INTRODUCTION
Sports-related concussion and mild traumatic brain injury (mTBI) are increasingly visible as underdiagnosed and undertreated public health burdens (Coronado et al., Reference Coronado, McGuire, Sarmiento, Bell, Lionbarger, Jones and . . . Xu2012; Faul, Xu, Wald, & Coronado, Reference Faul, Xu, Wald and Coronado2010; Langlois, Rutland-Brown, & Wald, Reference Langlois, Rutland-Brown and Wald2006; Rutland-Brown, Langlois, Thomas, & Xi, Reference Rutland-Brown, Langlois, Thomas and Xi2006). Tests that assess mild brain injury and brain functioning directly may be sensitive to changes in brain health and may be able to improve clinical prognoses as well as patient outcomes. This report examined brain functioning subsequent to concussion using theta-band functional connectivity to measure cognitive control.
Cognitive Control
Cognitive control facilitates adaptive action selection, emotion regulation, and concentration. Cognitive control is analogous to the construct of executive functioning: both constructs are putatively instantiated in frontal-lobe circuitry, are important for action selection and behavioral adaptation, and may be especially vulnerable to mTBI/concussion (Bigler, Reference Bigler2007; Eierud et al., Reference Eierud, Craddock, Fletcher, Aulakh, King-Casas, Kuehl and LaConte2014; Karr, Areshenkoff, & Garcia-Barrera, Reference Karr, Areshenkoff and Garcia-Barrera2014). Because a major component of cognitive control is adaptive behavioral responding, tasks like the Eriksen flanker, that require participants to inhibit habitual or prepotent behaviors are often used to assess individual differences in cognitive control (especially the sub-domains of response conflict or response inhibition; Zelazo et al., Reference Zelazo, Anderson, Richler, Wallner-Allen, Beaumont, Conway and . . . Weintraub2014). Moreover, patients with damage to the frontal lobes show impaired flanker performance including diminished neural indices of cognitive control, more erroneous responses, and impaired post-error slowing (PES) (e.g., Gehring and Knight, Reference Gehring and Knight2000; Hogan, Vargha-Khadem, Saunders, Kirkham, & Baldeweg, Reference Hogan, Vargha-Khadem, Saunders, Kirkham and Baldeweg2006; Stemmer, Segalowitz, Witzke, & Schönle, Reference Stemmer, Segalowitz, Witzke and Schönle2004; Wessel, Klein, Ott, & Ullsperger, Reference Wessel, Klein, Ott and Ullsperger2014; Ullsperger and von Cramon, Reference Ullsperger and von Cramon2006; Wessel et al., Reference Wessel, Ullsperger, Obrig, Villringer, Quinque, Schroeter and Klein2016).
PES happens when participants make an error on the flanker test, and then adapt their behavioral strategy toward slower and more cautious responding on subsequent trials. In healthy participants, PES has also been linked to neural activity, such that at the single-trial level a larger neural response following an error predicts greater slowing on the subsequent trial (Cavanagh, Cohen, & Allen, Reference Cavanagh, Cohen and Allen2009; Cavanagh, Meyer, & Hajcak, Reference Cavanagh, Meyer and Hajcak2017; Debener et al., Reference Debener, Ullsperger, Siegel, Fiehler, Von Cramon and Engel2005), ostensibly because that neural response reflects an instantiation of a cognitive control circuit (Cavanagh and Shackman, Reference Cavanagh and Shackman2015; Cohen, Reference Cohen2011a; Kerns et al., Reference Kerns, Cohen, MacDonald, Cho, Stenger and Carter2004). More specifically, increased activity in the medial-prefrontal cortex (mPFC), as well as connectivity between the mPFC and lateral-prefrontal cortex (lPFC) predicts slower reaction times (RTs) following errors, and may implement adaptive response inhibition and cognitive control (Cavanagh and Frank Reference Cavanagh and Frank2014; Cavanagh and Shackman, Reference Cavanagh and Shackman2015, Cohen, Reference Cohen2011a).
Error-Related Theta Dynamics
Oscillatory theta dynamics following errors are important for cognitive control inasmuch as they predict downstream cognitive control (Cavanagh et al., Reference Cavanagh, Cohen and Allen2009, Reference Cavanagh, Meyer and Hajcak2017; Cavanagh and Shackman, Reference Cavanagh and Shackman2015; Cohen and Cavanagh, Reference Cohen and Cavanagh2011; Van de Vijver, Ridderinkhof, & Cohen, Reference Van de Vijver, Ridderinkhof and Cohen2011). Event-related potentials characterized by midfrontal theta-band dynamics (e.g., the error-related negativity [ERN] and error-positivity) predict aspects of cognitive control, including PES (Gehring, Goss, Coles, Meyer, & Donchin, Reference Gehring, Goss, Coles, Meyer and Donchin1993; Hajcak, McDonald, & Simons, Reference Hajcak, McDonald and Simons2003), and are lower in mTBI than non-mTBI control groups (De Beaumont, Beauchemin, Beaulieu, & Jolicoeur, Reference De Beaumont, Beauchemin, Beaulieu and Jolicoeur2013; Moore et al., Reference Moore, Pindus, Drolette, Scudder, Raine and Hillman2015; Pontifex, O’Connor, Broglio, & Hillman, Reference Pontifex, O’Connor, Broglio and Hillman2009; although see Larson, Clayson, & Farrer, Reference Larson, Clayson and Farrer2012 for comparison). Similarly, midfrontal theta power following errors also predicts PES and cognitive control (Cavanagh et al., Reference Cavanagh, Cohen and Allen2009, Reference Cavanagh, Meyer and Hajcak2017; Van de Vijver et al., Reference Van de Vijver, Ridderinkhof and Cohen2011), and the ERN and midfrontal theta are presumed to have a common generator (Debener et al., Reference Debener, Ullsperger, Siegel, Fiehler, Von Cramon and Engel2005). It is believed that theta oscillations reverberate across the brain, facilitating functional connectivity and coordination between distal brain regions (Cavanagh and Frank, Reference Cavanagh and Frank2014, Cohen, Reference Cohen2011a). For example, synchronous theta-rhythms between mPFC and lPFC predicts participants’ cognitive control following errors (Cavanagh et al., Reference Cavanagh, Cohen and Allen2009, Reference Cavanagh, Meyer and Hajcak2017), ostensibly by facilitating communication between mPFC conflict-detection systems and lPFC response inhibition systems (Aron, Robbins, & Poldrack, Reference Aron, Robbins and Poldrack2014; Cohen, Reference Cohen2011a; Kerns et al., Reference Kerns, Cohen, MacDonald, Cho, Stenger and Carter2004).
Despite the importance of oscillatory theta dynamics for error processing and cognitive control, the relationship between synchronous theta activity and brain injury has not been previously investigated. Because connectivity between mPFC and lPFC is important for cognitive control, it stands to reason that disrupted connectivity may also result in disrupted cognitive control (operationalized as correlations between theta-band connectivity and PES). Thus, although the ERP literature is suggestive of mTBI-induced disruption to neural activity that implements cognitive control, specifically post-error theta-band connectivity has not yet been examined in relation to mTBI (cf. Cohen, Reference Cohen2011a; Wessel et al., Reference Wessel, Ullsperger, Obrig, Villringer, Quinque, Schroeter and Klein2016). Therefore, the present report will examine whether theta-band functional connectivity between frontal brain regions can serve as a potential indicator of concussion/mTBI.
Present Report
In Study 1, it was hypothesized that head injury would disrupt mPFC-lPFC theta-band connectivity (operationalized as theta-band synchrony between FCz and F6), and that head injury would disrupt cognitive control (operationalized as the single-trial correlation coefficient between connectivity and PES). We also expected that connectivity and cognitive control would be diminished in participants with more head injuries (i.e., a dose-response relationship). Differences in behavioral performance between injured athletes and healthy athletes (e.g., accuracy, RT, PES, and response efficiency) were not expected, consistent with previous reports that found differences in neural, but not behavioral data (De Beaumont et al., Reference De Beaumont, Beauchemin, Beaulieu and Jolicoeur2013). It was expected that the positive correlation previously observed in healthy participants between theta-band connectivity and post-error RTs (e.g., Cavanagh et al., Reference Cavanagh, Cohen and Allen2009, Reference Cavanagh, Meyer and Hajcak2017) would be stronger in healthy athletes than athletes with any history of concussion.
In Study 2, we aimed to extend the results of Study 1 by examining within-subjects’ comparisons over the course of a season of play in contact sports. A replication of the findings from Study 1 using within-subjects’ analyses would more directly link alterations in theta dynamics to frontal brain health. It was hypothesized that a season of high-contact athletic play (and presumed subconcussive injury) would be associated with a decline in connectivity strength as described above for Study 1.
METHODs
Participants
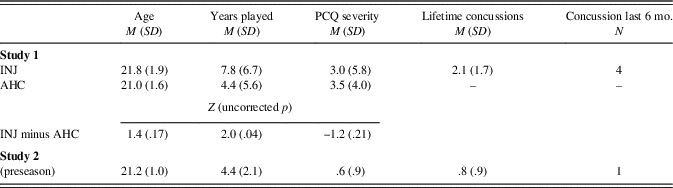
For Study 1, a total of 41 student athletes were recruited from intramural contact-collision sports at a large southwestern university. Participants’ concussion history was collected from athlete self-report and from certified athletic trainers that monitor athlete health. An athletic trainer conducted a clinical interview with participants as part of their admission into an intramural sports club, and participants reported concussion history to the trainer at that time. Athletes that had concussions under the supervision of the athletic trainer were interviewed again and monitored for recovery. Experimenters also administered the Post-Concussion Questionnaire (PCQ) from the Sports Concussion Assessment Tool - 2 (SCAT-2, McCrory et al., Reference McCrory, Meeuwisse, Johnston, Dvorak, Aubry, Molloy and Cantu2009) to participants before electroencephalograph (EEG) recording, and asked participants about their entire history of head injury, as well as head injury in the past 6 months (i.e., “When was the last time you were diagnosed with a concussion?”). Participant characteristics are shown in Table 1.
Table 1 Group characteristics

There was one group of athletic-healthy-control participants (AHC, N=16; 4 females) and another group of injured (INJ) participants. Data for one of the INJ participants were not analyzed because the subject only committed one error on the flanker task (analyzed N for INJ=24; 4 females). All other participants had at least seven useable error trials for analysis. Prior work suggests that error-related brain activity may be reliable with as few as six trials (Cavanagh et al., Reference Cavanagh, Meyer and Hajcak2017; Olvet and Hajcak, Reference Olvet and Hajcak2009). Participants in the INJ group had a mean of 2.1 lifetime concussions (SD=1.7; range, 1–7). Age and concussion symptoms (i.e., PCQ total) did not statistically differ between the AHC and INJ groups.
Participants in Study 2 (N=10) were athletes from Study 1 that had one preseason and one postseason EEG recording. These 10 participants were concussion-free across the season.
All data were collected in accord with ethical guidelines for human subject’s research and under approval of an institutional review board. All athletes were provided with both written and verbal informed consent with regard to study procedures, risks/benefits, and voluntariness of participation.
Procedure and Experimental Task
Participants first completed a short interview with a trained experimenter, then completed the PCQ. EEG sensors were applied, and participants had their resting-state recording (6 min) collected before completing a variant of the flanker task. The modified Eriksen flanker task used different letter strings for different blocks (e.g., MMNMM; FFEFF; QQOQQ; VVUVV; IITII). There were a total of 320 trials distributed evenly across 8 blocks. Participants were encouraged to respond with both speed and accuracy. The flanker task was the same as the task used in Zambrano-Vazquez and Allen (Reference Zambrano-Vazquez and Allen2014) and is described in detail there (also see Supplementary Figure 1).
EEG Recording and Preprocessing
EEG was recorded from 60 Ag/AgCl scalp electrodes and two mastoid electrodes using an ElectrodeArrays EEG cap (El Paso, Tx) and Neuroscan Synamps2 amplifiers (Charlotte, NC). EEG was recorded to an online reference electrode between Cz and CPz in AC mode with an online bandpass filter (.5–100 Hz), a sampling rate of 500 Hz, and all impedances were below 10 kΩ. In addition, two bipolar channels were recorded to monitor horizontal and vertical ocular movements.
EEG segments with discontinuities and paroxysmal artifacts were visually identified and removed (see Smith, Reznick, Stewart, & Allen, Reference Smith, Reznik, Stewart and Allen2017). Data were bandpass filtered 1–100 Hz and notch filtered 55–65 Hz using a custom zero-phase shift optimal FIR filter generated following the recommendations of Cook and Miller (Reference Cook and Miller1992). Channels marked as bad by human raters were removed. Response-locked epochs were created (−2500 ms to 2000 ms). The data were cleaned using ICA-based methods with the FastICA toolbox and ADJUST (Mognon, Jovicich, Bruzzone, & Buiatti, Reference Mognon, Jovicich, Bruzzone and Buiatti2011). Bad channels were then interpolated using spherical splines (EEGlab v13.4.4 function eeg_interp). The artifact-free data were transformed to the current-source density montage / surface laplacian using the laplacian_perrinX function included with Cohen (Reference Cohen2014) and based on the spherical spline approach summarized by Perrin, Pernier, Bertrand, and Echallier (Reference Perrin, Pernier, Bertrand and Echallier1989, Reference Perrin, Pernier, Bertrand and Echallier1990).
Trials where participants made two errors on the same trial were excluded. For single-trial correlations between synchrony and post-error RT, only errors with RTs >100 ms and only errors followed by correct trials were examined. The median number of useable error trials was 23.5 (SD=24.3; range=7–95).
Time-Frequency Decomposition and Functional Connectivity
A Morlet wavelet procedure (Cohen, Reference Cohen2014) was used to extract the analytic signals, which are the basis for calculating inter-site phase clustering (ISPC), a measure of functional connectivity between brain regions. A family of logarithmically-spaced Morlet wavelets from 2 to 80 Hz was created for extraction of analytic signals; wavelet cycles were also logarithmically spaced and ranged from 3 to 10, with lower frequencies having fewer cycles. The “theta-band” was defined as the average over 4 to 8 Hz. ISPC indicates synchrony between time-series, and ISPC is hypothesized to indicate functional connectivity between brain regions. ISPC-trials is calculated as the consistency of phase angle (φ) differences between two electrodes (x and y) for a given time-point (t) and frequency (f) over trials (n):
![]() ${\rm ISPC}_{f} {\equals} \left| {n^{{{\minus}1}} \mathop \sum\limits_{t{\equals}1}^n e^{{i(\varphi _{{xt}} {\minus}\varphi _{{yt}} )}} } \right|$
; for example, ISPC-trials indicates synchrony between electrodes that was stable at a certain TF point across many trials.
${\rm ISPC}_{f} {\equals} \left| {n^{{{\minus}1}} \mathop \sum\limits_{t{\equals}1}^n e^{{i(\varphi _{{xt}} {\minus}\varphi _{{yt}} )}} } \right|$
; for example, ISPC-trials indicates synchrony between electrodes that was stable at a certain TF point across many trials.
ISPC-trials coefficients for error trials were based on consistency in phase angle differences between FCz and F6 (Cavanagh et al., Reference Cavanagh, Cohen and Allen2009, Reference Cavanagh, Meyer and Hajcak2017) averaged across trials at individual TF points. We focused analyses on the relationship between medial (FCz) and right-lateral PFC (F6) because right-lateral PFC has been associated with response inhibition in multiple reports (see Aron et al., Reference Aron, Robbins and Poldrack2014 for review), and because previous reports have shown that the relationship between mPFC-lPFC synchrony and PES is greatest at this site (see Figure 4 in Cavanagh et al., Reference Cavanagh, Cohen and Allen2009).
By comparison, ISPC-time (n in the formula above is now time points and t is trial) is the consistency of several time-frequency points for a single trial, and it indicates the synchrony between electrodes for a given time period on a single-trial. This ISPC-time metric can be used for within-subject correlations between behavior and neural activity on single-trials. Thus, ISPC-trials was used for between-subjects’ comparisons, whereas ISPC-time was used for within-subjects / single-trial correlations. A 100-ms moving window was passed over each frequency band and correlated with post-error RT, thus creating an ISPC-time series (i.e., Figure 1).
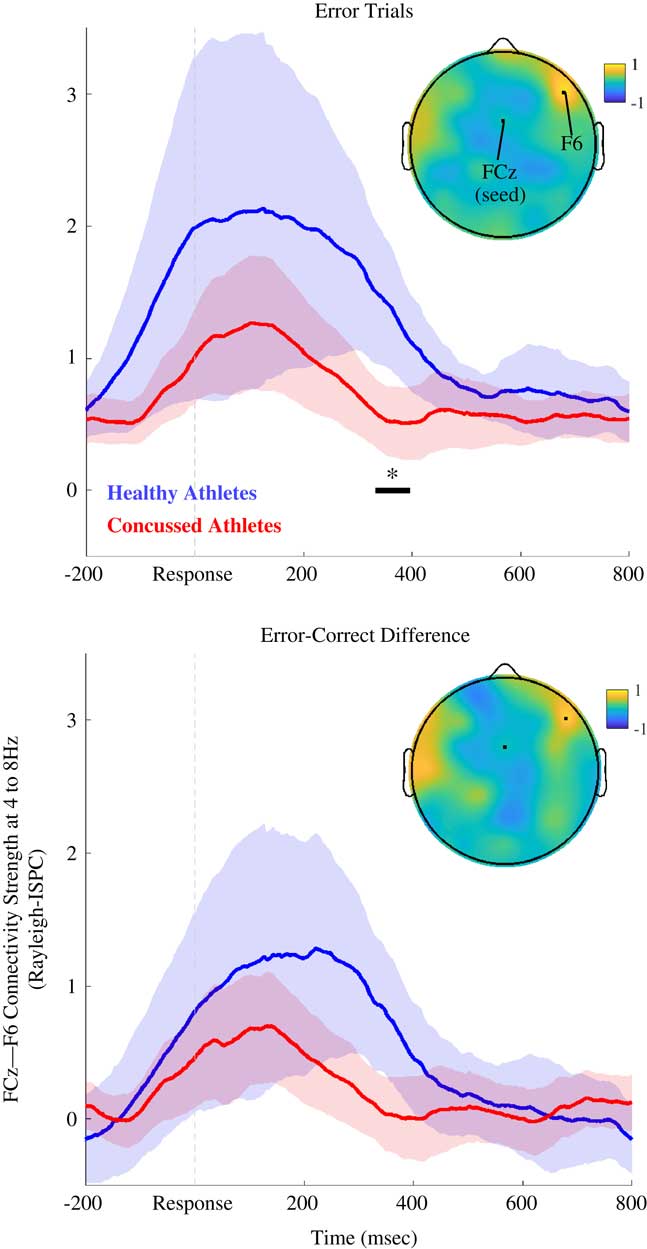
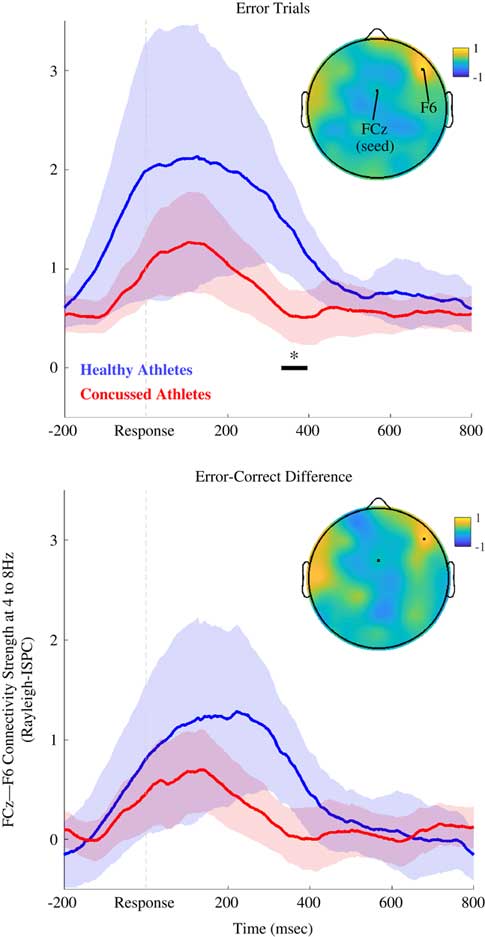
Fig. 1 Theta-band (4 to 8 Hz) connectivity between FCz and F6 (Study 1). Time series of theta-band connectivity (Rayleigh ISPC-trials) between FCz and F6. Blue time series are data for AHC participants. Red time series are data for INJ participants. Shaded regions depict bootstrapped 95% confidence intervals for group means. Black bar and asterisk indicate times where connectivity significantly differed between groups (FDR-corrected p<.05). Topoplot depicts AHC-INJ difference for ISPC using a FCz seed, and the location of F6 is also noted. Top panel shows results for error trials, and bottom panel shows results for error-minus-correct difference. AHC participants were characterized by more FCz-F6 connectivity than INJ participants.
Cognitive control was operationalized as positive Spearman correlation coefficients between ISPC-time and PES. PES was operationalized as the difference between response times for one error trial (trial N) and the subsequent correct trial ([trial N+1]; Cavanagh et al., Reference Cavanagh, Cohen and Allen2009); in other words, PES is the difference of post-error RTs and error RTs for pairs of single-trials. Significant correlations between PES and connectivity can result from more/less PES, more/less post-error speeding, or both depending on whether the post-error RT minus error RT differences are all positive (slowing on every post-error trial), are all negative (speeding on every post-error trial), or some combination of positive and negative (slowing and speeding on post-error trials). RTs are meaningful in this regard and can change the interpretation of a significant single-trial connectivity-RT correlation. The post-error RT minus error RT differences were evaluated for single trials and indicated that participants were most likely to demonstrate slowed responses on individual post-error trials (68% of trials for Study 1, and 73% of trials for Study 2), suggesting that positive single trial correlations between connectivity and PES (post-error RT minus error RT; Cavanagh et al., Reference Cavanagh, Cohen and Allen2009) were indicative of more connectivity predicting more slowing, whereas negative correlations were indicative of more connectivity predicting less slowing (because most trials demonstrated PES). ISPC-trials were adjusted to Rayleigh-ISPC / Rayleigh-Z
![]() $$\left( {N_{{trials}} {\asterisk}ISPC^{2} } \right)$$
to mitigate spurious inflation of ISPC due to low trial count (Cohen, Reference Cohen2014, p. 249).
$$\left( {N_{{trials}} {\asterisk}ISPC^{2} } \right)$$
to mitigate spurious inflation of ISPC due to low trial count (Cohen, Reference Cohen2014, p. 249).
Statistical Analysis
Statistics were computed over time series (−200 ms to 800 ms) of theta-band synchrony (i.e., 4 Hz to 8 Hz ISPC, Figure 1) extracted from TF decomposition. The false-discovery rate method (FDR; Benjamini and Hochberg, Reference Benjamini and Hochberg1995) was used to correct for multiple comparisons over time series (e.g., several hundred time points). FDR-corrected p-values smaller than .05 were considered statistically significant. Maximum Z-scores from Wilcoxon rank-sum tests are reported for AHC versus INJ contrasts. Wilcoxon signed-rank tests were calculated for one-sample tests (connectivity-PES correlations difference from zero), and for preseason versus postseason contrasts.
Spearman rank-order correlations were calculated to measure associations between continuous variables (e.g., number of lifetime concussions, years played, and age, with FCz-F6 connectivity; see Supplementary Figure 2). A robust multiple regression analysis (fitlm function in Matlab 2017a with default robust fitting option, i.e., iteratively reweighted least squares) was used to calculate R
2. Then, significance testing for an incremental improvement in R
2 (i.e., hierarchical regression) was calculated according to the recommendations of Cohen and Cohen (Reference Cohen and Cohen1983;
![]() $F{\equals}{{(R_{{Y \cdot AB}}^{2} {\minus}R_{{Y \cdot A}}^{2} )\,/\,kB} \over {(1{\minus}R_{{Y \cdot AB}}^{2} )\,/\,(n{\minus}k_{A} {\minus}k_{B} {\minus}1)}}$
). Data were natural log-transformed before effect size calculation (Cohen’s d) to mitigate effects of outliers and skew.
$F{\equals}{{(R_{{Y \cdot AB}}^{2} {\minus}R_{{Y \cdot A}}^{2} )\,/\,kB} \over {(1{\minus}R_{{Y \cdot AB}}^{2} )\,/\,(n{\minus}k_{A} {\minus}k_{B} {\minus}1)}}$
). Data were natural log-transformed before effect size calculation (Cohen’s d) to mitigate effects of outliers and skew.
STUDY 1 RESULTS
Behavior
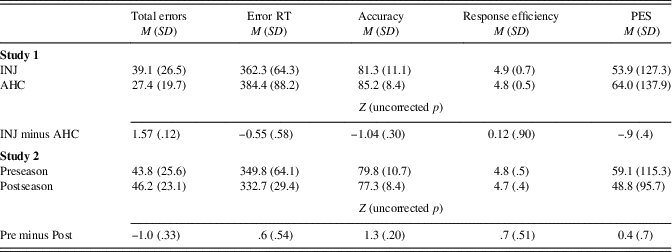
Behavioral performance was similar across groups. RTs, accuracy, PES (post-error RT minus error RT; e.g., Cavanagh et al., Reference Cavanagh, Cohen and Allen2009), and response efficiency (median response time / percentage of accurate trials, lower numbers indicate greater efficiency) were similar between AHC and INJ participants, and are shown in Table 2.
Table 2 Behavioral performance

Functional Connectivity
Figure 1 shows post-error FCz-F6 connectivity for the theta band. As expected, theta-band ISPC-trials between medial (FCz) and lateral frontal (F6) electrodes was diminished for INJ compared to AHC on error trials (Z=3.41; corrected p=.044, uncorrected p<.001; d=1.01 at 356 ms), and for the error-minus-correct difference (Z=2.72; corrected p>.10; uncorrected p=.007; d=.455 at 346 ms). ISPC for error trials was not significantly related to the number of lifetime concussions or years played for INJ participants; ISPC was also unrelated to athletes’ age or years played across the entire sample (see Supplementary Figure 2).
The ISPC results for the group contrast (ISPC on error trials for AHC vs. INJ groups) were similar (Z=3.05; corrected p=.063; uncorrected p=.002; d=.997 at 350 ms) when examining only participants that had at least 20 error trials (AHC, N=10; INJ, N=19).
As an operationalization of cognitive control, the single-trial correlation coefficients between ISPC-time and post-error RT were examined. After correcting for multiple comparisons, statistics were at trend-level for single-trial correlations in AHC (Z=1.91; corrected p>.10; uncorrected p=.056; d=2.19, at 582 ms) and INJ participants (Z=−1.66; corrected p>.10; uncorrected p=.098; d=2.41; at 306 ms). There was a trend for AHC participants to demonstrate greater cognitive control than INJ participants (Z=1.67; corrected p>.10; uncorrected p=.095; d=.19 at 310 ms).
It may be the case that INJ participants are characterized by reduced connectivity across all electrode pairs or that diminished FCz-F6 connectivity is not specific to the functioning of a hypothesized right-frontal mPFC-lPFC circuit. Hierarchical regression and a control variable (e.g., FCz-CP4 connectivity) can test for this possibility. CP4 was selected as a control electrode in prior work (Cavanagh et al., Reference Cavanagh, Cohen and Allen2009), and CP4 and F6 are equidistant with FCz. FCz-F6 peak post-error connectivity (i.e., at 356 ms) remained a significant predictor of group membership after accounting for FCz-CP4 connectivity (F(2,35)=4.00; p=.027; ΔR 2=.16).
A separate regression showed FCz-F6 connectivity predicted group membership after accounting for FCz-F5 connectivity (F(2,35)=3.13; p=.056; ΔR 2=.13), concordant with the notion that FCz-F6 phase synchrony denotes activation of a right-lateralized mPFC-lPFC circuit important for self-monitoring and response inhibition (Aron et al., Reference Aron, Robbins and Poldrack2014; Cavanagh and Shackman, Reference Cavanagh and Shackman2015; Kerns et al., Reference Kerns, Cohen, MacDonald, Cho, Stenger and Carter2004). Supplementary Figures 3 and 4 also show that, although FCz-F6 connectivity accounted for unique variance predictive of INJ participants, there is some overlapping variance for connectivity between different pairs of electrodes.
Participants in the AHC group had fewer years played in contact-collision sports (Z=−2.04; p=.04) than the INJ group, and years played and lifetime concussion history were correlated (r(39)=.40; p=.01), suggesting that years played could be a potentially confounding third variable (see Table 1). Hierarchical regression was used to assess the predictive contribution of group status above and beyond number of years played. After accounting for years played, group status was still a significant predictor of peak FCz-F6 connectivity on error trials (F(2,35)=5.88; p=.006; ΔR 2=.24).
STUDY 2 RESULTS
Study 2: Pre- vsersus Postseason ISPC
Behavior
Accuracy, RTs, PES, and response efficiency were unchanged pre- versus postseason (ps >.05; see Table 2).
Functional Connectivity
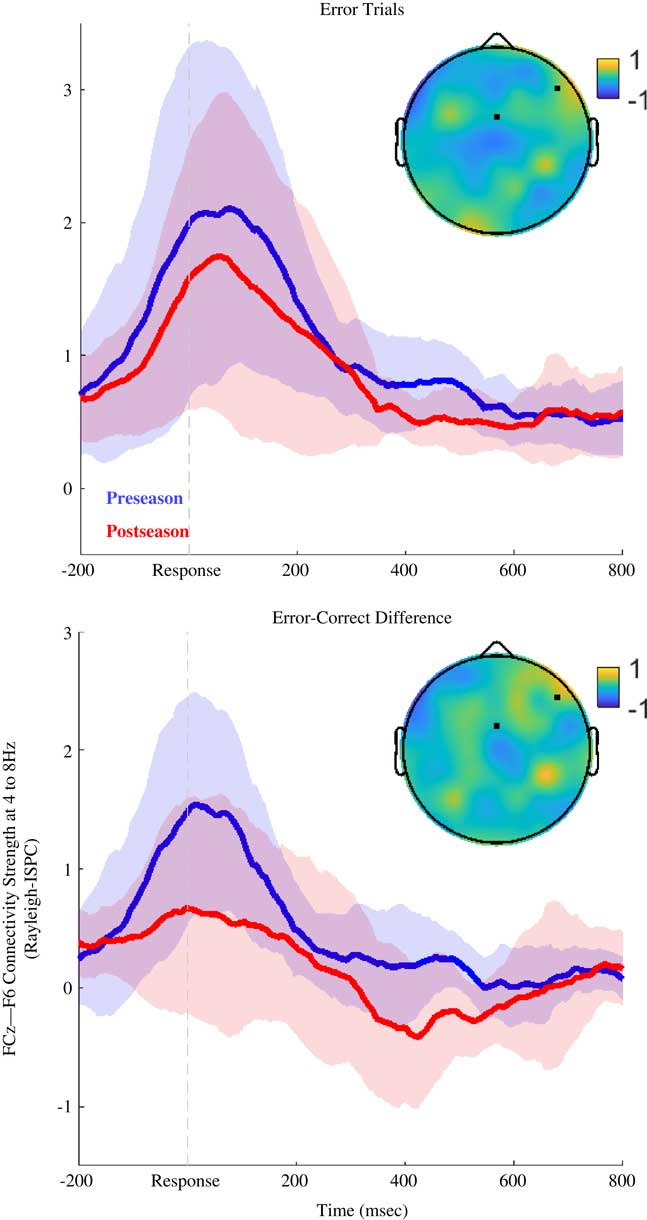
After correcting for multiple comparisons, there were no significant differences in ISPC or connectivity-PES correlations for preseason versus postseason recordings. Figure 2 shows that there were trends for greater post-error ISPC at preseason compared to postseason (Z=1.38; corrected p>.10; uncorrected p=.169; d=.574 at 466 ms), as well as greater error-modulated ISPC (the error-correct difference) for preseason recordings (Z=2.09; corrected p>.10; uncorrected p=.036; d=.490 at 518 ms).

Fig. 2 Theta-band (4 to 8 Hz) connectivity between FCz and F6 (Study 2). Time series of theta-band connectivity (Rayleigh ISPC-trials) between FCz and F6. Blue time series are preseason data. Red time series are postseason data. Shaded regions depict bootstrapped 95% confidence intervals for condition means. After multiple comparisons corrections (FDR-method), there were no significant differences between the groups (corrected ps>.10). Topoplot depicts preseason-postseason difference for ISPC using a FCz seed, and the location of F6 is also noted. Top panel shows results for error trials, and bottom panel shows results for error-minus-correct difference. At preseason there was a trend for participants to show stronger FCz-F6 connectivity than at postseason.
There were trends for connectivity to predict less PES at preseason (Z=−1.99; corrected p>.10; uncorrected p=.047; d=3.34 at 476 ms), and for connectivity to predict greater PES at postseason (Z=2.80; corrected p=.089; uncorrected p=.005; d=3.12 at 396 ms). Athletes tended to show a more negative correlation between connectivity and post-error RT in the preseason than postseason (Z=−2.80; corrected p>.10; uncorrected p=.005; d=.158 at 408 ms), indicating that connectivity strength predicted less PES in preseason recordings, whereas connectivity predicted more PES in postseason recordings.
DISCUSSION
Findings and Context
Study 1 showed that connectivity between mPFC and lPFC was lower in athletes with any history of concussion compared to a healthy athletic cohort. There was a similar pattern of results for Study 2: athletes showed a trend-level decrease in connectivity following a season of contact/collision play even though no athletes in Study 2 were diagnosed with a concussion over the course of the season.
These studies provide initial evidence that a theorized cognitive control circuit may be useful as a measure of brain health. Participants with concussion/mTBI were characterized by diminished mPFC-lPFC theta-band connectivity, circuitry important for implementing cognitive control, following errors compared to athletic control participants. There were also trends for diminished modulation of brain activity following errors (i.e., error-minus-correct difference score), and altered relationships between brain activity and response inhibition (i.e., single-trial relationships between connectivity and PES). These group differences in connectivity and cognitive control emerged late in the time series (>300 ms) and may indicate that complex cognitive processes such as error awareness (Nieuwenhuis, Ridderinkhof, Blom, Band, & Kok, Reference Nieuwenhuis, Ridderinkhof, Blom, Band and Kok2001) and learning from errors (Cavanagh, Frank, Klein, & Allen, Reference Cavanagh, Frank, Klein and Allen2010; Cohen and van Gaal, Reference Cohen and van Gaal2013) may be especially vulnerable to concussion and functioning of cortical-cortical pathways (e.g., Miller, Hayes, Lafleche, Salat, & Verfaellie, Reference Miller, Hayes, Lafleche, Salat and Verfaellie2017; Orr et al., Reference Orr, Albaugh, Watts, Garavan, Andrews, Nickerson and . . . Hudziak2016).
Moreover, FCz-F6 connectivity demonstrated some specificity with regard to prediction of mTBI (e.g., Supplementary Figures 3 and 4), suggestive that FCz-F6 connectivity assesses functioning of a vulnerable right-lateralized frontal lobe network that is important for behavioral inhibition (Aron et al., Reference Aron, Robbins and Poldrack2014). Yet, there was also some overlap between connectivity among electrode pairs, supportive of the notion that theta-band connectivity can facilitate simultaneous information flow between multiple brain regions (e.g., Buzsáki, Reference Buzsáki2006; Cavanagh and Shackman, Reference Cavanagh and Shackman2015). Although the connectivity-PES single-trial correlation differentiated AHC and INJ groups at a trend level in Study 1, the single-trial correlations between PES and synchrony were not significantly different from zero for either group in Study 1. Van de Vijver and colleagues (2011) also reported a null single-trial connectivity-PES relationship in healthy participants, and it may be the case that the connectivity-PES relationship is characterized by a low signal-to-noise ratio and may be difficult to detect without numerous error trials.
The connectivity-PES correlation was in the opposite of expected direction for Study 2 (a more positive connectivity-PES relationship in postseason recordings than preseason recordings), indicating that participants were likely to demonstrate less PES following strong mPFC-lPFC post-error connectivity. Other reports have also observed positive correlations between ISPC and post-error RTs (Cavanagh et al., Reference Cavanagh, Meyer and Hajcak2017), and it is likely the case that participants adopt different strategies to complete the task. For example, participants demonstrating post-error speeding may be using a proactive inhibitory strategy, whereas participants demonstrating PES may be using a reactive inhibitory strategy (Buzzell et al., Reference Buzzell, Barker, Troller-Renfree, Bernat, Bowers, Morales and Fox2018; Cavanagh et al., Reference Cavanagh, Meyer and Hajcak2017; Narayanan, Cavanagh, Frank, & Laubach, Reference Narayanan, Cavanagh, Frank and Laubach2013). Moreover, compensatory mechanisms, effort, anxiety, or depression (see Cavanagh et al., Reference Cavanagh, Meyer and Hajcak2017; Gehring et al., Reference Gehring, Goss, Coles, Meyer and Donchin1993; Hill, Samuel, & Foti, Reference Hill, Samuel and Foti2016; and Olvet, Klein, & Hajcak, 2010 for examples of moderators of error-related brain activity) can also modulate mPFC-lPFC connectivity and may have contributed to the pattern of results in this study.
Alternatively, postseason EEG recordings would have been relatively recent to any unreported injuries (at most within 6 months) during the season, and connectivity is sometimes stronger for the first several days and weeks post-injury (Eierud et al., Reference Eierud, Craddock, Fletcher, Aulakh, King-Casas, Kuehl and LaConte2014). These potential moderators, along with a limited range of injuries, may explain why there was no apparent relationship between theta-band connectivity and lifetime number of concussions or years played (e.g., there was no apparent dose-response relationship). Overall, the results suggest that theta dynamics may be ripe for investigation especially with regard to processing efficiency in neural systems important for cognitive control and learning from mistakes.
The present results align with an emerging consensus that late-stage neural processes (>200 ms post stimulus or response) important for cognitive processes like monitoring, adaptation, and error awareness (see Polich, Reference Polich2007) reliably correlate with behavioral performance and concussion/mTBI. Specifically, mTBI participants frequently demonstrate evoked potentials that are delayed and weaker compared to peers (Broglio, Pontifex, O’Connor, & Hillman, Reference Pontifex, O’Connor, Broglio and Hillman2009; Duncan, Kosmidis, & Mirsky, Reference Duncan, Kosmidis and Mirsky2003; Larson, Farrer, & Clayson, Reference Larson, Farrer and Clayson2011). Yet only a few reports have examined group differences in response-locked functional connectivity, for example, during set-shifting (Pang, Dunkley, Doesburg, da Costa, & Taylor, Reference Pang, Dunkley, Doesburg, da Costa and Taylor2016), target detection (Reches et al., Reference Reches, Kutcher, Elbin, Or-Ly, Sadeh, Greer and . . . Kontos2017), working memory (Bailey et al., Reference Bailey, Rogasch, Hoy, Maller, Segrave, Sullivan and Fitzgerald2017; Kumar, Rao, Chandramouli, & Pillai, Reference Kumar, Rao, Chandramouli and Pillai2009), and learning tasks (Tsirka et al., Reference Tsirka, Simos, Vakis, Kanatsouli, Vourkas, Erimaki and . . . Micheloyannis2011).
Specifically, Tsirka and colleagues (2011) reported correlations between graph theoretic metrics of functional connectivity and cognitive performance, finding that diminished clustering of theta-band connectivity in mTBI participants predicted diminished recognition memory. The results of Tsirka et al. (Reference Tsirka, Simos, Vakis, Kanatsouli, Vourkas, Erimaki and . . . Micheloyannis2011) and the present study align with a theoretical model of theta-band activity as a hub-like signal that facilitates cognitive control (e.g. Cavanagh, Zambrano-Vazquez, & Allen, 2012; Cohen, Reference Cohen2011a; Cohen & van Gaal, Reference Cohen and van Gaal2013; Wessel, Reference Wessel2018). Overall, the present results fit with a literature that has characterized synchronous theta dynamics as important for cognitive control and behavioral adaptation, and has demonstrated that error-related neural activity may be sensitive to neural function following mTBI.
Limitations
This study was limited in scope, and findings should be interpreted as exploratory or “proof-of-concept.” Sample size and characterization, study design (i.e., cross-sectional vs longitudinal data), and behavioral testing can all be improved in future investigations. In this regard, replication and extension of the present findings are warranted before strong claims can be made regarding study hypotheses. Nonetheless, the present work is interesting and supports continued investigation.
The study was particularly limited in terms of testing participants with a mild and restricted range of injury, narrow demographics, very few psychological or medical data, and no paper-and-pencil neuropsychological measures. For example, factors like time since injury, severity, chronicity, medication, effort, strategy, mood, and/or premorbid conditions (e.g., ADHD, neurodevelopmental disorder, pain, depression, impulsivity, reduced processing speed), could be more likely in the INJ group, potentially confounding results and interpretation. In particular, it may be the case that preexisting factors more prevalent in the INJ group, that is, developmental disability and impulsivity, contribute to diminished connectivity/ISPC and put participants at greater risk of getting a concussion (Iverson et al., Reference Iverson, Wojtowicz, Brooks, Maxwell, Atkins, Zafonte and Berkner2016). It may also be the case that less-than-ideal characterization of head injury history contributed to null dose-response correlations because of noise in self-reports, or self-reports that are confounded by impression management or over/under-reporting. Accounting for these potential moderators in a comprehensive longitudinal study that includes a detailed head injury interview and objective measures of brain structure will be important for future work.
Practice effects in Study 2 are another potential limitation: it is unclear to what extent repeated testing accounted for changes in neurobehavioral performance. Yet, there were no significant improvements in behavioral performance (i.e., practice effects) pre versus postseason, and practice effects would not account for the between-subjects’ differences observed in Study 1. Moreover, practice effects for the flanker test trend towards small effect sizes over a 2-week interval (d=.27 in Zelazo et al., Reference Zelazo, Anderson, Richler, Wallner-Allen, Beaumont, Conway and . . . Weintraub2014). Nonetheless, an important goal for future work will be to follow cohorts of high-risk participants over days and weeks to characterize the stability of connectivity and connectivity-PES metrics.
The present study was also limited in terms of connectivity estimation: low trial counts can inflate ISPC-based measures of connectivity. Yet, results were relatively unchanged when only examining participants with at least 20 trials, commensurate with trial counts from a previous report (i.e., Cavanagh et al., Reference Cavanagh, Cohen and Allen2009). The observation that neither AHC nor INJ participants’ connectivity-PES correlations differed from zero complicates any interpretation of group differences. On one hand, the flanker test is frequently used for cueing error related brain activity and is comparable with a large amount of published literature (Cavanagh et al., Reference Cavanagh, Cohen and Allen2009; Olvet and Hajcak, Reference Olvet and Hajcak2009; Zelazo et al., Reference Zelazo, Anderson, Richler, Wallner-Allen, Beaumont, Conway and . . . Weintraub2014). On the other hand, future work should consider using response inhibition tasks that elicit higher error rates, and that are otherwise similar to the flanker test, as it would improve signal-to-noise ratio for connectivity estimation. Aggregate data from multiple response inhibition tests would also improve characterization and reliability of measures of synchronous brain activity in future reports (e.g., Cavanagh et al., Reference Cavanagh, Zambrano‐Vazquez and Allen2012; Riesel, Weinberg, Endrass, Meyer, & Hajcak, Reference Riesel, Weinberg, Endrass, Meyer and Hajcak2013).
CONCLUSION
Concussion was associated with weaker theta-band connectivity in a brain circuit hypothesized to be important for cognitive control and response inhibition in a limited study of college athletes. The results align with previous work showing that theta-band connectivity in an mPFC-lPFC network is activated during tasks that necessitate cognitive control. The present results are also suggestive that interarea connectivity and connectivity-PES relationships may be sensitive indicators of cognitive control inefficiency following an mTBI. It is hypothesized that these findings result from disruption in phase-based coordination of cell assemblies that depend on white matter health/integrity in the frontal lobes (Cohen, Reference Cohen2011a, Reference Cohen2011b; Fries, Reference Fries2005; Sponheim et al., Reference Sponheim, McGuire, Kang, Davenport, Aviyente, Bernat and Lim2011).
These results demonstrate proof-of-concept for theoretically guided neurocognitive metrics, but are qualified by several limitations in study design and available data. Disrupted theta dynamics occurred at a later point in the time series than expected, putatively during error awareness and working memory updating; one interesting hypothesis for a future report might be that disrupted late-latency (>300 ms) theta dynamics in mTBI participants mediates rates of improvement in behavioral performance over trials (e.g., learning rate). Overall, the present results support the continued investigation of brain connectivity measures as potential tools for assessing brain health and cognitive functioning.
ACKNOWLEDGMENTS
Many thanks to the athletes who participated in the study; the project would not have been possible without them. Also, special thanks to the Dianne Goodridge, ATC, for her support of the project and her support of student-athletes. Finally, several research assistants were crucial to the completion of this project, especially Margaret Tobias, Jamie Velo, and Kenia Carrera. This work was funded by the National Collegiate Athletics Association (NCAA) and the Graduate and Professional Student Council of the University of Arizona. None of the authors have potential conflicts of interest to be disclosed.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/S135561771800108X