INTRODUCTION
Attention Deficit/Hyperactivity Disorder (ADHD) is a neurodevelopmental disorder characterized by excessive symptoms of hyperactivity, impulsivity, and difficulties with sustaining attention. The disorder is highly prevalent in the United States of America, affecting 9.4% of children aged 2–17, according to a national survey conducted in 2016 (Centers for Disease Control and Prevention, 2019), and can have profound impacts on function across school, home, and social environments. In addition to the core behavioral features that define ADHD, children with ADHD also show atypical motor development that can impact functioning in school and social settings (e.g., handwriting, playing instruments, athletic abilities). Motor impairments often observed in children with ADHD include patterns of disinhibited motor control, in particular excessive motor overflow, which have parallels with disinhibited behavioral control characteristics of ADHD, as well as an increased variability in motor task execution.
Motor overflow is a developmental phenomenon defined as unintentional motor movements that mimic intentional movements being executed. This phenomenon is thought to be developmental in nature because motor overflow is common in early childhood and typically decreases as children age into adolescence and as the motor system matures (Szatmari & Taylor, Reference Szatmari and Taylor1984). One specific pattern of motor overflow is mirror overflow, which refers to unintentional movements in homologous muscles on the opposite side of the body (MacNeil, et al., Reference Macneil, Xavier, Garvey, Gilbert, Ranta, Denckla and Mostofsky2011). Neurophysiologic findings investigating ipsilateral cortical silent period using transcranial magnetic stimulation (TMS) suggest that excessive mirror overflow in children with ADHD results from a lack of transcallosal inhibitory signaling between motor cortices (Hoy, Fitzgerald, Bradshaw, Armatas, & Georgiou-Karistianis, Reference Hoy, Fitzgerald, Bradshaw, Armatas and Georgiou-Karistianis2004; Wu, Gilbert, Shahana, Huddleston, & Mostofsky, Reference Wu, Gilbert, Shahana, Huddleston and Mostofsky2012).
Consistent with these neurophysiologic findings suggesting that ADHD-associated overflow movements reflect delayed and/or anomalous development of inhibitory capacity, excessive motor overflow (both mirror and proximal) have been found to be associated with patterns of disinhibited behavioral and cognitive control characteristic of ADHD. This includes studies among general populations of children revealing that excessive motor overflow correlates with greater severity of hyperactive, impulsive, and inattentive symptoms (Denckla & Rudel, Reference Denckla and Rudel1978; Szatmari & Taylor, Reference Szatmari and Taylor1984; Waber, Mann, & Merola, Reference Waber, Mann and Merola1985). Further, studies among children with ADHD reveal that excessive motor overflow correlates with neuropsychological measures of inhibitory control (Mostofsky, Newshaffer, & Denckla, Reference Mostofsky, Newschaffer and Denckla2003).
Traditionally, over several decades, motor overflow in children had solely been assessed using a principally qualitative approach; often through the use of the Physical and Neurological Examination for Subtle Signs (PANESS; Denckla, Reference Denckla1985). The PANESS assesses for developmental motor signs, including motor overflow, during the performance of a range of maneuvers including gait maneuvers (heel walking, toe walking, walking on the sides of feet, and tandem gait) and timed repetitive and sequential movements of the hands and feet. For each of these maneuvers, motor overflow is recorded as being present or absent, with the summation of “overflow-present” maneuvers providing an ordinal measure of “total overflow”, introducing an ordinal (rather than interval) limitation of the PANESS-based overflow measure. In addition to these disadvantages, the qualitative nature of this PANESS-based assessment of motor function also falls victim to rater subjectivity.
Recognizing the need for a more objective, interval-based quantitative measure of motor overflow, we pioneered methods for better quantifying motor control, specifically mirror overflow during finger sequencing, using finger twitch transducers (Biopac Systems Inc., Goleta, CA, USA; MacNeil, et al., Reference Macneil, Xavier, Garvey, Gilbert, Ranta, Denckla and Mostofsky2011). Our published findings reveal this approach provides a highly reliable, quantitative measure of mirror overflow during both left-hand and right-hand finger sequencing, as well as providing other quantitative measures of motor control including mean tap time and standard deviation (SD) of tap time (MacNeil, et al., Reference Macneil, Xavier, Garvey, Gilbert, Ranta, Denckla and Mostofsky2011). This allowed us to move beyond the PANESS and other clinical exam-based approaches that rely on observational, dichotomous coding of motor control.
In a previously published study using the finger twitch transducers (MacNeil, et al., Reference Macneil, Xavier, Garvey, Gilbert, Ranta, Denckla and Mostofsky2011), children with ADHD (aged 8–12) showed increased mirror overflow compared to their TD peers, with these quantitative measures of mirror overflow correlating with the qualitative assessments of overflow such as the PANESS. Of note, the findings of increased mirror overflow were particularly robust in boys with ADHD (as compared with their TD peers), with weaker findings in girls with ADHD (MacNeil, et al., Reference Macneil, Xavier, Garvey, Gilbert, Ranta, Denckla and Mostofsky2011). This observation is consistent with qualitative reports using the PANESS in which school-age boys with ADHD show higher amounts of motor overflow than do girls with ADHD (Mostofsky, et al., Reference Mostofsky, Newschaffer and Denckla2003; Cole, Mostofsky, Larson, Denckla, & Mahone, Reference Cole, Mostofsky, Larson, Denckla and Mahone2008).
In another study examining variability in motor and cognitive control in boys with ADHD and their TD peers, findings from the finger twitch transducer task revealed slower and more variable tap times in those with ADHD. Further, more variable tap time correlated with parent ratings of Hyperactive/Impulsive symptoms (Shiels Rosch, Dirlikov, & Mostofsky, Reference Shiels Rosch, Dirlikov and Mostofsky2012). These motor variability findings paralleled those from a Go/No-go task, such that boys with ADHD also showed slower and more variable reaction times than did TD boys, with reaction time variability correlating with parent ratings of Hyperactive/Impulsive symptoms.
These studies of motor control in ADHD have, thus far, principally focused on school-age children, with limited examination during early school age, which is a crucial period for motor development. A broader age range, inclusive of early school-age boys and girls, would provide increased understanding of the early expression of motor control impairments in children with ADHD, and whether sex impacts that expression. To date, there are no published studies using quantitative approaches to examine motor control in children younger than school age. One recent study used the more qualitative PANESS-based approach (Sweeney, et al., Reference Sweeney, Ryan, Schneider, Ferenc, Denckla and Mahone2018) in a longitudinal design (three annual visits) to examine developmental changes in motor overflow and speed of repetitive finger tapping and finger sequencing in 4–7 years old children with ADHD (n = 21, 10 girls) compared with TD children (n = 26, 7 girls). Children with ADHD consistently showed a slower speed of repetitive finger tapping than TD children that differentially improved with age among the ADHD group. In contrast, overflow was not significantly increased among young children with ADHD, although the group effect was marginal (p = .065). In addition, age-related improvements in motor overflow were observed across diagnostic groups. The investigators were not able to examine the effects of sex due to the small sample of girls included in the study, however, sex effects were examined by O’Brien, Dowell, Mostofsky, Denckla, and Mahone (Reference O’Brien, Dowell, Mostofsky, Denckla and Mahone2010) in a cross-sectional study also using measures from the PANESS. Findings revealed that girls with ADHD, but not boys with ADHD, showed greater motor overflow than their same-sex peers. Moreover, girls and boys with ADHD did not differ from each other on motor overflow, suggesting that the within-sex diagnostic differences observed were driven by the TD children for which TD boys showed greater motor overflow than TD girls.
The current study expands on the existing literature, applying quantitative (finger twitch transducer) methods to examine how abnormalities in overflow and motor variability are expressed in boys and girls with ADHD across a broader (5–12 years old) age range. Moderation analysis was used to examine whether diagnosis, sex, and their interaction moderate the association between age and these motor variables. We hypothesized that: (1) Across all children aged 5–12, mirror overflow and tap variability would decrease with age; (2) Across all ages, there would be significant effects of diagnosis, such that children with ADHD would show increased mirror overflow and tap variability compared with their TD peers; (3) Across all ages, there would be significant diagnosis-by-sex interactions, such that boys, but not girls, with ADHD would show increased mirror overflow and tap variability compared with their TD peers; and (4) The relationship between age and mirror overflow would differ among girls and boys with ADHD compared to same-sex TD peers, with boys with ADHD showing the strongest negative association (i.e., greatest decrease in overflow) with age.
METHODS
Participants
This study included 63 early school-age (age range =5.0–7.9 years) and 197 school-age (age range = 8.0–12.9) children. The total sample included 148 children with ADHD (45 girls, mean age = 9.2, SD = 2.1) and 112 TD children (41 girls, mean age = 9.4, SD = 2.2). Both TD participants and participants with ADHD were enrolled in ongoing studies of ADHD at the Kenney Krieger Institute, and were principally recruited through local public schools, but also through communitywide advertisement, volunteer organizations, medical institutions, daycare centers, websites, and word of mouth. These studies were approved by the Johns Hopkins University Institutional Review Board. Participants signed informed consent and children provided verbal assent. Participants were screened via parent telephone interview to determine eligibility.
All parents completed a telephone screening to determine initial eligibility. Children with histories of neurologic illness or injury, genetic disorders, seizures, or intellectual disability were excluded from the study. Parents of participants that remained eligible for either the ADHD or TD group after the telephone screening were then administered diagnostic interview (discussed below) over the phone to determine whether their child diagnostic criteria for ADHD and/or other psychiatric diagnoses. The diagnostic interview was also used to establish that TD participants did not meet the criteria for any psychiatric diagnoses. Eligible participants then attended a study visit to complete IQ and achievement testing and parents completed ADHD symptom rating scales. Children were excluded if they demonstrated a full-scale IQ less than 80 on the Wechsler Intelligence Scale for Children, Fourth or Fifth Edition (WISC-IV or WISC-V; Wechsler, Reference Wechsler2003; Wechsler, Reference Wechsler2014), or the Wechsler Preschool and Primary Scale of Intelligence, Third Edition (WPPSI-III; Wechsler, Reference Wechsler2002; see Table 1). Additionally, 8–12 years old children completed the Word Reading subtest from the Wechsler Individual Achievement Test, Third Edition (Wechsler, Reference Wechsler2009) and were excluded if they received a score below 85. Children younger than 8 years old were not excluded based on achievement testing.
Table 1. Participant demographic information

p-values were calculated using Pearson’s chi squared and independent-samples t tests. Early school age was defined as 5.0–7.9 years old and school age was defined as 8.0–12.9 years old. Full-scale intelligence quotients included scores from the WISC (TD n = 87, ADHD n = 115) and the WPPSI (TD n = 21, ADHD n = 28). Conners T-scores of Inattention and Hyperactivity/Impulsivity included scores from the Conners – Revised (TD n = 72, ADHD n = 74) and the Conners-3 (TD n = 38, ADHD n = 70).
ADHD Diagnosis
A previous diagnosis of ADHD was not required to be eligible for study participation. Instead, ADHD diagnosis was determined using the Diagnostic and Statistical Manual of Mental Disorders Fourth or Fifth Edition (DSM-IV or -DSM-V; APA, 2000; APA, 2013) criteria and confirmed using a parent interview, either the Diagnostic Interview for Children and Adolescents-IV (DICA-IV; Reich, Welner, & Herjanic, Reference Reich, Welner and Herjanic1997) or the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS; Kaufman, et al., Reference Kaufman, Birmaher, Axelson, Perepletchikova, Brent and Ryan2016), as well as parent and teachers report on either the Conners – Revised or Conners-3 Rating Scale (Conners, Reference Conners1997; Conners, Reference Conners2008). Diagnosis was verified by a board-certified child neurologist (SHM) or licensed clinical psychologists (KSR, KES) all with extensive experience in the clinical assessment of children with ADHD and related disorders.
TD children and children with ADHD were excluded from the study if they met the criteria, based on the DICA-IV or the K-SADS, for active psychosis, major depression, bipolar disorder, conduct disorder, adjustment disorder, or anxiety disorders, including generalized anxiety disorder, separation anxiety disorder, simple and social phobias, and obsessive-compulsive disorder. Children in both groups were not required to self-report any diagnoses prior to enrolling in the study. Children with ADHD were allowed to have a comorbid oppositional defiant disorder (n = 39).
Children in the TD group were ineligible if they were taking any psychotropic medications such as stimulants or mood stabilizers. Children taking non-stimulant medications, SSRIs, or other psychotropic medications were also excluded. Those children with ADHD taking stimulant medications (34%) were asked to withhold their medications the day of and the day prior to participating in the study to avoid the effects of stimulants on cognitive, behavioral, and motor measures.
Finger Twitch Transducers
TSD131 finger twitch transducers (Biopac Systems Inc., Goleta, CA, USA) were used to quantify mirror overflow in degrees of displacement from a baseline position. Transducers were affixed to the index and ring fingers of the left and right hands over the metacarpophalangeal joint to capture finger extension and flexion (see Figure 1a). Attaching transducers to only these two fingers still provides an accurate reflection of mirror overflow in the entire hand while also increasing comfort and ease of mobility for participants (MacNeil et al., Reference Macneil, Xavier, Garvey, Gilbert, Ranta, Denckla and Mostofsky2011). The transducers were then calibrated at 0° and 45° using AcqKnowledge software 4.2v (Biopac Systems Inc., Goleta, CA, USA) prior to beginning the task.

Fig. 1. (a) Finger twitch transducers affixed over the metacarpophalangeal joint of the index and ring fingers with tape and Velcro straps. (b) Finger twitch transducer data are collected in AcqKnowledge software during left-hand finger sequencing. The left finger channels show sequential tapping movements; the right finger channels show mirror overflow movements.
Finger Sequencing Task
All participants were asked to complete a sequential finger tapping task to assess tap speed and variability (in seconds) in the tapping hand and mirror overflow in the non-tapping hand (see Figure 1b). Participants were counterbalanced within diagnosis and sex for starting hand (i.e., begin with a left-hand sequencing block or a right-hand sequencing block). This counterbalancing, as well as summing mirror overflow across all left-hand and right-hand sequencing blocks, helped to account for any potential differences in performance between left-preferred and right-preferred handedness. Ten blocks (five per hand, alternating hands) of finger sequencing were collected. Correct positioning showed the tapping hand positioned upright and facing a camera in front of the participant and the non-tapping hand resting over a pillow on the participants’ lap so that extension and flexion in their fingers were not restricted, thus allowing for overflow movements. Ten seconds of baseline were collected before each block, where the tapping hand was held upright and flat. Participants were instructed to tap the pads of their fingers to their thumb in sequence (one sequence: index–middle–ring–pinky finger) “as big and fast” as possible to ensure valid, independent taps. Eleven sequences plus one ending tap to their index finger (45 taps total) were collected per block. Mirror overflow in the non-tapping hand was measured and summed for each block.
Measures
Completion of this task provides measures of mirror overflow, mean tap time, and SD of tap time for each block. Overflow was defined as any change in angle from the baseline position in the non-tapping hand and was measured in units of degrees. To calculate overflow scores, measures of mirror overflow in the non-tapping hand were summed across all left- and right-hand sequencing blocks (total overflow). Total overflow measures did not differ by left- or right-preferred handedness across diagnostic groups (p = .140) nor within diagnostic groups (TD: p = .567; ADHD: p = 194). To calculate scores of mean tap time and SD of tap time, mean and SD of tap times (in seconds) were averaged across all blocks (total mean tap time; total SD of tap time).
Statistical Analyses
Within a diagnostic group, outliers, defined as two or more SD away from the mean, were excluded for each dependent variable. Pearson’s correlations were conducted to examine associations between age, total overflow, mean tap time, and SD of tap time both across and within diagnostic groups. To test for effects of diagnosis, sex, and their interaction across the age range, multivariate analysis of covariance (MANCOVA) was employed with age as a covariate and total overflow, mean, and SD of tap time as dependent variables. These models were also examined with and without covarying for IQ and preferred handedness and similar results were obtained. Therefore, results without IQ and handedness are reported and any change in results is noted. To test our specific hypotheses regarding developmental changes in ADHD-related sex differences in motor function, three moderation models were employed to assess whether diagnosis, sex, or their interaction moderate the associations between age and total overflow, mean tap time, and SD of tap time.
RESULTS
Demographic information for the sample is provided in Table 1. Diagnostic groups did not differ in age or starting hand for the finger sequencing tasks. As is often observed in studies of children with ADHD, full-scale intelligence quotients were lower in children with ADHD compared to TD children (see Table 1.). Typically developing (TD) children and children with ADHD did not differ in preferred handedness across (p = .712) nor within sex (Girls: p = .758; Boys: p = .868).
Age-Related Change in Motor Function
Across measures, total overflow, mean, and SD of tap time improved with age when examined within the whole group and separately among ADHD and TD groups across and within sex (see Table 2 and Figure 2). The only notable change in results when controlling for preferred handedness was the relationship between age and SD tap time in girls with ADHD, which became only marginally significant (r = −.336, p = .024 to r = −.276, p = .070).
Table 2. Motor performance measures by age correlations

Pearson’s correlations examining associations between age and total overflow, mean tap time, and standard deviation tap time in the whole group and separately among ADHD and TD across and within sex.

Fig. 2. Both children with ADHD and TD control children show significant correlations between age and total overflow (ADHD: r = −.375, p < .0001; TD: r = −.610, p < .0001), mean tap time (ADHD: r = −.453, p < .0001; TD: r = −.537, p < .0001), and standard deviation of tap time (ADHD: r = −.453, p < .0001; TD: r = −.430, p < .0001). These associations suggest that as children age between 5 and 12 years old tap speed, tap variability, and mirror overflow decrease.
Diagnostic Group and Sex Comparisons of Motor Function
Results of the MANCOVA testing the main and interactive effects of diagnosis and sex on total overflow, mean and SD of tap time revealed a significant multivariate main effect of diagnosis, F(1, 258) = 5.3, p = .002, n p 2 = .060. Examination of the effect of diagnosis for each of the motor variables revealed that children with ADHD, compared to TD controls, (averaged across sex) demonstrated significantly greater total overflow, F(1, 258) = 11.7, p = .001, n p 2 = .044, and SD of tap time, F(1, 258) = 6.3, p = .013, n p 2 = .024, as well as a trend for greater mean tap time, F(1, 258) = 3.5, p = .064, n p 2 = .014 (see Table 3). There was a trending multivariate Diagnosis*Sex interaction, F(1, 258) = 2.5, p = .059, n p 2 = .029, and no multivariate main effect of sex, F(1, 258) = 1.0, p = .390, n p 2 = .012.
Table 3. Motor performance measures by diagnostic group – adjusted for age

Mean and standard deviation of total overflow, mean tap time, and SD of tap time. Statistics examining for the effect of diagnostic group were calculated using a MANCOVA controlling for age.
Further analyses of the Diagnosis*Sex interaction for each of the motor variables, given our hypothesized sex differences, showed a significant Diagnosis*Sex interaction for SD of tap time, F(1, 258) = 6.9, p = .009, n p 2 = .027, but not for total overflow, F(1, 258) = 0.1, p = .730, n p 2 < .001, or mean tap time, F(1, 258) = 0.8, p = .374, n p 2 = .003. Post hoc tests probing the Diagnosis*Sex interaction for SD of tap time were performed to further understand the direction of the interaction. The tests revealed greater SD of tap time among boys with ADHD compared to TD boys, F(1, 172) = 17.1, p < .001, n p 2 = .063, but not among girls with ADHD compared to TD girls, F(1, 84) < .01, p = .942, n p 2 < .001 (see Figure 3). Given this pattern of sex-specific diagnostic effects, we also performed unprotected post hoc tests examining the effects of diagnosis on total overflow and mean tap time within-sex groups. This revealed a diagnostic difference in boys for total overflow, F(1, 172) = 9.2, p = .003, n p 2 = .035, such that boys with ADHD exhibit more overflow than their same-sex TD peers. Similarly, there was a trending diagnostic difference in girls for total overflow, F(1, 84) = 3.8, p = .052, n p 2 = .015, such that girls with ADHD demonstrate trends toward greater overflow than their same-sex peers. A trending diagnostic difference was also found in boys for mean tap time, F(1, 172) = 4.9, p = .028, n p 2 = .019, such that boys with ADHD show slower tap times compared to TD boys. However, no diagnostic difference was observed in girls for mean tap time, F(1, 84) = 0.4, p = .538, n p 2 = .002.
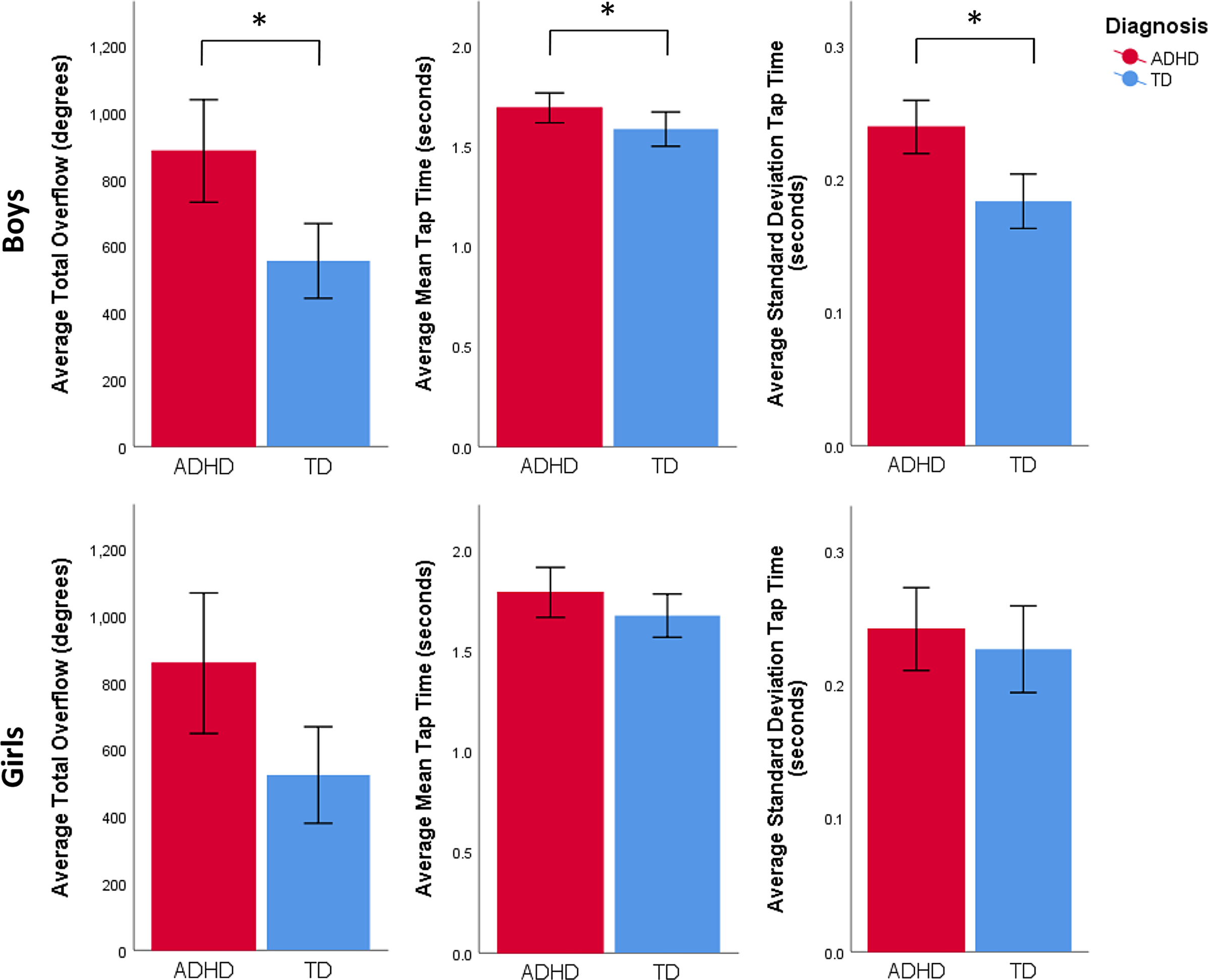
Fig. 3. Boys with ADHD show more total overflow than their TD peers, F(1, 172) = 9.2, p = .003, n p 2 = .035. Girls with ADHD show a trend for more total overflow than their TD peers, F(1, 84) = 3.8, p = .052, n p 2 = .015. Boys with ADHD exhibit longer mean tap times than their TD peers, F(1, 172) = 4.9, p = .028, n p 2 = .019. Girls with ADHD do not exhibit longer mean tap times than their TD peers, F(1, 84) = 0.4, p = .538, n p 2 = .002. Boys with ADHD demonstrate more variable tap times compared to TD boys, F(1, 172) = 17.1, p < .001, n p 2 = .063, whereas girls with ADHD do not, F(1, 84) < 0.01, p = .942, n p 2 < .001.
Diagnosis and Sex as Moderators of Association between Age and Motor Function
Neither diagnosis, sex, nor their interaction proved to moderate associations between age and total overflow, mean tap time, and SD of tap time (see Table 4.). Moderation tests for SD of tap time were strongest (see Figure 4), but still not significant, for the effects of Diagnosis*Age (p = .116) and Diagnosis*Sex*Age (p = .150) for predicting SD of tap time.
Table 4. Moderation of diagnosis and sex on age and motor performance measures

Statistics examining for whether diagnosis, sex, and their interaction moderate the association between age and total overflow, mean tap time, and SD of tap time.

Fig. 4. Standard deviation of tap times at the mean age and one standard deviation above and below the mean age within sex and diagnosis. This Diagnosis × Sex interaction does not moderate the relationship between age and standard deviation of tap time (p = .150).
DISCUSSION
In this study, we applied a highly reliable quantitative measure of mirror overflow and finger tapping variability to examine the associations with age and the impact of ADHD diagnosis and sex on these developmental motor phenomena in children 5–12 years of age performing a finger sequencing task. Consistent with our first hypothesis, we found that across girls and boys with and without ADHD, mirror overflow, finger tapping speed, and tapping variability, all improved with age. Consistent with our second hypothesis, we found that across the 5–12 years old age range, children with ADHD show greater mirror overflow and finger tapping variability than do their TD peers. Our third hypothesis was only partially borne out: Across the age range, we found a significant diagnosis-by-sex interaction for tap variability, but not mirror overflow or mean tap time, such that boys, but not girls, with ADHD showed increased tap variability compared with their same-sex TD peers. Finally, inconsistent with our last hypothesis, we did not find that diagnosis, sex, or interactions thereof moderated associations between age and mirror overflow or tap time variability; however, there was some suggestion that we had insufficient power to adequately address that question.
A significant effect of age was seen on mirror overflow and tap variability in children with and without ADHD, such that motor performance improves with age in children 5–12 years old. Similar results were found when performing partial Pearson’s correlations between age and motor performance controlling for preferred handedness. These findings add to the extant literature, confirming the age-related improvements in motor control seen in similar TD children of a similar age range, 7–14 years old (Gidley Larson, et al., Reference Gidley Larson, Mostofsky, Goldberg, Cutting, Denckla and Mahone2007). Current findings also add to the existing literature by expanding the age-related findings across early school-age and school-age children. A recent longitudinal study using the more qualitatively scored PANESS found similar age-related reductions in motor overflow and finger sequencing speed during finger sequencing improving in children aged 4–7 (Sweeney et al., Reference Sweeney, Ryan, Schneider, Ferenc, Denckla and Mahone2018). Specifically, Sweeney et al. (Reference Sweeney, Ryan, Schneider, Ferenc, Denckla and Mahone2018) found that both children with ADHD and TD children showed a reduction in motor overflow between ages 4 and 6 years; however, only children with ADHD showed a significant reduction in overflow across ages 4–7 years. Moreover, no diagnostic group differences were observed at any time point, which could result from a great amount of variability in overflow observed in the TD children.
Our findings of ADHD-associated elevations in motor overflow and tapping variability are consistent with those from several prior studies. Previous studies have revealed increased overall cumulative motor overflow in school-age children with ADHD as compared to their TD peers (Mostofsky et al., Reference Mostofsky, Newschaffer and Denckla2003; Cole et al., Reference Cole, Mostofsky, Larson, Denckla and Mahone2008; MacNeil et al., Reference Macneil, Xavier, Garvey, Gilbert, Ranta, Denckla and Mostofsky2011), with one study specifically revealing increased mirror overflow (MacNeil, et al., Reference Macneil, Xavier, Garvey, Gilbert, Ranta, Denckla and Mostofsky2011). Investigators employing a range of neuroimaging modalities (fMRI, EEG, and TMS) have found that these ADHD-associated increases in mirror overflow are associated with reduced motor cortex activation, with some suggestion for specific failed recruitment of inhibitory signaling (Mostofsky et al., Reference Mostofsky, Rimrodt, Schafer, Boyce, Goldberg, Pekar and Denckla2006; Gaddis, et al., Reference Gaddis, Rosch, Dirlikov, Crocetti, Macneil, Barber and Mostofsky2015; McAuliffe, et al., Reference Mcauliffe, Hirabayashi, Adamek, Luo, Crocetti, Pillai and Ewen2019; Wu, et al., Reference Wu, Gilbert, Shahana, Huddleston and Mostofsky2012). As with overflow, prior studies have also revealed increased tapping variability in children with ADHD. This includes previously published PANESS findings of increased dysrhythmia during finger sequencing (Cole et al., Reference Cole, Mostofsky, Larson, Denckla and Mahone2008; Sweeney et al., Reference Sweeney, Ryan, Schneider, Ferenc, Denckla and Mahone2018) as well as findings of increased finger tapping variability in school-age boys during finger sequencing using finger twitch transducers (Shiels Rosch, et al., Reference Shiels Rosch, Dirlikov and Mostofsky2012). The latter study also revealed significant associations between tapping variability and symptom severity, such that boys with more variable tapping showed more severe hyperactivity/impulsivity symptoms (Shiels Rosch, et al., Reference Shiels Rosch, Dirlikov and Mostofsky2012). The current study not only confirms this diagnostic effect, but also expands it to include girls with ADHD across a wider age range that includes early school-age and school-age children.
Regarding the impact of sex on ADHD-associated impairments in motor control, multiple prior studies have been consistent in revealing that, when compared with their TD peers, boys with ADHD show greater impairment in motor control than do girls with the disorder. This effect of sex was first reported by Mostofsky et al., who found greater levels of mirror overflow in boys with ADHD than in girls with ADHD and TD children (Mostofsky, et al., Reference Mostofsky, Newschaffer and Denckla2003). Since then, other studies have revealed findings of diagnostic differences in mirror overflow in boys, but not girls, with findings of boys demonstrating more mirror overflow than girls across diagnosis (MacNeil et al., Reference Macneil, Xavier, Garvey, Gilbert, Ranta, Denckla and Mostofsky2011; Cole et al., Reference Cole, Mostofsky, Larson, Denckla and Mahone2008). Conversely, one study found that it was girls, not boys, with ADHD who revealed more motor overflow than their same-sex TD peers, although the findings were driven by sex effects in TD children, with TD boys showing more overflow than TD girls (O’Brien, et al., Reference O’Brien, Dowell, Mostofsky, Denckla and Mahone2010). The current study, revealing no significant interaction of sex and ADHD diagnosis on mirror overflow, appears inconsistent with findings of sex effects in prior reports, and suggests that boys and girls aged 5–12 show similar levels of mirror overflow. This discrepancy could, in part, reflect the broader age range of early school-age and school-age children included in the current study, such that while younger early school-age TD children and children with ADHD demonstrate high levels of mirror overflow, ADHD-associated increases in mirror overflow span both boys and girls, with girls with ADHD then “outgrowing” mirror overflow earlier than boys with the disorder. Larger and longitudinal cohorts would help to address this hypothesis and aid in determining how sex should be considered when providing therapies to children with ADHD.
The current study also leaves open the question of developmental changes in the mechanisms of mirror overflow in boys and girls with ADHD beyond 12 years old and into adolescence. In this study, we examined age-related change, but we did not examine developmental trajectories given our analysis of cross-sectional rather than longitudinal data. One possibility, supported by Cole, et al. (Reference Cole, Mostofsky, Larson, Denckla and Mahone2008), is that ADHD-associated improvements in mirror overflow occur at a slower rate for boys than for girls, such that boys will eventually reach typical levels of overflow at a later age than do girls with ADHD. Another possibility is that increased levels of mirror overflow in boys with ADHD will persist into adolescence. Martins et al. (Reference Martins, Lauterbach, Slade, Luís, DeRouen, Martin and Townes2008) sought to answer this question by examining neurological soft signs across five time points in younger and older male and female adolescents. Results showed that male adolescents, particularly younger male adolescents, had increased neurological soft signs across time points compared to younger and older female adolescents. However, all four sex-age subgroups reached a near absence of neurological soft signs at the fifth time point (mean age of 17 years). These results suggest that rather than overflow/neurological soft signs improving at a slower rate in boys than girls, boys show increased neurological soft signs throughout adolescence, but improve at a faster rate than girls, reaching the same low levels of neurological soft signs as girls by the end of adolescence. An expansion of the current sample to include adolescent data from girls and boys with and with ADHD is needed to confirm whether or not a similar trajectory of motor improvements is observed in male and female adolescents with ADHD.
Published neuroimaging studies support this sexually dimorphic pattern of boys with ADHD have greater difficulties with motor control than girls with ADHD. Specifically, structural analyses of the frontal cortex reveal that boys with ADHD show reduced surface area localized to motor/premotor volumes compared with their TD peers, while girls with ADHD show reduced surface area in prefrontal regions (Dirlikov et al., Reference Dirlikov, Shiels Rosch, Crocetti, Denckla, Mahone and Mostofsky2015) suggesting different mechanisms of dysfunction in boys and girls with ADHD. Additionally, diffusion tensor imaging analyses reveal a similar pattern, with boys, but not girls, with ADHD showing reduced fractional anisotropy compared to their same-sex peers in primary motor white matter, with this reduced primary motor fractional anisotropy correlating with increased Go/No-go reaction time variability (Jacobson, et al., Reference Jacobson, Peterson, Rosch, Crocetti, Mori and Mostofsky2015). Taken together, these neuroimaging findings suggest that abnormalities in motor/premotor cortical and underlying white matter structure may contribute to the higher degree of motor impairment in boys with ADHD compared to their same-sex peers and to girls with ADHD. Studies directly examining these brain–behavior associations would help to address this hypothesis.
Limitations of the current study include cross-sectional rather than longitudinal data, preventing analysis of developmental mechanisms and trajectories. In addition, the inclusion of a larger sample size of girls that is comparable to that of boys will provide greater statistical power for testing diagnosis by sex interactions. Also, controlling for ADHD subtype across girls and boys would allow for a more accurate analysis of diagnosis by sex interactions on motor performance. Additionally, affixing finger twitch transducers to only two fingers (index and ring fingers) on each hand, while providing for greater ease of movement, leads to the potential of not capturing overflow movements occurring elsewhere. During data collection, overflow movements were observed in other fingers, mainly the middle and pinky fingers, and sometimes observed in the feet through extensions and flexions of the ankles and toes. Future studies quantifiably measuring overflow movements should consider improved methodologies to capture all exhibited overflow movements. Lastly, the current study measures the speed of tapping by calculating the time it takes to complete one tap sequence. This method does not account for tapping errors that occur during a sequence such as tapping a finger twice. While the speed of the individual taps remains the same, these tapping errors can slow the total time of the sequence, thus falsely suggesting that the participant is tapping at a slower speed. Accounting for the number of tapping errors in analyses and measuring the time of individual taps rather than tap sequences could resolve this issue.
In conclusion, we found that excessive overflow is observed in both boys and girls with ADHD, whereas anomalous motor variability is specific to boys with ADHD. Future studies should seek the inclusion of a larger sample of girls to provide greater power to test ADHD-related sex differences in motor overflow and variability. Inclusion of a broader age range extending into adolescence would also provide a greater understanding of developmental changes in motor overflow for girls and boys with ADHD compared to TD children. Lastly, incorporating measures of functional and structural connectivity in motor overflow analyses would expand current knowledge of the neurobiological foundation of motor overflow in ADHD and could help inform therapies that focus more specifically on the anomalous neurobiology associated with motor overflow.
ACKNOWLEDGMENTS
This work was prepared while Karen Seymour was employed at Johns Hopkins University and Kennedy Krieger Institute. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
FINANCIAL SUPPORT
This study was supported by the National Institutes of Health (MH078160, HD068425, MH104651, U54 HD079123, MH085328, K32 MH101322, K23 MH107734); and the Brain and Behavior Research Foundation (NARSAD Young Investigators Grant #23825).
CONFLICTS OF INTEREST
S. H. Mostofsky has a US patent (No: US10,410,041 B2).










