Introduction
The anterior cingulate cortex (ACC) has been implicated in several cognitive processes including emotion (Bush, Luu, & Posner, Reference Bush, Luu and Posner2000; Etkin & Schatzberg, Reference Etkin and Schatzberg2011), attention (Fassbender & Schweitzer, Reference Fassbender and Schweitzer2006; Posner & Petersen, Reference Posner and Petersen1990; Posner & Rothbart, Reference Posner and Rothbart1998), cognitive control (Carter et al., Reference Carter, Braver, Barch, Botvinick, Noll and Cohen1998; Clayson & Larson, Reference Clayson and Larson2011), reinforcement learning (Holroyd & Coles, Reference Holroyd and Coles2002), reward processing (Hajcak, MacNamara, & Olvet, Reference Hajcak, MacNamara and Olvet2010; Krebs, Boehler, Roberts, Song, & Woldorff, Reference Krebs, Boehler, Roberts, Song and Woldorff2012), and error processing (Gehring, Goss, Coles, Meyer, & Donchin, Reference Gehring, Goss, Coles, Meyer and Donchin1993; Overbeek, Nieuwenhuis, & Ridderinkhof, Reference Overbeek, Nieuwenhuis and Ridderinkhof2005). Taking into account the number of functions associated with the ACC, it is not surprising that the region is relatively heterogeneous in its cytoarchitecture and functional connectivity (Vogt, Finch, & Olson, Reference Vogt, Finch and Olson1992; Vogt, Nimchinsky, Vogt, & Hof, Reference Vogt, Nimchinsky, Vogt and Hof1995). Whereas caudal regions are thought to play a role in attention and cognitive control (Bush et al., Reference Bush, Luu and Posner2000; Posner & Petersen, Reference Posner and Petersen1990), rostral and subcallosal portions appear more involved in processing emotion (Bush et al., Reference Bush, Luu and Posner2000; Etkin, Egner, Peraza, Kandel, & Hirsch, Reference Etkin, Egner, Peraza, Kandel and Hirsch2006); however, a clear division of cognitive and emotional functions may be less likely than previously proposed (Etkin & Schatzberg, Reference Etkin and Schatzberg2011; Shackman et al., Reference Shackman, Salomons, Slagter, Fox, Winter and Davidson2011). Indeed, functional connectivity findings suggest that the differentiation in rostral/caudal ACC connectivity may be represented by more finely-graded transition regions than was indicated by prior neuroimaging studies (Margulies et al., Reference Margulies, Kelly, Uddin, Biswal, Castellanos and Milham2007). No neural region or system functions in isolation, however, and brain networks require complex interaction involving a multiplicity of neural systems to carry out a particular function (Collin, Sporns, Mandl, & van den Heuvel, Reference Collin, Sporns, Mandl and van den Heuvel2013; van den Heuvel & Sporns, Reference van den Heuvel and Sporns2011). Thus, while the cingulate may be part of a major hub in a particular network, it is but one aspect of the network (Kennedy & Adolphs, Reference Kennedy and Adolphs2012; Weible, Reference Weible2013).
Damage to the ACC as a result of traumatic brain injury (TBI) may explain several common TBI-related sequelae, which include altered emotional functioning, memory, and executive control (Hinnant, Reference Hinnant1999; Levin, Reference Levin1995); however, damage from TBI is multifaceted and the cingulate gyrus is never injured in isolation in trauma. Nonetheless, despite its apparently protected position in the depths of the longitudinal fissure, injury to the ACC may be relatively common in TBI (Stamatakis, Wilson, Hadley, & Wyper, Reference Stamatakis, Wilson, Hadley and Wyper2002) resulting in frontotemporolimbic susceptibility to injury, including the cingulate portion of the limbic system along with the global, nonspecific effects of TBI (Bigler, Reference Bigler2007). The proximity of the ACC to the ridged surface of the falx cerebri places it at risk for compression and grating, and it is vulnerable to secondary effects related to loss of afferent and efferent connections with other brain regions (Bigler, Reference Bigler2007). In addition, the cingulum bundle (composed of white matter fibers that course the length of the cingulate gyrus) is vulnerable to axonal injury due to rotational and shearing forces (Bendlin et al., Reference Bendlin, Ries, Lazar, Alexander, Dempsey, Rowley and Johnson2008; Kraus et al., Reference Kraus, Susmaras, Caughlin, Walker, Sweeney and Little2007; Rutgers et al., Reference Rutgers, Toulgoat, Cazejust, Fillard, Lasjaunias and Ducreux2008; Wu et al., Reference Wu, Wilde, Bigler, Yallampalli, McCauley, Troyanskaya and Levin2010).
Despite the prominent role of the ACC in cognition and its susceptibility to injury, surprisingly few studies have examined the effects of volume loss in the ACC following TBI and the effects of ACC-related volume changes on behavioral outcomes. Yount et al. (Reference Yount, Raschke, Biru, Tate, Miller, Abildskov and Bigler2002) measured surface area of the right and left cingulate gyrus and found significant decrease in surface area, but only in the posterior cingulate gyrus, where increased injury severity was related to reduced size; however, no neuropsychological measures were significantly correlated with cingulate volume in the study. Limitations of the study also include small sample size, variability in operator-controlled hand tracing methods, and reliance on clinically-based MRI scans. Recently, comparing MRI studies close to the time of injury with those 6-months post mild-to-severe TBI, Hudak et al. (Reference Hudak, Warner, Marquez de la Plata, Moore, Harper and Diaz-Arrastia2011) reported significant volume loss over time in the cingulate, but only in the left rostral anterior cingulate. Zhou et al. (Reference Zhou, Kierans, Kenul, Ge, Rath, Reaume and Lui2013) observed structural brain changes 1 year after a single concussive episode, with the anterior cingulum bundle and cingulate gyrus isthmus being particularly vulnerable. Additionally, they found that white matter volume loss in the anterior cingulum bundle was associated with changes in memory and attention.
Recent studies have evaluated changes in the ACC and related functional deficits in individuals with TBI. Wilde et al. (Reference Wilde, Newsome, Bigler, Pertab, Merkley, Hanten and Levin2011) explored the effect of TBI on working memory (WM) in children and adolescents across several brain regions using MRI volumetrics, fMRI, and DTI. Their findings indicated that, across modalities, the cingulate cortex emerged as a common structure related to WM performance in children and adolescents suffering from TBI. Furthermore, in a recent event-related fMRI study, severe TBI patients showed greater magnitude error-related activation in the ACC, which could either reflect compensatory plasticity following injury, or the inefficient use of additional neural resources to perform a task-switching cued-Stroop task (Sozda, Larson, Kaufman, Schmalfuss, & Perlstein, Reference Sozda, Larson, Kaufman, Schmalfuss and Perlstein2011).
Despite the association between cingulate function and attention (including WM), no known studies have investigated the functional relationships between TBI-related volumetric changes of the ACC and measures of attention and WM in adults. In the current study, WM and attention were evaluated using the Auditory Consonant Trigrams (ACT) task and a single trial variant of the Stroop Color Word task, in which response expectancy is varied per trial instead of per block as in the traditional “card” Stroop (Cohen, Barch, Carter, & Servan-Schreiber, Reference Cohen, Barch, Carter and Servan-Schreiber1999; Perlstein, Larson, Dotson, & Kelly, Reference Perlstein, Larson, Dotson and Kelly2006; Seignourel et al., Reference Seignourel, Robins, Larson, Demery, Cole and Perlstein2005). As a result, the test is a putative measure of context maintenance, WM, and cognitive flexibility in addition to the attention and processing speed aspects attributed to the more traditional Stroop task (Boone, Pontón, Gorsuch, González, & Miller, Reference Boone, Pontón, Gorsuch, González and Miller1998; Heflin et al., Reference Heflin, Laluz, Jang, Ketelle, Miller and Kramer2011). The ACT is a task of WM and dual task management that was originally developed by Brown and Peterson (Brown, Reference Brown1958; Peterson & Peterson, Reference Peterson and Peterson1959). Factor analytic studies of the measure have found high loadings with short-term memory and/or WM (Boone et al., Reference Boone, Pontón, Gorsuch, González and Miller1998; Mertens, Gagnon, Coulombe, & Messier, Reference Mertens, Gagnon, Coulombe and Messier2006) and on dual information processing and complex attention (Mertens et al., Reference Mertens, Gagnon, Coulombe and Messier2006).
Overall cingulate volume would be expected to be less in a severe TBI group when compared to controls; however, given that volume loss in TBI is uneven (Bigler, 2007; Levine et al., 2008), the range of cingulate volumes in those with TBI would be expected to overlap with control volumes. Combining individuals with and without a TBI therefore ensures a distribution of cingulate volumes from abnormally small (as a result of trauma), mixed with those unaffected by the injury, and the normal distribution of cingulate volume in those not injured. Furthermore, simple comparisons of task performance without a focus on whole-group and interactive effects can lead to spurious findings and conclusions (see Nieuwenhuis, Forstmann, & Wagenmakers, Reference Nieuwenhuis, Forstmann and Wagenmakers2011). Thus, to study the influence of cingulate volume on neuropsychological performance, this type of combined distribution provides a method to assess relations between cingulate volume and neuropsychological performance on measures like the ACT or Stroop. Accordingly, in the current study, cingulate volume was assessed with two methods from Freesurfer (Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston; http://ftp.nmr.mgh.harvard.edu/) automated image analysis tools: (1) region of interest (ROI) cingulate volume morphometry and (2) statistical maps displaying both whole-brain group contrasts (TBI vs. control) of cortical volume and also correlation of neuropsychological variables in relation to cortical volume.
Using the above quantitative neuroimaging techniques, we examined several hypotheses. With regard to volumetry, we hypothesized that ACC volume would be correlated with a modified Stroop task and with ACT performance, with decreased ACC volumes associated with reduced accuracy on both tasks; however, given that the TBI participants in this study had severe TBI, which commonly results in global atrophy (Bigler & Maxwell, Reference Bigler and Maxwell2011), we did not expect that trauma-related cingulate volume loss would occur in isolation, independent of atrophic changes elsewhere in the brain. To determine how unique cingulate versus non-cingulate effects of TBI were, we used the ventricle-to-brain ratio, an established metric reflective of global brain volume loss that corrects for head size differences (Tate, Khedraki, Neeley, Ryser, & Bigler, Reference Tate, Khedraki, Neeley, Ryser and Bigler2011) and the statistical brain map to simultaneously assess where cortical volume differences between TBI and controls were. We assumed that, in this sample of severe TBI patients, the expected general volume loss would be confirmed, as reflected by increased ventricle to brain ratio (VBR) as well as cortical volume loss in the expected frontotemporal distribution on the statistical brain map (Levine et al., 2008; Merkley et al., Reference Merkley, Bigler, Wilde, McCauley, Hunter and Levin2008). For these analyses, non-directional hypotheses were explored. Lastly, since focal injuries may occur within the cingulate gyrus, imaging studies were reviewed for focal pathology (using a semi-structured clinical rating method).
Methods
The Institutional Review Board at the University of Florida Health Science Center approved all study procedures in accordance with the Helsinki Declaration. Participants provided written informed consent and were compensated for participation in the study. Study procedures, including scanning and cognitive evaluation, were completed in a single session.
Participants
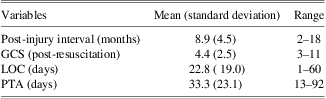
Individuals with TBI were recruited through the Florida Brain Injury Association, the Brain and Spinal Cord Injury Program of Florida, the Brooks Rehabilitation Hospital (Jacksonville), and local Florida Brain Injury Association support groups. The TBI group consisted of 12 adults who had sustained a severe TBI in vehicle accidents (9 motor vehicle accidents, 2 motorcycle accidents, 1 boating accident) and were in the post-acute injury phase at the time of evaluation (M = 8.9 ± 4.5 months post-injury; range 2 to 18 months). Severity of TBI was determined by medical record review of lowest post-resuscitation Glasgow Coma Scale (GCS) score (Teasdale & Jennett, 1974) and indices of injury severity. Severe TBI was defined as either a GCS score <9 or loss of consciousness (LOC) > 6 hr and post-traumatic amnesia (PTA) > 7 days. Duration of PTA and LOC were acquired from medical record review or from structured participant and significant other interview (King et al., Reference King, Crawford, Wenden, Moss, Wade and Caldwell1997; McMillan, Jongen, & Greenwood, Reference McMillan, Jongen and Greenwood1996). Indicators of injury severity are presented in Table 1. Demographic characteristics of the TBI and control participants are presented in Table 2.
Table 1 Indicators of TBI severity
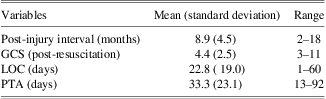
Note. GCS = Glasgow Coma Scale; LOC = loss of consciousness; PTA = post-traumatic amnesia.
Table 2 Demographic characteristics, neurobehavioral, and cognitive functioning of TBI and control participants

Note. Median values are reported, where applicable. For gender, M = male, F = female; For handedness, R = right, L = left; N/A = data not applicable. STAI = State-Trait Anxiety Inventory; BDI-II = Beck Depression Inventory, 2nd Edition; NRS-R = Neurobehavioral Rating Scale-Revised; DEX = Dysexecutive Questionnaire; NAART = North American Adult Reading Test; FSIQ = Full Scale Intelligence; ACT = Auditory Consonant Trigrams. Using Cohen's criteria, r ≥ 0.50 indicates a large effect size; 0.30 – 0.49 indicates a moderate effect size; 0.10–0.29 indicates a small effect size (Cohen, 1988).
The control participants included 18 never-injured individuals who were demographically similar in age, gender and parental level of education (see Table 2). The median level of education was higher for the control group as compared to the TBI group (p = .03). All participants were right-hand dominant and had normal or corrected-to-normal vision and no orthopedic impairments that would preclude task completion. Participants were administered the North American Adult Reading Test (NAART; Blair & Spreen, 1989) as a measure of pre-morbid intelligence. Participants with TBI made significantly more errors resulting in significantly lower pre-morbid IQ estimates, although estimated pre-morbid intelligence levels for both groups were within the average range of intelligence.
Magnetic Resonance Imaging Acquisition
All participants underwent magnetic resonance imaging (MRI) without sedation on a Siemens Allegra 3-Tesla MRI head scanner equipped with a standard head radio frequency coil. T1-weighted MP-RAGE high-resolution three-dimensional (3D) anatomical images were acquired (160 1-mm-thick slices; repetition time [TR] = 2000 ms; echo time [TE] = 4.13 ms; flip angle [FA] = 8°; matrix = 512 × 512 voxels; field of view [FOV] = 24 cm) for evaluation of structural abnormalities.
MRI Processing
Surface reconstruction and cortical parcellation were performed with the Freesurfer neuroimage analysis suite version 4.5.0 (http://surfer.nmr.mgh.harvard.edu/). Details of the procedure are described in previous publications (see Bigler et al., Reference Bigler, Abildskov, Wilde, McCauley, Li, Merkley and Levin2010; Merkley et al., Reference Merkley, Bigler, Wilde, McCauley, Hunter and Levin2008) and the following additional details of morphometric processing are extracted from the written description of morphometric procedures provided at the Freesurfer website, modified for the specific purposes of this study. Results of the cortical surface reconstruction for each participant were inspected for accuracy, and manual edits were performed to optimize the results where necessary. The cortical surface models were registered to a spherical atlas, using individual cortical folding patterns to correspond with cortical geometry across subjects (Fischl, Sereno, Tootell, & Dale, Reference Fischl, Sereno, Tootell and Dale1999), and the cortical surface was parcellated into regions according to the gyral and sulcal structure as described in Desikan et al. (Reference Desikan, Segonne, Fischl, Quinn, Dickerson, Blacker and Killiany2006). Briefly, the rostral anterior cingulate (rACC) was delineated by the rostral extent of the cingulate sulcus (rostral boundary), the genu of the corpus callosum (caudal boundary), medial aspect of the cortex (medial boundary), superior frontal gyrus (supero-lateral boundary), and the medial division of the orbitofrontal gyrus (inferolateral boundary). The cACC was circumscribed by the genu of the corpus callosum (rostral boundary), the mammillary bodies (caudal boundary), the medial aspect of the cortex (medial boundary), and the superior frontal gyrus (lateral boundary). The posterior division of the cingulate was defined rostrally and caudally by the caudal anterior and the isthmus regions of the cingulate cortex, respectively. It was bounded by the corpus callosum medially and the superior frontal gyrus and/or paracentral lobule laterally. The isthmus region of the cingulate was defined rostrally by the posterior division of the cingulate, caudally by the parahippocampal gyrus, medially by the medial wall and laterally by the precuneus. Parcellation of the cingulate is illustrated in Figure 1.

Fig. 1 Depiction of the cingulate gyrus, with rostral anterior cingulate gyrus marked in red, caudal anterior cingulate in yellow, posterior cingulate in blue, and the isthmus cingulate marked in green.
Cortical thickness was calculated as the closest distance between the reconstructed surface of the pia mater and the gray/white matter boundary at each point on the cortical mantle. Cortical volumes of the cingulate regions were computed as the product of the surface area of these parcellated regions and the cortical thickness. Intracranial volume (ICV) was derived from the automated atlas-based head size normalization approach (Buckner et al., Reference Buckner, Head, Parker, Fotenos, Marcus, Morris and Snyder2004) used by the Freesurfer workflow.
We subsequently conducted qualitative ratings of the TBI scans to identify the presence or absence of lesions, laterality, and location using methods outlined in Bigler (Reference Bigler2006), and Bigler and Maxwell (Reference Bigler and Maxwell2011). Specifically, two raters coded lesion presence, focal encephalomacia, white matter signal abnormalities, and atrophy from frontal, temporal, and parieto-occipital regions in each hemisphere. We also specifically coded any lesion to the cingulate gyrus or cingulum bundle. Disagreement was resolved by consensus between the raters adjudicated by the second author.
Cognitive Tasks
Stroop task
Participants completed a single-trial Stroop task, originally developed by Cohen and colleagues (Cohen et al., Reference Cohen, Barch, Carter and Servan-Schreiber1999) following completion of the 3D structural images in the scanner for a separate functional imaging study that has been previously published (Sozda et al., Reference Sozda, Larson, Kaufman, Schmalfuss and Perlstein2011). The study by Sozda et al. focused on the neural correlates of error-related performance monitoring. Full details of the task are provided in the study by Sozda et al. Briefly, however, for each trial, participants were presented an instructional cue (the word “color” or “word”) that was followed by the probe (i.e., Stroop) stimulus after a short delay. Participants performed either a word reading task or color naming task as prompted by the instructional cue. When prompted for the “word” task, participants responded with a button press to one of three color-coded response keys using the index, middle, and ring fingers of their right hand indicating the probe word; for the “color” task, they responded with a button press to the printed color of the probe word. All participants were trained in color-button response mapping to at least 80% before beginning the task. Three font colors and color words (red, green, blue) were presented in each of two possible congruency conditions (congruent, incongruent). In the congruent condition, one of the three color names was displayed in its own color (e.g., “BLUE” printed in blue); incongruent stimuli consisted of a color name displayed in one of the two remaining colors (e.g., “BLUE” printed in red). Response times and error commissions were recorded.
For timing, there was a 12.5-s delay from the instructional cue to the Stroop stimulus with a 10-s inter-trial interval. Participants performed a total of 192 trials consisting of 16 blocks of 12 trials each. Trial conditions were equally distributed across task conditions (i.e., color naming/word reading and congruent/incongruent) and were presented pseudo-randomly with an equal number of conditions per block. Stimuli generation and behavioral response recordings of accuracy and reaction time were conducted with E-Prime software 1.1 (Psychology Software Tools, Pittsburgh, PA). Additional details of this single-trial version of the Stroop task are described in prior publications (Cohen et al., Reference Cohen, Barch, Carter and Servan-Schreiber1999; Perlstein et al., Reference Perlstein, Larson, Dotson and Kelly2006; Seignourel et al., Reference Seignourel, Robins, Larson, Demery, Cole and Perlstein2005; Sozda et al., Reference Sozda, Larson, Kaufman, Schmalfuss and Perlstein2011). To simplify analyses, we chose to focus only on error rates (see Perlstein et al., 1998; Seignourel et al., Reference Seignourel, Robins, Larson, Demery, Cole and Perlstein2005). Interested readers can view precise response time information in the study by Sozda et al. (Reference Sozda, Larson, Kaufman, Schmalfuss and Perlstein2011) that is from this same TBI sample.
Auditory Consonant Trigrams (ACT)
The Auditory Consonant Trigrams task, also known as the Brown-Peterson task (Brown, Reference Brown1958; Peterson & Peterson, Reference Peterson and Peterson1959), is a WM task in which the examinee is instructed to remember three-letter trigrams (consonants) while verbally counting backward by 3's from a given number. The ACT was administered just before MRI acquisition outside of the scanner. After a specified interval delay (3-, 9-, or 18-s), the examinee is signaled to provide the three letters. The number of correct letters for each of the 3-, 9-, or 18-s interval delay conditions is summed over five trials for each condition, and, a total correct score (ACTSum) is computed (of a possible total of 45 points).
Behavioral Measures
In addition to the cognitive tasks, the participants completed several questionnaires to assess anxiety symptoms (State-Trait Anxiety Inventory (STAI); Spielberger, Reference Spielberger1983), presence of common sequelae of head injury (Neurobehavioral Rating Scale-Revised (NRS-R) (Vanier, Mazaux, Lambert, Dassa, & Levin, Reference Vanier, Mazaux, Lambert, Dassa and Levin2000), and symptoms of dysexecutive syndrome (Dysexecutive Questionnaire (DEX) (Burgess, Alderman, Wilson, Evans, & Emslie, Reference Burgess, Alderman, Wilson, Evans and Emslie1996). Self-report versions of the NRS-R and DEX were administered to both the TBI and control participants, whereas informant versions of the questionnaires were completed only by individuals familiar with the TBI participants (e.g., spouse, significant other, or parent).
Statistical Analysis
Between-group differences for demographic and clinical characteristics were evaluated using χ 2 for categorical variables and Mann-Whitney U tests for continuous variables due to the small sample size. Volumetric between-group differences of the cingulate cortex as a whole and by individual sub-regions were investigated by first transforming the cortical regional volume of interest into a percentage of intracranial volume (to correct for variability in head size). Between-group differences of cortical volume were then examined with Mann-Whitney U tests. Between-group differences on cognitive and behavioral measures, as well as more generalized volumetric quantities [e.g., total brain volume (TBV), total ventricular volume (TVV), and VBR] were also examined with Mann-Whitney U tests. The relation between cognitive performance measures (Stroop accuracy and ACT overall total sum) and cortical volume of anterior and total cingulate regions (as a percentage of ICV) were investigated by means of Pearson correlations. Correction for multiple tests (for between-group differences on brain-based region of interest measures and clinical variables, and also for Pearson correlations) was performed via false discovery rate (FDR) correction. Statistical maps of cortical volume differences were created by fitting a between-subject general linear model at each surface vertex for (1) cortical volume differences between groups, and (2) the relation of cortical volume to performance on the cognitive tasks. Correction for multiple tests for the statistical maps was applied using a Monte Carlo simulation (Hagler, Saygin, & Sereno, Reference Hagler, Saygin and Sereno2006) using a vertex-wise threshold of p < .05. Qualitative scan ratings present number of individuals with pathology for frontal, temporal, parieto-occipital areas, as well as the cingulate gyrus and cingulum bundle, for location and laterality.
Results
Group Comparisons of Behavioral and Cognitive Functioning
Demographic and task performance variables are summarized in Table 2. The TBI group did not report high levels of anxiety, depression, or executive impairment. Relative to the control group, most comparisons on these measures were non-significant with small effect sizes, and thus likely not clinically significant. The control group demonstrated higher estimated intellectual functioning as compared to the TBI group as assessed by the North American Adult Reading Test (p < .01), although parental education did not differ between the groups. As expected, the TBI group achieved lower scores on the ACTSum (p = .01) and committed more errors on the Stroop task (p < .01) relative to control participants.
Group Comparisons of Region of Interest Volumes
Table 3 presents the results of group comparisons of brain volumes of interest. Except for the region of the left isthmus of the cingulate, the TBI group had smaller cingulate volumes with generally small-to-medium effect size differences, although only the right cACC survived FDR correction for the multiple ROIs cingulate comparisons performed (where Pi < 0.05i/13) (see Benjamini & Hochberg, Reference Benjamini and Hochberg1995). VBR was significantly increased in the TBI group (p < .01). The statistical map of between-group differences in cortical volume indicated cortical volume reductions in the TBI group in frontal, temporal, and inferior parietal regions bilaterally (see Figure 2). Left rostral and caudal anterior cingulate were among the regions that demonstrated reduced cortical volume in the TBI group, whereas regions in the cingulate sulcus bordering on the anterior cingulate in the right hemisphere showed similar reductions.
Table 3 Between-groups differences for cingulate cortical and total brain volumes

Note. Cortical volumes are reported as median percentage of intracranial volume. Total brain volume and total ventricular volume are reported as median raw values for reference purposes. Using Cohen's criteria, r ≥ 0.50 indicates a large effect size; 0.30 – 0.49 indicates a moderate effect size; 0.10 – 0.29 indicates a small effect size (Cohen, 1988).
TBI = traumatic brain injury; cACC = caudal anterior cingulate cortex; rACC = rostral anterior cingulate cortex.

Fig. 2 Statistical map of between-group differences in cortical volume. Blue regions indicate significantly reduced cortical volume in the traumatic brain injury (TBI) group as compared to controls.
Correlations Between Volumetric Data and Cognitive Variables
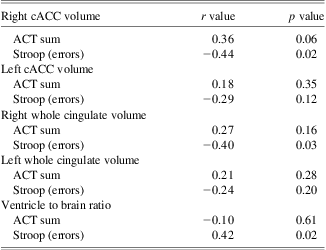
<PROI correlational results are summarized in Table 4. As indicated above, only the right cACC ROI survived FDR correction and therefore only the right cACC plots for Stroop and ACT performance are presented in Figure 3. Right cACC volume was negatively correlated with error rates on a single-trial version of the Stroop task (p = .02), and there was a trend for positive correlation with performance on the ACT task (p = .06 for ACTSum). Right cortical volume of the cingulate as a whole and VBR were also negatively correlated with error rates on the Stroop task (p = .03 and p = .02, respectively); however, none of these relationships survived FDR correction (where Pi < 0.05i/10, which yields FDR thresholds P1 < 0.005, P2 < 0.010, and P3 < 0.015 for the first 3 ordered observations).
Table 4 Relation of cingulate cortical volume to cognitive tasks
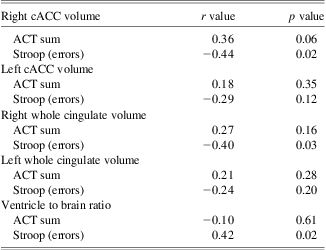
cACC = caudal anterior cingulate cortex; ACT = Auditory Consonant Trigrams.

Fig. 3 Correlations between cortical caudal anterior cingulate cortex (cACC) volume (right) and (A) Auditory Consonant Trigrams (ACT) sum of all trials and (B) number of Stroop errors. Traumatic brain injury (TBI) participants are represented in red, and control participants blue. Cortical volume was positively correlated with ACT accuracy, and negatively correlated with Stroop error commission.
The statistical brain map examining cortical volume correlation with Stroop performance (as shown in Figure 4) demonstrated widespread brain regions where cortical volume negatively correlated with Stroop error commission, including frontal, temporal, inferior parietal, and posterior cingulate regions, bilaterally. The blue regions shown in Figure 4 indicate areas of significant correlation with Stroop errors that withstood the Monte Carlo simulation. The statistical map of the correlation between cortical volume and ACTSum performance did not yield any clusters that withstood the Monte Carlo simulation; thus, those results are not displayed.

Fig. 4 Statistical map of correlation between Stroop error commission and cortical volume. Blue indicates regions of negative correlation.
Qualitative Scan Ratings
Across all individuals with TBI (total n = 12), lesions were most prevalent in the frontal lobes (9/12 participants; 75%), although 8/12 (67%) participants had temporal or parieto-occipital lesions. Only one participant had a clear lesion of the anterior cingulate gyrus on the left side. For laterality, seven of nine participants with frontal lesions were bilateral, of the other two, one was left and one was right. Two of eight individuals with temporal lesions were bilateral; two had lesions on the right and four had lesions on the left. Only one individual with a parieto-occipital lesion was bilateral. Four had lesions on the right and three had lesions on the right. There were no clear differences between right and left lesions across sites.
Discussion
As a group, TBI participants performed significantly more poorly than controls on the Stroop and ACT tasks, with medium effect size differences. Volumetric analyses demonstrated the expected smaller volume of both the right and left cingulate as well as less volume of all sub-regions of the cingulate except for the region of the isthmus on the left; however, at the level of specific ROI volumetric analyses, only the right cACC was significantly reduced in volume when corrected for FDR that included all of the different cingulate ROIs. In addition to using the ROI volumetry method, whole-brain maps of regional volume differences contrasting TBI with controls were also performed. The whole-brain maps showed distinct and significant cortical volume reductions, even after correction for multiple tests, particularly notable in the medial and anterior wall of both frontal lobes, but only reduced volume in the left ACC. Frontal volume reductions on the right bordered the ACC on the right but did not extend specifically into the right ACC. The disparities between the ROI cingulate volumetry method (that showed significant reduction in right cACC) and the whole brain mapping method suggest that the volume reductions in the TBI group were likely heterogeneous and not necessarily overlapping. Indeed, inspection of Figure 3 shows that whereas TBI participants overall had smaller cACC volumes, several had volumes clearly in a range indistinguishable from controls. This would suggest that while the cingulate is likely damaged in TBI, the damage is likely uniquely distributed on an individual basis resulting in no distinct uniformity in volume loss at a group level.
With regards to relations with Stroop and ACT, the right cACC volume was significantly related to Stroop errors, but so was the overall VBR measure at a similar magnitude. Furthermore, cortical mapping of volume loss in relation to Stroop performance did not replicate the right cACC finding using volumetry methods, and the statistical brain map identified no significant correlations between Stroop and ACC regions that survived correction for multiple tests. Taken together these findings suggest that the significantly reduced performance in TBI patients on the Stroop was not a specific finding related only to ACC volume differences. It is most likely that the ACC volume reduction occurs concomitantly with other frontal and whole brain volume loss changes in TBI, and the aggregate of these structural changes relate to diminished executive and WM deficits so common to TBI.
Brain injury and specifically damage involving the cingulate is known to be associated with changes in emotional and personality functioning; however, differences in this TBI sample appeared to be minimal as reflected in Table 2. Effect size differences were in the expected range suggesting that the TBI patients endorsed higher rates of symptoms traditionally associated with depression and anxiety, but the absolute values of the various self-rating metrics were minimal. For example, on the BDI-II the TBI group median score was 10, which would merit classification of minimal depression (less than mild).
As reviewed by Damasio, Anderson, and Tranel (Reference Damasio, Anderson and Tranel2011), there are substantial limits in attempting to relate specific frontal ROIs with neuropsychological test performance. While cACC volume relates to ACT and Stroop performance, deficits on these measures may be more related to network failure of which the ACC is but one part of, rather than damage specific to just the ACC. Volumetric analyses only reflect one dimension and it is very likely that combining structural image analyses that show where volumetric reductions have occurred in response to brain injury with techniques that better address network analysis (see Dennis et al., Reference Dennis, Simic, Bigler, Abildskov, Agostino, Taylor and Yeates2012; Hillary, Medaglia, Gates, Molenaar, & Good, Reference Hillary, Medaglia, Gates, Molenaar and Good2012; Pandit et al., Reference Pandit, Expert, Lambiotte, Bonnelle, Leech, Turkheimer and Sharp2013) will be able to more specifically examine the role of the cingulate in Stroop and ACT performance. Clearly, functional neuroimaging studies have provided substantial evidence for the role of the ACC as part of a network of brain regions involved in several cognitive control processes, including attention (Bush et al., Reference Bush, Frazier, Rauch, Seidman, Whalen, Jenike and Biederman1999, Reference Bush, Whalen, Rosen, Jenike, McInerney and Rauch1998; Nebel et al., Reference Nebel, Wiese, Stude, de Greiff, Diener and Keidel2005), conflict processing (Carter et al., Reference Carter, Macdonald, Botvinick, Ross, Stenger, Noll and Cohen2000; Kerns et al., Reference Kerns, Cohen, MacDonald, Cho, Stenger and Carter2004; Kim, Chung, & Kim, Reference Kim, Chung and Kim2010; Sohn, Albert, Jung, Carter, & Anderson, Reference Sohn, Albert, Jung, Carter and Anderson2007), WM (Kondo et al., Reference Kondo, Morishita, Osaka, Osaka, Fukuyama and Shibasaki2004; van der Wee et al., Reference van der Wee, Ramsey, Jansma, Denys, van Megen, Westenberg and Kahn2003), and cognitive flexibility (Gu et al., Reference Gu, Park, Kang, Lee, Yoo, Jo and Kwon2008; Shafritz, Kartheiser, & Belger, Reference Shafritz, Kartheiser and Belger2005). Furthermore, there is fMRI evidence that activity of the anterior cingulate during the Stroop task is reduced following TBI, which may reflect the degradation of neural networks secondary to diffuse axonal injury (Soeda et al., Reference Soeda, Nakashima, Okumura, Kuwata, Shinoda and Iwama2005). The current findings suggest that there may be structural changes that parallel these functional changes of the ACC, but additional techniques will be needed to fully characterize the relationship between structural and functional changes of the ACC following TBI.
Limitations
Limitations of the current study include the relatively small sample size. Whereas the groups were well matched for many demographic factors, they were not matched for education and estimated premorbid intellectual ability; however, maternal IQ has been cited as the single best predictor of child IQ in population-based studies (Sattler, 2008), and maternal education is also frequently used as an estimate of IQ (Meador et al., Reference Meador, Baker, Browning, Clayton-Smith, Cohen, Kalayjian and Loring2011). In our sample, maternal education did not significantly differ between the groups. Furthermore, while the TBI group demonstrated lower estimated IQ on the NAART, it is likely that basic reading skills may have been reduced as a result of the TBI itself, leading to an underestimation of premorbid IQ, as it has been observed that basic reading skills may be reduced in severe TBI (Mathias, Bowden, Bigler, & Rosenfeld, Reference Mathias, Bowden, Bigler and Rosenfeld2007). Finally, some previous studies do not explicitly differentiate between the anterior cingulate and the cACC, which makes it difficult to compare the current results to previous findings. Also, the inherent heterogeneity in the location and severity of injury sustained by the participants may obscure the full relationship between cingulate cortical measures and cognitive processes. The current study did not incorporate functional neuroimaging, diffusion tensor imaging or any type of network analysis, which clearly represent the next step in elucidating the potentially unique contribution of the cingulate gyrus in executive functioning.
Conclusions
The current findings are consistent with previous evidence that the cingulate gyrus is susceptible to atrophy following TBI, and the cACC may be preferentially vulnerable. However, atrophic changes occur against a backdrop of global atrophic changes and do not appear to be specific to just cACC atrophy in severe TBI. Atrophy of the right cACC likely contributes to reduced performance on executive function tasks, such as the Stroop and ACT, which are measures of executive control, divided attention, and WM, although this is likely but one node of an extensive brain network involved in these cognitive processes. An understanding of the functional role of TBI-vulnerable brain regions may provide insight into the common functional impairments that are observed subsequent to TBI. In addition to structural neuroimaging analysis techniques, future studies should incorporate functional neuroimaging, diffusion tensor imaging, and other techniques to investigate the relative roles of different brain network nodes in cognitive processes.
Acknowledgments
This research was supported by grants NIH F31 NS053335 to MJL and NIH R21 MH073076 to WMP, and by the Ira Fulton Foundation at Brigham Young University. Dr. Erin Bigler notes that he sees forensic neuropsychology cases. No other conflicts of interest are reported.