in which participants experienced a stimulus for a specific interval and then had to translate that experience into a time estimate using conventional units (Schmitter-Edgecombe & Rueda, Reference Schmitter-Edgecombe and Rueda2008). The studies found that accuracy of TBI patients decreased as a function of increased time and working memory demands (Meyers & Levin, Reference Meyers and Levin1992; Schmitter-Edgecombe & Rueda, Reference Schmitter-Edgecombe and Rueda2008). Moreover, TBI patients were reported to be more variable in their performance relative to healthy controls (Perbal et al., Reference Perbal, Couillet, Azouvi and Pouthas2003).
Thus, simultaneous monitoring behavior and time perception may be critical for accurate performance in time-based PM tasks. The objective of this study was to investigate time-based PM in TBI patients, and the relationship among time-based PM, time perception, and executive functions. To accomplish this objective we used four distinct measures including two executive functions tasks, a temporal task, and a time-based PM task. We hypothesized that (1) TBI patients would be less accurate than healthy controls on a time-based PM task, and that the frequency of monitoring behavior would correlate with time-based PM performance; and that (2) time perception would be involved in monitoring frequency performance, such that participants with better timing ability would display reduced monitoring frequency. To this end, participants were required to reproduce durations similar to the time elapsed between clock checking.
Method
Research Participants
Eighteen patients with severe TBI (12 men and 6 women), and 18 healthy participants (9 men and 9 women), matched for age and educational level, participated in the study. Demographic and clinical features of the patients were reported in Table 1. All patients referred to Modulo di Neuropsicologia Riabilitativa (Azienda Ospedaliero-Universitaria Ferrara, Italy). TBI patients were tested at least 6 months post their injury. All patients had available neuroimaging information (computed tomography, magnetic resonance imaging) that showed damage in a wide variety of cortical areas, with the majority of participants having frontal lesions. According to available clinical records, the participants were not densely amnesic or aphasic, and had no prior or current psychiatric pathology. We excluded participants who had motoric deficits, or history of drug or alcohol abuse.
Table 1 Demographic and clinical features of TBI patients. Cause referred to the cause of accident; MB = moto-bike; TPI = Time Post Injury (month); Injury site referred to the prevalent site of injury; F-P = Fronto-Parietal; DAI = Diffuse Axonal Injury; F = Frontal; F-T = Fronto-Temporal; LCF = Level of Cognitive Functioning (Hagen, Malkmus, Durham, & Bowman, Reference Hagen, Malkmus, Durham and Bowman1979): PTA = Post Traumatic Amnesia (day); GCS = Glasgow Coma Scale (Teasdale & Jennett, Reference Teasdale and Jennett1974); FIM/FAM = Functional Independence Measure/Functional Assessment Measure (Hall, Hamilton, Gordon, & Zasler, Reference Hall, Hamilton, Gordon and Zasler1993); TMT (msec) = difference between execution time on TMT part B and TMT part A; Action Programme and Zoo map score from the Behavioral Assessment of Dysexecutive Syndrom; WCST global = global score in the Wisconsin Card Sorting Test; Divided attention (msec) and GO/Nogo (msec) tasks from the Test of Attentional Performance

We evaluated cognitive competencies of the TBI cohort with the following neurocognitive measures: Trial Making Test (Bowie & Harvey, Reference Bowie and Harvey2006); Action Programme and Zoo Map subtasks from the Behavioral Assessment of Dysexecutive Syndrome (BADS; Wilson, Alderman, Burgess, Emslie, & Evans, Reference Wilson, Alderman, Burgess, Emslie and Evans1996); Wisconsin Card Sorting Test (Laiacona, Inzaghi, De Tanti, & Capitani, Reference Laiacona, Inzaghi, De Tanti and Capitani2000), and the Divided Attention and Go/Nogo subtasks from the Test of Attentional Performance (TAP, Zimmermann & Fimm, Reference Zimmermann and Fimm2002). Based on their performance of these screening measures, all TBI patients were found to be physically and mentally able to understand and complete the experimental tasks. All participants provided verbal consent to participate in this study that was conducted in accordance with the Helsinki Declaration (59th WMA General Assembly, Seoul, 2008).
The control group was matched to the TBI patient group on the basis of age and educational level: The mean age was 34.50 years (SD = 6.52; range = 22–52 years) and the mean level of education was 13.33 years (SD = 3.88; range = 8–18 years). The TBI group and the control group did not show significant differences with respect to age [t(34) = 1.20; p = .236] and education [t(34) = −.947; p = .347]. The difference in gender was also not significant, χ 2 = 1.02, df = 1, p = .50.
Procedures
TBI patients and six healthy control participants completed their study procedures in a quiet, distraction-free room at the Modulo di Neuropsicologia Riabilitativa (Ferrara, Italy). The other healthy control participants (12 students) completed their study in a similar testing environment at the University of Padova (Padova, Italy). All tasks were presented on a 15-inch computer monitor and participants were seated approximately 60 cm away from the computer screen.
Time-based PM Task
We used a time monitoring task (Mäntylä et al., Reference Mäntylä, Carelli and Forman2007) to test time-based PM performance. For this task, participants watched a 20-min cartoon-movie (Madagascar), and were instructed to mark the passage of time by pressing a key on a keyboard at every 5-min interval. Subjects were not informed of the duration of the movie. The participants had available two response buttons (red and green) and were instructed to press the red key every 5 min (at 5:00, 10:00, 15:00, and 20:00 min). Participants could press the green key at any time during the task to monitor the passage of time and the time appeared for 2 s on the right bottom portion of the computer screen. Importantly, participants were also told to watch the film carefully, because they would be asked to complete a 10-item questionnaire about the movie content. To clarify instructions and ensure familiarization with the task, participants completed a practice where they watched and responded to a 5-min movie (Sanpei). The experimenter demonstrated that the clock would start at 00:00 and that, for example, 2:00 means 2 min. No feedback was provided. Time-based PM performance was evaluated in terms of PM accuracy (target time response accuracy), monitoring frequency (number of clock checking), and on-going task performance (response accuracy about the movie content).
Time Reproduction Task
Participants were instructed to reproduce the duration of a stimulus previously seen. The stimulus (“smiley” face) appeared at the center of the computer screen for one of three durations (4, 9, or 14 s). Each duration was randomly presented 4 times for a total of 12 stimuli presentations. After a 2-s inter-stimulus interval, a question mark appeared on the computer screen and participants were instructed to press the spacebar for the same duration that the stimulus was on the screen. To prevent counting strategies (Baudouin, Vanneste, Pouthas, & Isingrini, Reference Baudouin, Vanneste, Pouthas and Isingrini2006) digits appeared at the center of the stimulus and participants should read these digits aloud. Digits ranged from 1 to 9 and were randomly presented with an inter-digit interval that varied from 400 to 1000 ms (Baudouin et al., Reference Baudouin, Vanneste, Pouthas and Isingrini2006). Participants completed a practice phase before beginning the task (one time each duration); no feedback was provided.
The durations used in this study were selected following Shum et al. (Reference Shum, Valentine and Cutmore1999) results: analyzing the monitoring frequency (in particular at the last minute closer to the target time) controls monitored on average every 12 s while TBI patients every 7 s. Following these results, participants were required to reproduce durations in the range of 4 to 14 s that are durations similar to the intervals between monitoring. Time reproduction data were analyzed in terms of relative errors and coefficient of variation (CV) (Perbal et al., Reference Perbal, Couillet, Azouvi and Pouthas2003). Relative error was obtained by dividing each participant's time reproduction by the time duration of the sample interval presented for that trial. This measure provided a standard score across the different time intervals, with coefficients above and below 1.0 indicative of overproductions and underproductions, respectively. The CV is computed by taking the ratio of the standard deviation (SD) over the reproduction mean. The CV index represents the variability in temporal judgment for each participant, and evaluates the consistency of time reproductions of the same target duration.
Executive Functions Tasks
We assessed inhibition and updating ability with the Stroop and n-back tasks, respectively. For the Stroop task, each trial contained three of four words (RED, YELLOW, GREEN, or BLUE) presented in 20 point size Arial font for 2 s in the center of a computer screen. The central word was colored in red, yellow, green, or blue and was the target stimulus, whereas the two lateral words were always black. Participants were instructed to identify the color of the central word by pressing a key on the keyboard marked with an arrow pointing either right (→) or left (←), depending on the position of the correct response word (in black). For example, if the answer was on the left, the (←) key had to be pressed with the left index finger, but if the answer was on the right, the (→) key had to be pressed with the right index finger. Two possible conditions, congruent and incongruent, were randomly presented on the screen. In the congruent condition, the color of the central word corresponded with the written word (e.g., the word “RED” appeared in red); whereas, in the incongruent condition, the written word appeared in a different font color (e.g., the word “RED” colored in green). Forty-eight stimuli were presented for each condition (for a total of 96 stimuli), and an equal number of correct responses were presented right or left (Del Missier, Mäntylä, & Bruine de Bruin, Reference Del Missier, Mäntylä and Bruine de Bruin2010). Participants performed 10 practice trials, 5 for the congruent and 5 for the incongruent condition, before completing the task. No feedback was provided. We analyzed the Stroop data in terms of reaction time (RT), and defined the Inhibition index as the difference between incongruent and congruent RT.
For the n-bask task (Miyake et al., Reference Miyake, Friedman, Emerson, Witzki, Howerter and Wager2000; Owen, McMillan, Laird, & Bullmore, Reference Owen, McMillan, Laird and Bullmore2005), common bi-syllabic word stimuli were presented centrally on the computer screen. The stimuli were displayed in white color, 36-point size Courier New font on a black background. Participants were instructed to press the left mouse key to indicate when they recognized a word that was the same as one of the 2 words back. The task comprised 48 target stimuli (24 different stimuli presented 2 times) and 48 non-target stimuli: 12 stimuli were presented once, 6 stimuli were 3-back presented two times, 6 stimuli were 4-back presented two times and 6 stimuli were 5-back presented two times. Participants completed a practice phase (5 target words and 10 non-target words) before completing the task. No feedback was provided. N-back data were analyzed in terms of number of errors (false alarms+omissions) and called Updating index.
Results
Time-Based PM
PM accuracy
To assess PM accuracy, we followed the methods of Einstein et al. (Reference Einstein, McDaniel, Richardson, Guynn and Cufer1995) and Shum et al., (Reference Shum, Valentine and Cutmore1999) and scored as accurate if participants responded within 10 s before or after the target time. TBI patients were less accurate than controls [t(33) = −1.83; p < .05; Cohen's d = −.67]. To further investigate PM accuracy we also computed latency responses. As such, participants’ responses were categorized and assigned a score that ranged from 1 to 4 (1 = from 0 to 2 s; 2 = from 3 to 5 s; 3 = from 6 to 8 s, and 4 = 9 s or over) depending on latency of the response. The TBI cohort was significantly less accurate [t(33) = 2.57; p < .05; Cohen's d = .86] and had lengthier latency response times relative to the healthy control group (4.05 vs. 1.61, respectively).
Monitoring frequency
We compared the groups regarding their monitoring behavior, by analyzing their monitoring frequency (e.g., number of clock checking) across 4 blocks (block 1 = 0–5 min; block 2 = 6–10 min; block 3 = 11–15 min, and block 4 = 16–20 min; Mäntylä et al., Reference Mäntylä, Carelli and Forman2007). To investigate learning effect across blocks a 2 × 4 analysis of variance (ANOVA) that included between-subject group (TBI, control) and the within-subject blocks (1st, 2nd, 3rd, and 4th block) was conducted. There was no effect for block (p = .21; η2p = .044), or interaction between group and block (p = .59; η2p = .019). Therefore, each block was collapsed and analyzed at each minute (see Figure 1). Data were subjected to a 2 × 5 ANOVA that included the between-subject group (TBI, control) and the within-subject minute (1st, 2nd, 3rd, 4th, and 5th min). The results identified a main group effect [F(1,33) = 7.28; p < .01], showing that TBI patients monitored more frequently than controls (1.88 vs. 1.05 times), and a main effect for minute [F(4,132) = 64.22; p < .0001], consistent with increased time monitoring frequency at the target time deadline (1st = .66; 2nd = .89; 3rd = 1; 4th = 1.63, and 5th = 3.25 times). The interaction between group and minute was also significant [F(4,132) = 2.44; p < .05]. The TBI group significantly increased their monitoring starting from the 2nd min, whereas the healthy control group increased their monitoring beginning at the 4th min.

Fig. 1 Clock-checking frequency collapsed across 5-min task intervals and error bars for traumatic brain injury (TBI) patients and controls.
On-going task performance
Data from on-going task performance were also scored and the numbers of correct answers on the 10-item movie content questionnaire were included in the analysis. The groups showed similar performance regarding their recall of the movie content as demonstrated in their responses to the questionnaire [t(28) = -1.94; p = .06, Cohen's d = −.71; .50 vs. .64 for patients and controls, respectively].
Time Reproduction Task
Two separate ANOVAs were conducted on relative errors and CV. The between-subject factor was group (TBI, control) and the within-subject factor was duration (4, 9, and 14 s). Analysis of relative errors revealed a significant main effect of duration [F(2,68) = 36.29; p < .001], increasing durations produced more under-reproduction errors (.95, .83, and .72, respectively). No group differences were found (p = .98; η2p = .001), and no significant interaction was found between group and duration [p = .97; η2p = .001].
The analysis of CV showed a main group effect [F(1,34) = 5.77; p < .001]; TBI patients showed more variability in their performance than controls (.24 vs. .15). There was no main effect of duration (p = .28; η2p = .036), or interaction between group and duration (p = .72; η2p = .009).
Executive functions tasks
The results of the Inhibition index (Stroop task) revealed no significant differences between groups [t(30) = 1.71; p = .097; Cohen's d = .58]. TBI and control participant were equally affected by the Stroop interference (184 vs. 136 ms). While, significant difference was found between groups on the Updating index (n-back task) [t(34) = 2.57; p < .01; Cohen's d = .86] showing that TBI patients produced more errors than controls (8.11 vs. 5.44).
Correlation Analyses
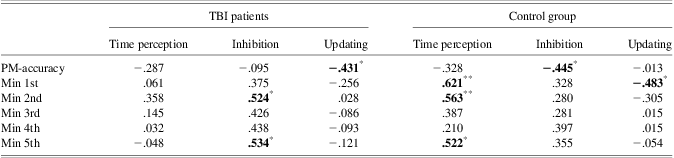
We calculated specific indices to examine the relationship between time-based PM, time perception and executive functions. The PM accuracy (PM-accuracy) index was calculated using the accuracy at the target time and monitoring frequency indices (Min 1st, Min 2nd, Min 3rd, Min 4th, and Min 5th) were calculated by adding participants’ monitoring frequency at each minute. We investigated monitoring behavior at each minute as this provides critical information regarding time-based PM performance (i.e., Einstein et al., Reference Einstein, McDaniel, Richardson, Guynn and Cufer1995; Shum et al., Reference Shum, Valentine and Cutmore1999). Time perception index was the mean of the participants’ CV in the time reproduction task. For the executive functions, the Inhibition index of the Stroop was the difference between RTs in incongruent and congruent trials, and the Updating index was the number of produced errors in the n-back task. Two separate, one-tailed Pearson correlation analyses were performed for TBI patients and controls. Moreover, to better understand the differences between the TBI and healthy control groups, we compared the two correlation analyses (Z Fisher) and in this case only significant results will be reported.
Correlation among time-based PM and monitoring frequency
No significant correlations were found between PM-accuracy and monitoring frequency indices in TBI patients. For controls, monitoring frequency in the 5th min correlated significantly with PM-accuracy (r = .398; p = .05). That is, controls with higher accuracy strategically increased their monitoring frequency closer to the target time.
Correlation among time-based PM, time perception, and executive functions tasks
In the TBI group, correlation analysis revealed a significant negative correlation between PM-accuracy and Updating suggesting that patients who performed better on the PM task made fewer errors on the n-back task (Table 2). In addition, monitoring frequency at the 2nd and 5th min significantly correlated with Inhibition indicating that TBI patients with higher numbers of clock-checking were less able to inhibit irrelevant information. For the control group, a significant negative correlation was found between PM-accuracy and Inhibition, suggesting that healthy controls with higher accuracy were less affected by interference. In addition, significant correlations were found between monitoring at the 1st, 2nd, and 5th min and Time perception, although healthy control participants with higher monitoring frequency were more variable in time reproduction task. Significant negative correlation was also found between the 1st min and the Updating indicating that healthy controls with higher monitoring frequency at the 1st min made less errors on the n-back task.
Table 2 Pearson's correlations between time-based PM, time reproduction tasks and executive functions tasks. PM-accuracy = accuracy at the target time; Min 1st, Min 2nd, Min 3rd, Min 4th and Min 5th = monitoring frequency; Time perception = mean of the participants’ CV in the concurrent reproduction task; Inhibition = difference between the RTs in incongruent and congruent trials in the Stroop task; Updating = number of errors in the n-back task
*p < .05; **p < .001.
Monitoring frequency at the 1st and 5th min differentially correlated with Time perception index in TBI patients and controls (Z = 1.82; p < .01 and Z = 1.45; p < .01 respectively). In particular, the monitoring at the 1st and 5th min significantly correlated with time reproduction in the control group whereas no significant correlations were found in the TBI group. Control participants with less monitoring frequency were also less variable in the time reproduction task.
Correlation among time perception and executive functions tasks
Significant positive correlations were found between Time perception and Updating in both groups (TBI r = .517 and controls r = .439). Participants that made more errors in n-back task were also more variable in time reproduction task.
Discussion
The present study investigated time-based PM in TBI patients and its relationship with time perception and executive functions. Compared to previous studies where participants were required to engage in high-load concurrent activities (i.e., performing the time-based prospective task within a word verification task, Kinch and McDonald, Reference Kinch and McDonald2001, or while answering a set of four-choice general-knowledge questions, Shum et al., Reference Shum, Valentine and Cutmore1999), our study asked participants to perform the time-based PM task while watching a movie. Despite the low-load concurrent activity, TBI patients showed poorer performance than controls confirming the time-based PM dysfunction in these patients.
The results suggested that healthy control participants performed significantly better than TBI patients, as they were more accurate in their responses closer to the target time. When the task was completed, participants were asked to repeat the instructions; all patients were able to recall the requirements confirming that the difficulties in the TBI group were related to performing the action at the target time rather than a failure to recall the task content. This might be because the cerebral areas commonly affected by TBI (i.e., frontal areas) are responsible for the neurocognitive processes involved in the PM tasks (initiation and execution of plan action, updating and interruption of ongoing activity) (McFarland & Glisky, Reference McFarland and Glisky2009).
Our study supports previous investigations and confirm the involvement of executive functions on time-based PM task (Kinch & McDonald, Reference Kinch and McDonald2001; Kliegel, Eschen, & Thöne-Otto, Reference Kliegel, Eschen and Thöne-Otto2004; Mathias & Mansfield, Reference Mathias and Mansfield2005). In particular, intact updating abilities were associated with better accuracy at target time in the TBI group (i.e., Carlesimo et al., Reference Carlesimo, Formisano, Bivona, Barba and Caltagirone2004; Maujean, Shum, & McQueen, Reference Maujean, Shum and McQueen2003), while PM accuracy was associated with inhibition abilities in the control group. Control participants who were more accurate at the target time were less affected by interference showing greater inhibition abilities (i.e., Gonneaud et al., Reference Gonneaud, Kalpouzos, Bon, Viader, Eustache and Desgranges2011). A possible explanation is that TBI PM accuracy is strongly influenced by working memory abilities, probably to keep constantly updating the PM task (e.g., press the key at the target time), whereas controls participants, with better overall cognitive abilities, probably do not rely only on their updating ability to constantly recall the task, but rather based their performance on inhibition of irrelevant task information.
In this study, monitoring behavior has been discussed as a critical index to investigate time-based PM because it is suitable to underlie differences between group performances (Carlesimo et al., Reference Carlesimo, Formisano, Bivona, Barba and Caltagirone2004; Einstein et al., Reference Einstein, McDaniel, Richardson, Guynn and Cufer1995, Shum et al., Reference Shum, Valentine and Cutmore1999). Time-based PM requires remembering the content of the future activity, inhibit the ongoing task, and strategically monitor the clock to accurately perform the prospective task. Deficits in inhibition and updating abilities could be compensated for increased monitoring frequency. Both groups increased their monitoring frequency as the target time approached and, in average, the TBI patients monitored more frequently than controls. TBI patients increased their monitoring frequency around the 2nd min, whereas the control group significantly increased their monitoring frequency around the 4th min. Patients with TBI may increase their monitoring frequency to compensate for executive and temporal dysfunction, and the healthy group may have not monitored as frequently due to their intact cognitive abilities. Despite the higher monitoring frequency TBI patients showed an inefficient monitoring strategy as they had lower PM accuracy. Based on the results of Einstein et al. (Reference Einstein, McDaniel, Richardson, Guynn and Cufer1995), it was expected that performance on the time-based task would be related to frequency of monitoring behavior, especially in the period most proximal to the target time. This was confirmed in the control group, in fact, a significant correlation was found between monitoring at the 5th min and accuracy. As noted above, the control group showed a more efficient strategic monitoring pattern than TBI participants, which may have resulted in a higher accuracy performance.
Prior research has found contrasting data in the monitoring behaviour of patients with TBI, with some (Shum et al., Reference Shum, Valentine and Cutmore1999) indicating similar monitoring rates between TBI and healthy controls, and others (Carlesimo et al., Reference Carlesimo, Formisano, Bivona, Barba and Caltagirone2004) finding it to be less in TBI groups. One possible explanation is that the studies used different time-based PM tasks with different instructions. In the study by Shum et al. (Reference Shum, Valentine and Cutmore1999), participants were asked to call the second experimenter every 5 min while engaged in a general-knowledge task, and they were instructed that they could press the “t” key on the keyboard to check the clock. In Carlesimo et al. (Reference Carlesimo, Formisano, Bivona, Barba and Caltagirone2004) study, participants performed eight triplets of actions at the occurrence of the target event or expiration of the established time, and they were not informed that they could reference a wall clock to benefit from a time cue. In our study, participants performed a training phase were they became familiar with the time monitoring task and they were instructed that they could use the keyboard to access a time cue. Previous studies have addressed the issue of task importance in PM and have shown that the strategic allocation of attention improves PM (Kliegel, Martin, McDaniel, & Einstein, Reference Kliegel, Martin, McDaniel and Einstein2001).
When engaged in a monitoring task (dual-task like paradigm), participants had to inhibit the irrelevant information to perform the prospective activity. Monitoring frequency was found to positively correlate with inhibition ability in both groups. Participants with low inhibition abilities checked the clock more frequently than did participants with more efficient inhibitory functions. Participants may have attempted to compensate for their difficulties in inhibition by relying on the external clock and monitor more frequently than did participants with more efficient inhibition functions. An interesting implication of this pattern of compensatory behavior is that increased monitoring should have direct effects on the PM task performance. That is, high monitoring frequency should facilitate accurate PM performance, but this was not the case for TBI patients. In fact, despite higher monitoring frequency, TBI patients were less accurate than controls which further highlights their inefficient use of a compensatory strategy.
Particularly interesting is the positive correlation between monitoring frequency and reproduction task in the control group. Controls that frequently checked the clock were more variable in the time reproduction task, but TBI participants who did not have adequate temporal abilities needed to more frequently monitor the time. These results suggest that temporal abilities are involved in the performance of time-based PM tasks, particularly in monitoring behavior. These results add further support the suggestion of Shum et al. (Reference Shum, Valentine and Cutmore1999) that differences in monitoring behavior between TBI patients and controls may not be explained by differences in strategic monitoring alone, but may also be due to differences in time estimation, suggesting that the ability to accurately estimate the passage of time may be critical for successful time-based PM performance.
In this study, we also investigated time perception in TBI patients. According to the Attentional-Gate model (Block & Zakay, Reference Block and Zakay2006) attentional and updating factors are critical to accurately perceive durations. Attentional and updating dysfunctions are often reported in TBI patients (Mathias & Wheaton, Reference Mathias and Wheaton2007; Vallat-Azouvi, Weber, Legrand, & Azouvi, Reference Vallat-Azouvi, Weber, Legrand and Azouvi2007), therefore, we expected temporal impairment in patients, particularly because time reproduction task is performed with a concurrent secondary task. Both groups under-reproduced the durations confirming the influence of divided attention on time judgment (Zakay & Block, Reference Zakay and Block1996). Particularly interesting are the findings on the variability index that showed greater variability in TBI patients than controls. This greater variability may be explained in terms of impaired updating (Nichelli, Clark, Hollnagel, & Grafman, Reference Nichelli, Clark, Hollnagel and Grafman1995; Perbal et al., Reference Perbal, Couillet, Azouvi and Pouthas2003). In time reproduction tasks, participants accumulate and store pulses during the presentation of the stimulus, retain the number of pulses in working memory while accumulating the new pulses corresponding to the current duration, and then compare the two numbers of pulses stored in working memory (Block, Zakay, & Hancock, Reference Block, Zakay and Hancock1998). Variability in temporal perception could come from variable representations of the duration in working memory; TBI patients may have difficulty in maintaining a stable representation of duration due to updating dysfunctions. Support for this notion comes from the correlation analysis that showed significant positive correlation between Time perception and Updating indices.
Strength of this study is that we investigated the involvement of time perception and executive functions on time-based PM tasks in TBI patients, which has received limited scientific investigation. The generalizability of our results may be limited by the sample size and the tasks used, particularly with regard to the domain of executive functions. Because executive functions are not a unitary system (Miyake et al., Reference Miyake, Friedman, Emerson, Witzki, Howerter and Wager2000), additional study is needed to examine if other executive functions (e.g., problem solving) are involved with PM tasks. Future studies may also consider enrolling patients based on the location of cortical lesions (e.g., frontal vs. non-frontal). Our data are interesting and may have important implications for PM rehabilitation. In fact, the results suggested that improving temporal abilities in TBI patients might improve TBI time-based PM performance.
In conclusion, our results support the hypothesis that executive functions, particularly inhibition and updating abilities, are strongly related to time-based PM (Cockburn, Reference Cockburn1996; Kinch & McDonald, Reference Kinch and McDonald2001; McDaniel et al., Reference McDaniel, Guynn, Glisky, Rubin and Routhieaux1999) and provide additional information to their relative contribution to monitoring behavior and performance on time-based PM tasks. Participants with better inhibition and updating abilities showed better performance on time-based PM. Moreover, monitoring frequency was found to correlate with inhibition and time perception performance. TBI patients relied more on executive functions than temporal components, while control participants engaged both executive functions and temporal abilities. TBI patients with higher temporal variability may not feel confident with their temporal abilities and, therefore, base their PM performance on working memory and compensate by increasing their monitoring frequency. Controls were more confident with their temporal abilities and, therefore, could perform time-based PM task also on the grounds of this additional cognitive component.
Acknowledgments
The information in this manuscript and the manuscript itself has never been published either electronically or in print. There are no financial or other relationships that could be interpreted as a conflict of interest affecting this manuscript. This research received no specific grant from any funding agency, commercial or not-for-profit sectors. Part of this study has been presented at the 3rd International Conference on Prospective Memory 28–30 July 2010, Vancouver, BC, Canada. The authors gratefully acknowledge Silvia Zambelli, the staff of the Modulo di Neuropsicologia Riabilitativa (Azienda Ospedaliero-Universitaria Ferrara, Italy) and the participants and their families and friends who co-operated in this study.





