Introduction
Severe traumatic brain injury (TBI) results in significant neuropsychological and psychosocial sequelae with devastating consequences both for the individual and for their family (Tate, Broe, & Lulham, Reference Tate, Broe and Lulham1989). However, it is the disruption to social behavior after TBI that is often reported as being the most disabling and distressing for family and for the community (Brooks & McKinlay, Reference Brooks and McKinlay1983; McKinlay, Brooks, Bond, Martinage, & Marshall, Reference McKinlay, Brooks, Bond, Martinage and Marshall1981). A particularly debilitating behavior change commonly reported after TBI is social disinhibition, which refers to “socially inappropriate verbal, physical or sexual acts which reflect a loss of inhibition or an inability to conform to social or cultural behavioral norms” (Arciniegas & Wortzel, Reference Arciniegas and Wortzel2014, p. 39). This inappropriate social behavior may contribute to the well-documented trouble people with TBI have in maintaining social relationships post-injury, leading to social isolation and psychiatric illness such as depression and anxiety (Gould, Ponsford, Johnston, & Schonberger, Reference Gould, Ponsford, Johnston and Schonberger2011).
Socially disinhibited behavior after TBI has been linked with damage to the orbitofrontal cortex (OFC) and its connections with other brain regions (Lipszyc et al., Reference Lipszyc, Levin, Hanten, Hunter, Dennis and Schachar2014; Namiki et al., Reference Namiki, Yamada, Yoshida, Hanakawa, Fukuyama and Murai2008). Furthermore, evidence from lesions studies in both humans (Barrash, Tranel, & Anderson, Reference Barrash, Tranel and Anderson2000; Blair & Cipolotti, Reference Blair and Cipolotti2000; Namiki et al., Reference Namiki, Yamada, Yoshida, Hanakawa, Fukuyama and Murai2008) and monkeys (Butter, Mishkin, & Mirsky, Reference Butter, Mishkin and Mirsky1968; Franzen & Myers, Reference Franzen and Myers1973; Machado & Bachevalier, Reference Machado and Bachevalier2006), as well as studies of neurodegenerative disease (Hornberger, Geng, & Hodges, Reference Hornberger, Geng and Hodges2011; Krueger et al., Reference Krueger, Laluz, Rosen, Neuhaus, Miller and Kramer2011), also consistently demonstrate an association between OFC damage and social disinhibition. The orbitofrontal region is particularly susceptible following TBI (Mattson & Levin, Reference Mattson and Levin1990) due to abrasion of the ventral surfaces of the frontal lobes as they scrape across the bony floor of the anterior fossa in response to the acceleration-deceleration forces associated with the trauma (Bigler, Reference Bigler2007). Damage to frontal white matter tracts, which connect the orbitofrontal region with other brain regions has also been shown to be a common outcome of TBI (Kinnunen et al., Reference Kinnunen, Greenwood, Powell, Leech, Hawkins, Bonnelle and Sharp2011). Despite a general consensus in the literature that damage to the OFC mediates acquired social disinhibition, it is unknown what specific mechanism is involved.
Reversal learning impairment, or an impaired ability to update responding when reward contingencies change, is a neuropsychological hallmark of OFC damage (Schoenbaum, Takahashi, Liu, & McDannald, Reference Schoenbaum, Takahashi, Liu and McDannald2011). This well-documented deficit has generally been demonstrated using a visual discrimination test of reversal learning which involves the subject learning, based on reward and punishment, to respond to one of two visual stimuli presented, until, when a criterion level performance is reached, the reinforcement contingencies are swapped without warning. Human subjects with damage to the OFC, but not those with damage outside the OFC, have been found to exhibit deficient performance on such tasks (Fellows & Farah, Reference Fellows and Farah2003; Hornak et al., Reference Hornak, O’Doherty, Bramham, Rolls, Morris, Bullock and Polkey2004). Furthermore, patients with frontal variant fronto-temporal dementia (fv-FTD), characterized by neurodegeneration which preferentially affects the OFC (Gregory, Serra-Mestres, & Hodges, Reference Gregory, Serra-Mestres and Hodges1999), similarly demonstrate an impairment in reversal learning (Rahman, Sahakian, Hodges, Rogers, & Robbins, Reference Rahman, Sahakian, Hodges, Rogers and Robbins1999). Finally, people with TBI have also been found to perform poorly on reversal learning tasks (Rolls, Hornak, Wade, & McGrath, Reference Rolls, Hornak, Wade and McGrath1994). This impairment in the ability to flexibly adapt responding in an environment of changing social reinforcement contingencies may underlie acquired social disinhibition (Bachevalier & Loveland, Reference Bachevalier and Loveland2006). While reversal learning impairment has been documented in people with TBI and other clinical groups with OFC damage, no studies have yet demonstrated an impairment of reversal of social reinforcement contingencies after TBI. Thus, the first aim of the current study was to determine whether participants with TBI are impaired on a social reversal learning task and whether this impairment is related to social disinhibition.
Although it is clear that the OFC is crucial for reversal learning, the precise role it plays has been the subject of debate. Schoenbaum, Roesch, Stalnaker, and Takahashi (Reference Schoenbaum, Roesch, Stalnaker and Takahashi2009) argued that the role of the orbitofrontal cortex in reversal learning behavior is its contribution to the generation of reward prediction error signals which indicate the need for behavioral change when an outcome is worse than expected (Walsh & Anderson, Reference Walsh and Anderson2011a). Specifically, Schoenbaum et al. (Reference Schoenbaum, Roesch, Stalnaker and Takahashi2009) suggests that the OFC provides important information about the value of the expected outcome which is used in the generation of these reward prediction error signals in the dopaminergic midbrain. Evidence from neural recording studies (Gottfried, O’Doherty, & Dolan, Reference Gottfried, O’Doherty and Dolan2003; Hikosaka & Watanabe, Reference Hikosaka and Watanabe2004; Padoa-Schioppa & Assad, Reference Padoa-Schioppa and Assad2006) and behavioral studies (Izquierdo, Suda, & Murray, Reference Izquierdo, Suda and Murray2004) in animals support the role of the OFC in signaling expected outcomes. Crucially, in a reversal learning task reward prediction errors are necessary to signal the need to update behavior when negative feedback is delivered. Thus, the current study focused also on the role of reward prediction error signals in reversal learning and socially disinhibited behavior.
In humans, feedback-related negativity (FRN), an event related potential (ERP) component of the electroencephalogram (EEG) occurring approximately 200 to 400 ms after feedback onset, is thought to reflect reward prediction error signals (Nieuwenhuis, Holroyd, Mol, & Coles, Reference Nieuwenhuis, Holroyd, Mol and Coles2004). The FRN originates at the ACC, where it is hypothesized that the reward prediction error signals are used to update behavior such as is required in reversal learning tasks. The FRN is theorized to reflect the influence of midbrain dopaminergic reward prediction error signals on the ACC, such that a more negative FRN reflects a negative reward prediction error and a more positive FRN reflects a positive reward prediction error (Holroyd & Coles, Reference Holroyd and Coles2002).
This is evidenced by the finding that FRN amplitudes are most negative following unpredicted non-reward and least negative following unpredicted reward, and only occur when error feedback is not expected or probable (Hajcak, Moser, Holroyd, & Simons, Reference Hajcak, Moser, Holroyd and Simons2007; Holroyd & Coles, Reference Holroyd and Coles2002; Holroyd, Krigolson, Baker, Lee, & Gibson, Reference Holroyd, Krigolson, Baker, Lee and Gibson2009; Holroyd, Nieuwenhuis, Yeung, & Cohen, Reference Holroyd, Nieuwenhuis, Yeung and Cohen2003; Walsh & Anderson, Reference Walsh and Anderson2011a, Reference Walsh and Anderson2011b). Studies demonstrating that FRN can predict behavioral change (Cohen & Ranganath, Reference Cohen and Ranganath2007; Holroyd & Krigolson, Reference Holroyd and Krigolson2007; van der Helden, Boksem, & Blom, Reference van der Helden, Boksem and Blom2010) support the assumption that the FRN reflects the dopaminergic signaling of reward prediction errors which guide behavioral adaptation when an outcome is worse than expected. If the role of the OFC in reversal learning is its contribution to the generation of reward prediction error signals as Schoenbaum et al. (Reference Schoenbaum, Roesch, Stalnaker and Takahashi2009) suggests, it would be expected that an impaired ability to generate FRN signals to social feedback would be related to social disinhibition after TBI.
The current research compared the performance of a group of participants with TBI to a control group on both a social and a non-social reversal learning task. Feedback-related negativities elicited by negative feedback on the reversal learning tasks were also measured. To determine whether reversal impairments were related to social disinhibition, participants with TBI were also rated by two independent, blind-raters on their level of social disinhibition based on a video-taped interview. It was predicted that participants with TBI would make more reversal errors and have attenuated feedback-related negativities compared to controls on both the non-social and the social task. Furthermore, if reversal learning deficits play a role in acquired social disinhibition, those TBI participants high on social disinhibition should demonstrate an impairment compared to those low on social disinhibition in the ability to update responding when social reinforcement contingencies change in the social reversal learning task. Finally, it was hypothesized that attenuated feedback-related negativity amplitudes elicited by negative social feedback would be observed for the participants with TBI high on social disinhibition compared with those low on social disinhibition.
Method
Participants
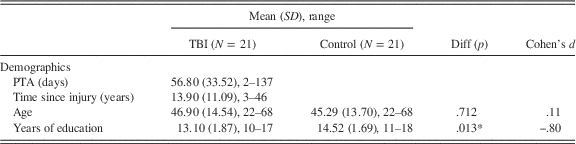
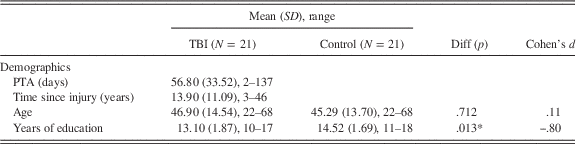
Twenty-one adults (19 males) who had sustained a severe traumatic brain injury (TBI) of mean age 46.90 years (SD=14.54, range: 22 to 68 years) with an average of 13.10 years of formal education (SD=1.87, range: 10 to 17) participated. Participants were recruited from the outpatient records of three metropolitan brain injury units in Sydney. Included participants met the following criteria: they had sustained a severe TBI resulting in at least one day of altered consciousness (Russell & Smith, 1961), were discharged from hospital and living in the community, were proficient in English and had no substance abuse or dependence. The participants with TBI had experienced post-traumatic amnesia (PTA) ranging from 2 to 137 days (Mean=56.8; SD=33.52), and time post-injury ranging from 3 to 46 years (Mean=13.90; Median=12.0; SD=11.09). PTA scores were obtained from patient medical records, with an exception of one participant whose records were unavailable. In this case, the injury was recorded as severe because coma duration exceeded 24 hr (Corrigan, Selassie, & Orman, Reference Corrigan, Selassie and Orman2010).
The participants’ injuries were sustained as a consequence of motor vehicle accidents (n=11), falls (n=8) and assaults (n=2). Computed tomography (CT) scans from the clinical records showed that injuries were left hemisphere focused (n=4), right hemisphere focused (n=5) and bilateral (n=11). A CT scan was not available for one participant. Specific frontal lobe injuries were reported in 12 participants. However, traditional imaging technology is not a reliable indicator of orbitofrontal damage. Orbitofrontal damage has been found using high resolution MRI in patients with behavioral change despite no obvious frontal lesions detected by traditional imaging technology (Namiki et al., Reference Namiki, Yamada, Yoshida, Hanakawa, Fukuyama and Murai2008). Furthermore, frontal white matter damage has been identified using diffusion tensor imaging in patients with little cortical damage evident using standard imaging (Kinnunen et al., Reference Kinnunen, Greenwood, Powell, Leech, Hawkins, Bonnelle and Sharp2011).
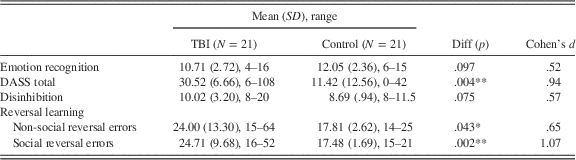
Control participants were 21 adults (18 males) without brain injury with a mean age of 45.29 (SD=13.70; range: 22 to 68 years) and an average of 14.52 years of education (SD=1.69; range: 11 to 18). Controls were recruited from the community via online and local newspaper advertisements. The control group did not differ significantly from the TBI group with respect to age, t(40)=.37, p=.712, d=.11, or with respect to emotion recognition scores, t(40)=−1.70, p=.097, d=−.52. However, the control group did differ from the TBI group in terms of number of years of education, t(40)=−2.60, p=.013, d=−.80 and Depression, Anxiety and Stress Scale (DASS; Lovibond & Lovibond, Reference Lovibond and Lovibond1995) total score, t(40)=3.07, p=.004, d=.94. To address these differences between groups in analyses, years of education was entered into the behavioral analyses as a covariate since it correlated with the outcome measure. Furthermore, emotion recognition scores were entered as a covariate as they were theoretically relevant. Table 1 provides demographic information for the TBI and control group.
Table 1 Means, standard deviations, ranges, and results of group comparisons for the TBI and comparison groups
*p<.05.
Materials
Reversal learning task
Participants were told that they could gain points in the task by selecting symbols displayed on the screen. As in Chase, Swainson, Durham, Benham, and Cools (Reference Chase, Swainson, Durham, Benham and Cools2011), on each trial, two different hiragana symbols appeared on the screen and participants made a selection using a left or right mouse click. Participants learned by trial and error which of these symbols was correct and which was incorrect. Selection of the correct symbol was rewarded by the delivery of the text “You WIN 1 point!”, while selection of the incorrect symbol was punished by the delivery of the text “You LOSE 1 point” in red font. The position of the symbols on the screen was randomized. Once the participant reached a criterion level of performance, the reinforcement contingency swapped, without warning, such that the previously correct symbol became incorrect and the previously incorrect symbol became correct. The contingencies continued to switch at the beginning of each block for a total of 16 blocks. The criterion level of performance to be reached before the reinforcement contingencies were reversed differed for each block, but was between 7 and 11 consecutive correct responses. This was to prevent participants from anticipating the reversal. If an error was made, the count toward the criterion level of performance for that block began again from zero. Thus, the number of trials per block depended on the performance of the individual. Each block had a maximum of 30 trials, after which the reward contingencies reversed whether or not the participant had reached criterion. Feedback presentation was displayed for 1000 ms and the inter-trial interval was 500 ms. Stimuli remained on the screen until a selection was made.
Social reversal learning task
The social reversal learning task was based on that described by Kringelbach and Rolls (Reference Kringelbach and Rolls2003). This task ran identically to the non-social reversal learning task described above, except that the stimuli were black and white photographs of two faces with neutral expressions and the feedback consisted of a happy or angry expression of the photographed actor appearing in the place of the neutral expression. The first eight blocks used two female faces and the second eight blocks used two male faces. The design of this task is represented in Figure 1. In this task, participants were not told that they were to gain points throughout the task but were just told to figure out which face to select at any given time. These instructions were designed to avoid the possibility of participants applying a rule such as “a happy expression means I have gained a point” and thus to make reinforcement as close to natural social feedback as possible. The design of this task is represented in Figure 1. The order in which the participants received the social and the non-social reversal learning tasks was counterbalanced to minimize the impact of practice effects, since it been suggested that reversal learning deficits disappear quickly with practice (Dias, Robbins, & Roberts, Reference Dias, Robbins and Roberts1997; Schoenbaum, Nugent, Saddoris, & Setlow, Reference Schoenbaum, Nugent, Saddoris and Setlow2002). Counterbalancing was achieved for the comparison between the TBI and control group as well as for comparison between the low disinhibition and high disinhibition group.

Fig. 1 Design of the social reversal learning task.
Social disinhibition interview task
The current study used an adaptation of the self-disclosure task developed by Beer, John, Scabini, and Knight (Reference Beer, John, Scabini and Knight2006). Participants were initially told that they would be asked several questions about themselves and their experiences, and that it was their choice how much information they wished to disclose and that they could skip any question at any time. These instructions were designed to minimize an expectation of excessive self-disclosure. Participants were then asked a series of nine questions, which included: “Tell me about an embarrassing moment you’ve had” and “Tell me about something someone has done to make you angry”. The interviews were videotaped and rated by two independent judges, blind to participant condition. Judges rated the frequency of the participants socially inappropriate behavior on a scale of 1 to 5 (where 1 represented “never” and 5 represented “always”) on the following items: “While talking with the interviewer, the participant spoke too candidly”, “Considering that they didn’t know the interviewer very well, the participant disclosed an inappropriate amount of information about themselves”, “The participant revealed more intimate details than most people would”, “The participant was rude”, “The participant made inappropriate jokes or remarks”, “The participant was impatient”, “The participant did not know when to stop talking”, “The participant was critical or argumentative”. These items were based on a thorough review of literature reporting socially inappropriate behaviors displayed by individuals with damage to the OFC. The inter-rater reliability for ratings across both TBI and control groups was analyzed with an intraclass coefficient (ICC) using a two factor mixed effect model. The inter-rater absolute agreement was good, ICC=.70, 95% CI [.43, .84]. The ICC was similar when looking at ratings for the TBI group alone, ICC=.70, 95% CI [.28, .87].
Emotion recognition task
Stimuli were 18 static images of one of four actors (two male and two female) portraying one of six emotions (happiness, surprise, sadness, anger, fear, and disgust). Stimuli were still images taken from the Emotion Recognition Task (ERT; Montagne, Kessels, De Haan, & Perrett, Reference Montagne, Kessels, De Haan and Perrett2007), a computer-generated program which shows a series of 216 video clips of facial expressions across different intensities. The stimuli were developed using algorithms (Benson & Perrett, Reference Benson and Perrett1991) which created intermediate morphed images between a neutral face (0% emotion) and a full-intensity expression (100% emotion). Data from a study by Rosenberg, McDonald, Dethier, Kessels, and Westbrook (Reference Rosenberg, McDonald, Dethier, Kessels and Westbrook2014), which used the ERT video stimuli, suggest that some emotions are much easier to recognize than others. Thus, to avoid floor and ceiling effects in recognition, 100% intensity of expression was used for fear, sadness and surprise stimuli, 80% intensity was used for anger and disgust stimuli, while 30% intensity was used for happy stimuli. Following the protocol of Heberlein, Padon, Gillihan, Farah, and Fellows (Reference Heberlein, Padon, Gillihan, Farah and Fellows2008), participants were asked to rate the intensity of each of six emotions they detected in each stimulus. For each participant, an accuracy score was derived by determining the number of trials on which participants correctly rated the expressed emotion as the most intense emotion in that stimulus. This task was included to determine whether poor performance on the social reversal learning task could be explained by poor emotion recognition.
Procedure
This study and its procedures were approved by the University of NSW Human Research Ethics Committee.
EEG acquisition
EEG data were acquired using a PC-based digital signal-processing hardware and software package from Neuroscan (Compumedics, Acquire Version 4.5). Continuous EEG was recorded from 64 scalp sites using the Neuroscan Quick-cap. Signals were then filtered with a bandpass of 0.1–30 Hz, referenced to the nose and grounded by the cap electrode. Tin cup electrodes were placed 2 cm above and below the left eye, and on the outer canthus of each eye, measuring vertical (vEOG) and horizontal (hEOG) eye movements, respectively. The maximum impedance was always below 5 kΩ for both EOG and cap electrodes.
EEG data analyses
Neuroscan Edit software (Compumedics 4.5) was used to calculate ERPs. The continuous data were bandpass filtered (0.01–30 Hz, zero-phase shift, down 24 db) and subjected to an EOG correction procedure (Semlitsch, Anderer, Schuster, & Presslich, Reference Semlitsch, Anderer, Schuster and Presslich1986). Waveforms were segmented into epochs 200 ms pre- and 600 ms post-feedback onset. The feedback-locked data were then baseline corrected by subtracting the average activity during the 200 ms preceding the feedback onset. For each participant, difference waves were computed by subtracting the average wave for correct feedback from the average wave for error feedback. The reversal learning tasks used ensured at least 15 errors were made by each participant across a minimum of 150 trials. As is conventional in the literature, the FRN was measured base-to-peak (Hajcak, Moser, Holroyd, & Simons, Reference Hajcak, Moser, Holroyd and Simons2006; Holroyd et al., Reference Holroyd, Nieuwenhuis, Yeung and Cohen2003; Yasuda, Sato, Miyawaki, Kumano, & Kuboki, Reference Yasuda, Sato, Miyawaki, Kumano and Kuboki2004).
The amplitude at the most negative peak between 200 and 500 ms were derived from the individual difference waves. This large window accommodated the large variance in latency found for participants with a TBI. The FRN component was defined as the difference in an individual’s difference wave between the negative peak identified and the preceding positive peak at medio-frontal channel FCZ. This electrode location was chosen because the FRN was largest at that site on examination of grand-averaged waveforms for the control group and based on previous studies showing the FRN is maximal at this medio-frontal site (Hajcak et al., Reference Hajcak, Moser, Holroyd and Simons2006; Holroyd, Larsen, & Cohen, Reference Holroyd, Larsen and Cohen2004; Holroyd et al., Reference Holroyd, Nieuwenhuis, Yeung and Cohen2003). For each participant, two FRNs were derived, one for the social task and one for the non-social task. One control participant’s EEG data for the social task were excluded due to faulty equipment. A task (social vs. non-social task) by group (TBI vs. control) repeated-measures analysis of variance (ANOVA) was performed with FRN amplitude as the dependent variable. The FRN was not correlated with years of education nor with DASS total score for either task. Thus, no covariates were entered in this analysis. In addition, because there is evidence of laterality of processing for social information in the literature, FRN amplitude at both FC3 (over the right hemisphere) and FC4 (over the left hemisphere) was reported.
Results
Behavioral Results
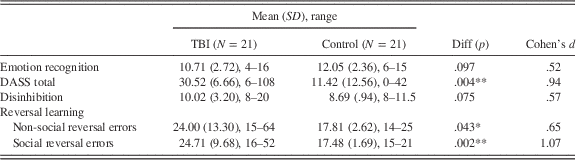
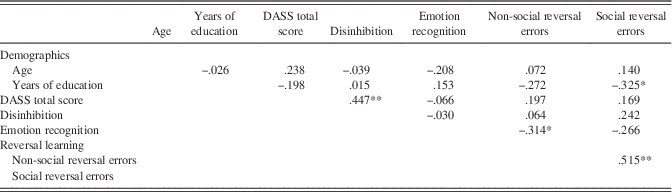
Emotion recognition, DASS, disinhibition, and reversal learning scores for both groups are outlined in Table 2. Correlations between these variables are provided in Table 3.
Table 2 Correlations between demographic variables, emotion functioning, disinhibition, emotion recognition, and reversal learning across the TBI and control group (N=42)
*p<.05.**p<.005.
Table 3 Correlations between demographic and experimental variables across the TBI and control group (N=42)

*Significant at p<.05.
**Significant at p<.001.
A 2×2 (task×group) repeated-measures analysis of variance (ANOVA) was conducted with number of reversal errors as the dependent variable. The analysis revealed a significant main effect of group, F(1,40)=9.54, p=.004, η2=.19, such that controls (M=17.64; SE=1.54) made fewer errors than did participants with TBI (M=24.36; SE=1.54). Group differences remained with the addition of years of education and emotion recognition as a covariate, F(1,38)=4.081, p=.05, indicating that these variables were not important factors in this effect. Mean reversal errors for both groups and both tasks are shown in Figure 2. There was no significant main effect of task, F(1,40)=.02, p=.892, and no significant interaction, F(1,40)=.14, p=.709.

Fig. 2 Mean number of errors on the social and the non-social reversal learning tasks for the TBI and control group.
Social disinhibition ratings were not normally distributed in the TBI group, with a significant positive skewness of 3.08 (SE=.37, p<.05; Cramer & Howitt, Reference Cramer and Howitt2004). To provide a meaningful metric based on these ratings individuals were categorized as low (n=10) on social disinhibition if they received the lowest possible social disinhibition rating of 8. They were categorized as high (n=11) on social disinhibition if they received a score of 9 or above. These two groups did not differ with regards to age (p=.396), years of education (p=.369), post-traumatic amnesia (p=.758), time since injury (p=.731) or DASS total score (p=.921). Figure 3 shows reversal errors on both tasks for TBI participants high on social disinhibition and TBI participants low on social disinhibition.

Fig. 3 Mean number of errors on the social and the non-social reversal learning tasks for TBI participants with high (n=11) and low (n=10) social disinhibition.
A repeated-measures 2×2 (task×disinhibition) ANOVA with number of reversal errors as the dependent variable revealed a trend toward a task by disinhibition interaction, F(1,19)=4.02, p=.059, η2=.18. This result was significant when years of education and emotion recognition were added as covariates, F(1,17)=7.48, p=.014, η2=.31. Because an a priori hypothesis was made about a specific relationship between the social reversal learning task and social disinhibition, univariate ANOVAs were carried out to determine whether differences between groups existed for each task separately. These analyses revealed that participants high on social disinhibition (M=29.18; SD=11.04) made significantly more errors than those low on social disinhibition (M=19.80; SD=4.66) on the social reversal learning task, F(1,21)=9.23, p=.007, η2=.34, but not on the non-social task, F(1,21)=.001, p=.971.
EEG Results
Figure 4 displays mean correct and incorrect waveforms, as well the difference waves (FRN), at electrode FCZ for each group and each task. Figure 5 displays the variance (SEM) contributing to the correct and incorrect wave forms for both groups and for both tasks. The repeated-measures 2×2 (task×group) ANOVA with FRN amplitude as the dependent variables revealed a significant main effect of group, F(1,39)=8.97, p=.005, η2=.19, such that controls (M=8.85; SE=.85) had higher FRN amplitudes than did the TBI group (M=5.29; SE=.83). There was also a main effect of task, F(1,39)=10.80, p=.002, η2=.22, such that FRN amplitudes were higher in the social task (M=8.63; SE=.92) than in the non-social task (M=5.51; SE=.57). There was no significant interaction, F(1,39)=1.13, p=.295.

Fig. 4 Average waveforms for the TBI and control group for correct and incorrect trials as well as the difference waveform. Waveforms for the non-social reversal learning task can be seen in the left panels and for the social reversal learning task in the right panels.

Fig. 5 Variance (SEM) contributing to the correct and incorrect wave forms for both groups and for both tasks.
To determine whether these results were affected by the inclusion of more correct trials than incorrect in the analysis, a separate analysis was run with equal number of trials. The above analysis was re-run on randomly selected 15 correct and 15 incorrect trials for each participant and each task and results remained the same. There was a significant group effect, F(1,39)=12.14, p=.001, η2=.24, and a significant task effect, F(1,39)=4.98, p=.031, η2=.11, but no interaction, F(1,39)=.79, p=.378.
Figure 6 depicts the FRN difference wave at FC3 (left hemisphere), FCZ (central) and FC4 (right hemisphere) and shows that the FRN was larger over the right hemisphere compared to central and left hemisphere sites for the social task. A repeated-measures 3 (electrode: FC3, FCZ, FC4)×2 (task) ANOVA revealed a significant electrode by task interaction, F(2,80)=10.09, p<.001. Follow-up tests of simple effects revealed that there was a main effect of electrode for the social task, F(2,80)=16.42, p<.001, but not for the non-social task, F(2,82)=1.25, p=.291. For the social task, pairwise comparisons with Bonferroni correction revealed that the FRN difference wave at FC4 was greater than at FC3 (M diff =1.92; p<.001) but not different than at FCZ (M diff =.63; p=.168).

Fig. 6 Feedback-related negativity at electrodes FC3, FCZ, and FC4 for the non-social task for (a) the control group and (b) the TBI group, as well as for the social task for (c), the control group and (d) the TBI group.
Finally, using only the TBI group, a repeated-measures 2×2 (task×disinhibition) ANOVA with FRN amplitude as the dependent variable revealed no significant effect of task, F(1,19)=3.51, p=.076, no significant main effect of disinhibition, F(1,19)=.588, p=.453, and no significant interaction, F(1,19)=.07, p=.789.
Discussion
The current study aimed to determine whether reversal learning deficits play a role in acquired social disinhibition after TBI by comparing performance of a group of people with TBI and a control group on a social and a non-social reversal learning task. As predicted, the TBI group made significantly more reversal errors across both versions of the reversal learning task than did controls, demonstrating an impaired ability to update behavior when reinforcement contingencies change. Although reversal learning impairment has been previously demonstrated in a brain-injured sample (Rolls et al., Reference Rolls, Hornak, Wade and McGrath1994), the current study was the first to show that TBI participants are also impaired at reversing responding when social reinforcement contingencies change. Furthermore, the current study found that TBI participants high on social disinhibition performed more poorly on the social reversal learning task than did those low on social disinhibition. This is consistent with Rolls et al. (Reference Rolls, Hornak, Wade and McGrath1994) report of a reversal learning deficit in TBI patients who displayed socially inappropriate behaviors as reported by caregivers.
The current research, however, is the first to demonstrate that reversal learning impairment is associated with social disinhibition observed in an experimental setting. Furthermore, this result could not be explained by poor emotion recognition in the high social disinhibition group. Together, these findings suggest that an inability to reverse social reinforcement contingencies may contribute to inappropriate social responding after TBI. Furthermore, the current results suggest that the social reversal learning task may be a useful neuropsychological tool for detecting susceptibility to social disinhibition after TBI. This is significant because past research has been unable to identify neuropsychological predictors of social disinhibition, often reporting that disinhibited individuals perform normally on neuropsychological tests (Cicerone & Tanenbaum, Reference Cicerone and Tanenbaum1997; Damasio, Grabowski, Frank, Galaburda, & Damasio, Reference Damasio, Grabowski, Frank, Galaburda and Damasio1994).
The current study also measured feedback-related negativity amplitudes evoked by negative feedback in both the non-social and social reversal learning tasks. FRNs are thought to reflect dopaminergic midbrain reward prediction error signals, which drive the updating of reinforcement contingencies and thus the updating of behavior (Holroyd & Coles, Reference Holroyd and Coles2002). Participants with TBI had attenuated FRN amplitudes compared with controls across both tasks, indicating an impaired ability to generate reward prediction error signals when negative social and non-social feedback is encountered. Consistent with this, previous research has shown that people with TBI did not differentiate reward from non-reward at an electrophysiological level (Larson, Kelly, Stigge-Kaufman, Schmalfuss, & Perlstein, Reference Larson, Kelly, Stigge-Kaufman, Schmalfuss and Perlstein2007).
Together these findings suggest that people with TBI are impaired at reward processing and thus at signaling when a predicted reward has not been delivered. This impairment in reward prediction error signaling was not, however, related to social disinhibition. This finding is contrary to the hypothesis that FRN amplitudes reflecting social reward prediction error signals drive changes in behavior to enable adaptive and context appropriate social behavior. It suggests that while these signals may be important in indicating when social feedback is worse than was expected, they may not necessarily correlate with updated behavior. In fact, while some studies have found a link between FRN amplitude and the updating of behavior (Cohen & Ranganath, Reference Cohen and Ranganath2007; Holroyd & Krigolson, Reference Holroyd and Krigolson2007; van der Helden et al., Reference van der Helden, Boksem and Blom2010), other studies have demonstrated that FRNs are generated when no behavioral adaptation is required (Gehring & Willoughby, Reference Gehring and Willoughby2004; Luu, Tucker, Derryberry, Reed, & Poulsen, Reference Luu, Tucker, Derryberry, Reed and Poulsen2003), suggesting that the FRN is not necessarily a signal used for learning. Thus, social reward prediction errors may not constitute sufficient information upon which to base a decision to change behavior.
Since the FRN has been widely reported to be maximal centrally, the right hemisphere lateralization of the FRN in the social task, illustrated in Figure 6, warrants discussion. Another study has similarly found a right-hemisphere lateralized “social FRN” elicited by unfair offers from other “players” in a computerized game (Boksem & De Cremer, Reference Boksem and De Cremer2010). Gehring and Willoughby (Reference Gehring and Willoughby2004) have suggested that lateralized contributing activity could result in a lateralized FRN. The right hemisphere lateralization of social FRNs, then, is in line with a pattern of literature documenting right hemisphere lateralization of social reward processing (Demaree, Everhart, Youngstrom, & Harrison, 2005). For example, right hemisphere dominance has been found for processing of negative emotional expressions (Adolphs, Damasio, Tranel, & Damasio, Reference Adolphs, Damasio, Tranel and Damasio1996; Nakamura et al., Reference Nakamura, Kawashima, Ito, Sugiura, Kato, Nakamura and Fukuda1999) and in responding to negative social feedback (Kaplan & Zaidel, Reference Kaplan and Zaidel2001). Thus, the right hemisphere lateralization of the FRN produced by negative social feedback in the current study likely results from right hemisphere dominance of negative social feedback processing.
A couple of limitations of the current study must be considered when interpreting the results. The TBI group had a slightly higher probability of experiencing error feedback in the reversal learning tasks than did controls. It is well established that a larger amplitude FRN is produced by less probable events (Sambrook & Goslin, Reference Sambrook and Goslin2015). This is because the more a reward comes to be expected, the greater the reward prediction error signal will be when the reward is not delivered. In the current study, the control group experienced error feedback on 11.5% of trials on average, while the TBI group experienced error feedback on 13.7% of trials. This seems a trivial difference in terms of participant’s perceptions of the probability of error feedback and is unlikely to be the source of group differences. Even so, future research should attempt to replicate this finding using a paradigm which equates number of errors as a percentage of total trials. Furthermore, despite ample evidence to suggest that reversal learning impairment and social disinhibition stem from OFC damage, the current study cannot confirm the origins of observed impairments in the TBI group. The use of high resolution imaging technology in combination with the measures used here could clarify these findings.
In summary, the current research found increased reversal errors and decreased FRN amplitudes elicited by error feedback in participants with TBI when compared with controls across both a social and a non-social reversal learning task. Furthermore, participants with TBI high on social disinhibition made more errors on the social reversal learning task than did those low on social disinhibition, supporting the hypothesis that reversal learning impairments underlie acquired social disinhibition after TBI. Attenuated FRN amplitudes in people with TBI indicate an impairment in feedback monitoring, possibly driven by an inability to differentiate reward from non-reward at an electrophysiological level. This impairment was not found to be a feature of socially disinhibited individuals specifically, suggesting that reward prediction error signals are not critical for behavioral adaptation in the social domain.
Acknowledgments
We express our gratitude to people with traumatic brain injuries who participated in the studies reported here as well as to our community control participants who gave willingly of their time. The authors have no competing or conflicts of interest to report.