The persistence of cognitive, emotional, and behavioral symptoms after mild traumatic brain injury (mTBI) has been recognized in the medical literature for close to 200 years (Evans, 1994). The term postconcussion syndrome (PCS) was first used in 1934 by Strauss and Savitsky to describe the subjective symptoms of headache, dizziness, fatigue and intolerance to “intoxicants, and vasomotor instability” that were reported to follow a blow to the head (Evans, 1994; Strauss & Savitsky, 1934).
The study of PCS has been made difficult because there is no commonly accepted definition (Binder, 1997). Two sets of diagnostic guidelines exist. According to the research criteria of the Diagnostic and Statistical Manual of the American Psychiatric Association fourth edition (DSM-IV; American Psychiatric Association, 1994), the essential feature is impairment in attention or memory, associated with three or more of the following postconcussion symptoms that occur after a significant cerebral concussion, or represent a considerable worsening of preexisting symptoms, and persist for at least 3 months: fatigue, sleep problems, headache, vertigo or dizziness, irritability, anxiety, headache or emotional lability, personality change, or apathy. The guidelines of the 10th revision, of the International Classification of Diseases (ICD-10; World Health Organization; WHO; 1993) are similar in requiring a history of head trauma with loss of consciousness, preceding the onset of symptoms by a period of up to 4 weeks. The ICD-10 guidelines require three or more features of various symptoms to be present. In contrast to DSM-IV, ICD-10 does not require evidence of a cognitive deficit. DSM-IV also calls for the postconcussion symptoms to be associated with deterioration in social or occupational functioning, and the symptoms must not be better explained by other disorders. Because of these differences, limited agreement has been found between DSM-IV and ICD-10 guidelines (Boake et al., 2004). As a result, the ICD-10, PCS symptom categories (Mittenberg & Strauman, 2000) and the similar DSM-IV symptom complaints (Luis et al., 2003) have been used instead of the diagnostic guidelines, in investigating the incidence of persistent PCS.
Most research, however, into the PCS has studied the number, type, and severity of PCS symptoms whose definitions have differed extensively among studies (see McCauley et al., 2001a). To add to the confusion, the term PCS has been used interchangeably with that of persistent PCS. For clarity, the term PCS is used in this study as it is defined by Alexander (1995), to describe the symptoms that develop in the first few days after mTBI and “persistent PCS” to describe symptomatic individuals who have not recovered after 3 to 12 months.
Opinion has been divided over whether PCS results from neurological or psychological factors (Cartlidge & Shaw, 1981; Karzmark et al., 1995; Lidvall et al., 1974; Lishman, 1988; Mittenberg & Strauman, 2000; Rutherford, 1989). The neurological versus psychological dichotomy is illustrated in the distinction made between early-enduring and late PCS symptoms (Karzmark et al., 1995; Lidvall et al., 1974; Rutherford, 1989). Early (neurological) symptoms, which occur immediately after the individual regains consciousness and on the following day are vomiting, nausea, and drowsiness, and headache and dizziness may endure. Anxiety, irritability, noise sensitivity, difficulty concentrating, subjective memory problems, and fatigue are late (psychological) symptoms, and they occur some weeks after discharge. In fact, Lidvall et al. (1974) considered anxiety to be the nucleus of the late PCS symptoms.
It is surprising given the literature on the association between persistent PCS and depression, posttraumatic stress disorder (PTSD), and general distress (Bryant & Harvey, 1999; Cattelani et al., 1996; Karzmark et al., 1995; McCauley et al., 2001a; Suhr & Gunstad, 2002) that so few studies have investigated the contribution of psychological symptoms in the development of the early PCS. One study by King (1996) reported strong, positive correlations between psychological measures and PCS symptoms in individuals 7 to 10 days after mTBI. No significant associations, however, were found between cognitive measures, posttraumatic amnesia (PTA), and PCS symptoms. Acute stress disorder (ASD) has been diagnosed in 14% of mTBI individuals in the first month post-injury (Harvey & Bryant, 1998); however the relationship between ASD and early PCS has not been explored.
A number of authors have investigated whether PCS symptoms following mTBI may form distinct groupings (Bohnen et al., 1992; Cicerone & Kalmar, 1995; Horn 1996; Levin et al., 1987a; Levin et al., 1987b; Lidvall et al., 1974; Suhr & Gunstad, 2002; Wrightson & Gronwall, 1981). Others have examined the PCS symptom construct in medical and psychiatric outpatients (Axelrod et al., 1996; Axelrod et al., 1998). Theoretically, three underlying factors, made up of cognitive, psychological, and physical symptoms have been suggested (Gouvier et al., 1988; Levin et al., 1987b).
Confirmation that the PCS symptoms are associated with three factors is mixed. Drawing from symptom checklists and structured interview containing different number, type, and definitions of PCS symptoms (McCauley et al., 2001a) measured at various points in time, two-factor (Bohnen et al., 1992), four-factor (Axelrod et al., 1996; Cicerone & Kalmar, 1995; Horn, 1996; Suhr & Gunstad, 2002), and five-factor models (Levin et al., 1987a; McCauley et al., 2001b) have been identified. One three-factor model of cognitive, psychological, and physical factors has been reported by Kay et al. (1995), and it was revealed in three of the four factors identified by Axelrod et al. and Cicerone and Kalmar.
Two studies have investigated the structure of early PCS symptoms. A two-factor (Bohnen et al., 1992; cognitive and emotional-vegetative factors) and a five-factor model has been reported (Levin et al., 1987a; cognitive-depression, somatic, sensory-sleep, gustatory-olfactory, and irritability-anxiety factors). Following cluster analysis based on the five factors, Levin et al. (1987a) identified one group in which symptoms of cognitive impairment and depression predominated, a second group with somatic symptoms, whereas the third and largest, reported minimal or no PCS symptoms. Bohnen et al. (1992) suggested emotional-vegetative complaints might be secondary and related to other factors such as previous psychiatric illness.
To further understand the relationship of psychological and cognitive factors in the development of the early PCS following mTBI, the association between PCS and neuropsychological and psychological outcome was investigated in an acutely hospitalized group of individuals. The participants were administered the Post Concussion Syndrome Checklist (PCSC; Gouvier et al., 1992), and a screening battery that included neuropsychological and psychological measures. The PCSC is a valid measure (Gouvier et al., 1992) that contains commonly reported PCS symptoms (Gasquoine, 1997; Lidvall et al., 1974; Strauss & Savitsky, 1934). In addition, the PCSC includes 6 of the 7 (fatigue, dizziness, poor concentration, memory problems, headache, and irritability) PCS symptoms, listed in ICD-10, reported to differentiate between mTBI and control groups at 1 month post-injury (Kashluba et al., 2006). As ICD-10 diagnostic criteria have been recommended for clinical purposes (Mittenberg & Strauman, 2000), classification of a current PCS was made based on the presence and frequency of three or more ICD-10, PCS symptom complaints. It was hypothesized that individuals classified with a PCS would perform more poorly on neuropsychological and psychological measures than those without a PCS. Moreover, the difference between participants with a PCS and those without would be greater on psychological measures than on neuropsychological measures.
There is limited research investigating the effects of opioid analgesia administered acutely following mTBI. The administration of opioids may create confound between opioid use and cognitive and psychological effects. Reductions in learning and memory, and impairments in information processing and sustained attention caused by opioids (Zacny, 1995) may be confused with cognitive deficits. Opioid analgesia may also have side effects that imitate psychological symptoms (e.g., dissociative symptoms associated with ASD) (O'Donnell et al., 2003). It was hypothesized that opioid analgesia would be associated with increased levels of psychological distress and decreased competency in neuropsychological function.
METHOD
Participants
Two hundred and seventy two participants who were consecutive clinical referrals to the mTBI Clinic, at a level 1 trauma hospital in Sydney, Australia were eligible for the study. Participants were general trauma cases, a number of whom were admitted because of orthopedic injury. The data had been collected over a 44-month period, between the years 2001 and 2004. All data included in this manuscript were obtained in compliance with hospital regulations. The study was approved by the hospital's Ethics Committee.
To be included, individuals were required to have sustained an uncomplicated mTBI. The criteria were based on the definition of the American Congress of Rehabilitation Medicine (1993) and the recommendations of the WHO Collaborating Task Force on mTBI (Carroll et al., 2004a), with the exception of including individuals who had sustained an intracranial lesion not requiring surgery. Because acute neuropsychological performance and outcome following mTBI complicated by an intracranial abnormality has been reported to be poorer than in those individuals with uncomplicated mTBI (Dikmen et al., 2001; Carroll et al., 2004b; Iverson, 2005) these participants were excluded.
To meet criteria required an acute brain injury that resulted from mechanical energy to the head from external physical forces and (i) one or more of the following: confusion or disorientation, loss of consciousness for 30 minutes or less, PTA for less than 24 hours, and/or other transient neurological abnormalities such as focal signs or seizure; (ii) a Glasgow Coma Scale score of 13 to 15 after 30 minutes, or on presentation for healthcare.
One hundred and twenty two individuals met the criteria. In addition, these individuals met the following criteria:
- They were not in PTA at the time of assessment based on retrospective evaluation of PTA (Gronwall & Wrightson, 1980), or identified by prospective measurement of PTA using the Westmead PTA Scale (Shores et al., 1986).
- Neuroradiological imaging, if performed, was normal. A computerized tomography (CT) brain scan was performed on 71.3% of participants within 24 hours of trauma. In these individuals there was no evidence of intracranial lesions.
- Time of assessment was not greater than 12 days post-injury.
- They were aged between 15 to 70 years at the time of the injury.
- Participants were required to have an IQ no less than 80.
- They were not forensic cases and were not currently in litigation. A number of individuals, however, may have been contemplating litigation at the time of assessment, or have gone on to engage in litigation.
- There was no evidence of preexisting cognitive impairment.
- Individuals needed adequate command of English to allow for valid test administration.
One hundred and fifty participants were excluded due to the following: 5 were in PTA, 41 sustained a mild complicated TBI, 39 sustained a severe TBI, 13 were assessed after 12 days post-injury, 3 were forensic cases, 4 were aged above 70 years, 7 were presumed to have preexisting cognitive impairment, 9 did not speak adequate English, 14 did not sustain a TBI, and 15 had insufficient data for analyses.
Individuals were not excluded if they had a prior mTBI, learning difficulty or psychiatric history, and alcohol or marijuana use because these factors may be related to a poorer outcome (Binder, 1997). Because not all participants may have had access to services for the formal diagnosis of learning disorder, they were asked whether they had a history of learning difficulty.
The majority of participants were men (73%). The mean age was 31.81 years (SD = 12.73, range = 15.11–65.01), and the mean years of education was 11.34 years (SD = 2.56, range = 6–18). The mean estimate of full scale IQ was 96.65 (SD = 9.09, range 81–130). Twenty-five percent of participants were born overseas, and the mean time of residence in Australia was 14.55 years (SD = 14.52, range = .1–52). English was the first language of most participants (77%), with the remainder speaking English and another language. At the time of the trauma, 59% was employed full time, 13.1% employed part-time or casually, 6.6% students, and 21.3% not employed (e.g., unemployed, retired, or home duties). The main cause of TBI was through road traffic accidents (78%). Of these the majority occurred through a motor vehicle accident to either a driver or passenger (56.6%), followed by motorcycle accident (11.5%), pedestrian struck by a motor vehicle (6.6%), and bicycle accident (3.3%). The remainder experienced a TBI through a fall (13.1%), assault (7.4%), and other (1.6%) such as sporting injury and work related accident.
Measures
Post Concussion Syndrome Checklist (PCSC; Gouvier et al., 1992) is a 10 item checklist. Participants were asked to rate the frequency of postconcussional symptoms since the accident, on a 5-point rating scale: 1 “Not at all,” 2 “Seldom,” 3 “Often,” 4 “Very Often,” 5 “All the time.”
Barona and Chastain's (1986) demographic equation was used to estimate premorbid intellectual capacity. When selecting region, the Northeast (weighted 1.59) was used in the equation because it was considered to be demographically comparable to the Southeast region in Australia.
Westmead Selective Reminding Test (WSRT; Shores 1995; Shores et al., 1986) is a 10-word, 10-trial selective reminding test that measures verbal new learning. The Consistent Long-Term Retrieval score (CLTR) has been found to be sensitive to the effects of traumatic brain injury (Shores, 1995; Shores et al., 1986). A total recall score (WSRT-Total Recall) was calculated, and a delayed-recall (WSRT-Delayed Recall) score was obtained after 30 minutes. All patients were administered one or other of two parallel forms. Because the data from the parallel forms were not normally distributed, nonparametric univariate analysis (Mann-Whitney U-test) was used for univariate comparisons. An alpha level of .05 was used. There was no significant difference revealed between the parallel forms, therefore scores were combined.
Symbol-Digit Modalities Test (SDMT; Smith, 1982), written and oral versions, was used to measure auditory and visual-motor response speed. The SDMT has been shown to be sensitive to the cognitive effects of traumatic brain injury and has been suggested for use in mTBI assessment (Hinton-Bayre et al., 1997; Ponsford & Kinsella, 1992; Raskin, Mateer & Tweeten, 1998). The raw data were converted into age and education adjusted z-scores (Smith, 1982).
California Computerized Assessment Package (CALCAP; Miller, 1999) is a computerised assessment of reaction time and speed of information processing. The abbreviated version which contains four tasks was administered. Reaction time tasks are considered to measure information processing (MacFlynn et al., 1984). Sequential Reaction Time of the CALCAP has been shown to discriminate between subgroups of mTBI individuals (Waterloo et al., 1997). Moreover, reduced reaction time has been demonstrated at 24 hours and up to 35 days following mTBI (McAllister et al., 1999; MacFlynn et al., 1984). Only the last two tasks Sequential Reaction Time 1 (RT1) and Sequential Reaction Time 2 (RT2) were analyzed.
Verbal fluency, which requires self-organized retrieval of words according to a given letter, in this case the letter “S,” was extracted from the Frontal Assessment Battery (Dubois et al., 2000; Spreen & Strauss, 1998). Fluency tests have been reported to be sensitive to the acute deficits following mTBI (Belanger et al., 2005; Zakzanis et al., 1999). The raw data were converted into z-scores (Spreen & Strauss, 1998).
The 21 item half-form of the Depression Anxiety Stress Scales (DASS; Lovibond & Lovibond, 1995) was used to measure the severity of symptoms of depression, anxiety, and stress. Scores were converted to full scale DASS scores.
Acute Stress Disorder Scale (ASDS; Bryant et al., 2000) is a 19 item self-report inventory that was used to identify posttraumatic stress reactions in the acute trauma phase (2 days to 28 days posttrauma). The dissociative score from the dissociative cluster and a cumulative score from the reexperiencing, avoidance, and arousal clusters (ASD-Total) were analyzed. Bryant and Harvey (1999) have suggested that the dissociative amnesia item from the dissociative cluster for ASD directly overlaps with PTA, therefore scores were examined for participants with the dissociative amnesia item (Have you been unable to recall important aspects of the trauma?) included and excluded.
Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 2001) is a 10-item questionnaire, which screens for current alcohol consumption, hazardous and harmful alcohol use, as well as possible dependence. The first three items ask an individual to rate: (i) frequency of drinking; (ii) the typical quantity; and (iii) frequency of heavy drinking (i.e., six or more drinks on one occasion). A combined score on questions 1, 2, and 3 was used to estimate hazardous alcohol consumption.
Procedures
The participants were referred to the mTBI Clinic for the purpose of neuropsychological screening. Medical details were obtained from the hospital record. These included the result of CT brain scan, cause of injury, GCS on acute admission, and a record of whether opioid analgesia was administered or not, within 24 hours of the trauma (including at the scene of the accident) and on the day of test administration. Participants were screened a mean 4.67 days (SD = 2.52, range 1–12) post-injury. Demographic information and history of prior mTBI, self-reported learning difficulty, psychiatric history, and marijuana use was obtained on interview. Available data on neuropsychological measures that had a visuographic component were reduced for participants who could not complete the written SDMT, Sequential RT1, and Sequential RT2 because of their physical injuries. The ASDS was not used in the mTBI Clinic until 2002; therefore not all participants have data from this test. The neuropsychological screens were conducted by Intern Clinical Neuropsychologists, under the supervision of the first author (SM) who was present for each assessment.
The majority of individuals presented with a GCS score of 15 (69.6%), whereas 27.9% had a GCS of 14 and 2.5% a GCS of 13. Duration of PTA was estimated retrospectively by asking individuals “What is the first event you can remember after the injury” followed by “And what happened then?” until their description reflected detailed and ongoing memories (Gronwall & Wrightson, 1980; Levin et al., 1979). Duration of PTA was estimated to be less than 5 minutes for 20.5% of participants, 6 to 60 minutes in 14.8%; 61 minutes to 12 hours in 29.5%, and 17.2% of participants had PTA of greater than 12 hours to 24 hours. Duration of PTA was unable to be retrospectively estimated for 22 individuals (18%) because of the effects of either opioid analgesia within the first 24 hours (n = 22), anesthesia (n = 14), intubation for intensive care treatment or aggression (n = 5), or a combination of these factors. A CT brain scan was performed on 20 of these 22 participants with a normal result reported. Ten of these 22 individuals presented with a GCS of 15, 10 had a GCS of 14, and two a GCS of 13. By review of medical notes none were found to have evidence of amnesia (e.g., repetitive questioning), confusion, or agitation after 24 hours post-injury.
RESULTS
Demographic and Preinjury Statistics For Groups With and Without Postconcussion Syndrome
Only those symptoms endorsed on the PCSC as occurring 3 “Often,” 4 “Very Often,” and 5 “All the time” were considered to characterize a current PCS symptom (Luis et al., 2003). As shown in Table 1, fatigue was the most frequently reported symptom followed by dizziness, anxiety, headache, and irritability. Less frequent were difficulty concentrating, visual disturbances, aggravated by noise, memory problems, and judgement problems. Mild TBI participants were divided into two groups based on whether or not they were experiencing a PCS. Individuals were classified according to ICD-10, PCS symptom criteria. They were required to subjectively report three or more current PCS symptoms. Forty-eight percent (n = 59) of participants were classified as having a PCS. The mean number of reported symptoms was 4.71 (SD = 1.85, range 3–9).
Rank order of frequency of reported postconcussion symptoms on the Post Concussion Syndrome Checklist (N = 122)
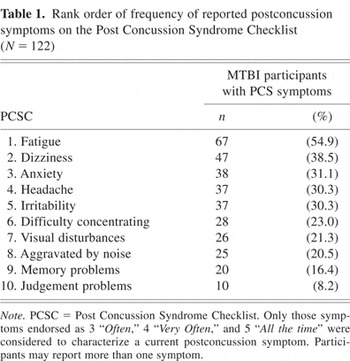
Differences between groups with and without a PCS were examined to investigate whether demographic and preinjury variables were related to whether a PCS was classified as being present or not. From Table 2 it can be seen that there were no significant differences between the groups in terms of age, sex, education, Barona full scale IQ scores, those reporting prior mTBI, a self-reported history of learning difficulty or psychiatric history, and alcohol or marijuana use.
Demographic and preinjury statistics for mTBI participants with and without a postconcussion syndrome

Group Differences in Neuropsychological and Psychological Variables
Univariate analyses were performed to examine whether individuals classified with a PCS performed more poorly on neuropsychological and psychological measures than those without a PCS. Mann-Whitney U-tests were carried out because the data were not normally distributed. Following the method of Iverson et al. (2004), independent t-tests were also performed to allow effect sizes to be calculated. Univariate analyses of participants with a PCS and those without were subjected to a Bonferroni adjustment where the alpha level was set at .004 to provide an overall rejection level of .05. No significant differences were present between the groups on neuropsychological measures (see Table 3 for independent t-test results). In contrast, there were significant differences between groups with and without a PCS on all psychological measures. On measures of depression, anxiety, stress, dissociative score, dissociative-DA score, and ASD total the PCS group reported greater psychological distress. Mann-Whitney U-tests and independent t-tests revealed identical levels of significance (see Table 4).
Means, standard deviations, effect sizes and percentage of overlap in the mTBI groups, with and without a postconcussion syndrome, on neuropsychological measures

Means, standard deviations, effect sizes and percentage overlap in the mTBI groups, with and without a postconcussion syndrome, on psychological measures

The magnitude of difference between the two group means was estimated. Effect sizes were calculated, together with overlap percentages (Cohen, 1992; Zakzanis, 2001). A medium effect size (.48) was found for verbal fluency S (see Table 3). Small effect sizes were found on WSRT-Total Recall, WSRT-Delayed Recall, written and oral SDMT, and Sequential RT1. In contrast, large effect sizes were present for all of the psychological measures, these ranged from .86 to 1.42. The largest effect sizes were found for the dissociative score, dissociative-DA, and ASD Total (1.25 to 1.42), with 31.9% to 34.7% overlap in the scores of the PCS group and the group without PCS (see Table 4). Therefore, 65.3% to 68.1% of participants classified with a PCS obtained scores on these measures that were not obtained by individuals without a PCS.
The Relationship of Opioid Analgesia on Psychological Symptoms and Cognitive Performance
Within the first 24 hours after trauma 92.6% of participants had been administered opioid analgesia to provide acute pain relief. A point-biserial correlation found a small but significant positive relationship with levels of depression rpb = .197, p = .05, anxiety, rpb = .27, p = .01, and stress, rpb = .25, p = .05 and opioid analgesia administered to participants within the first 24 hours of admission. No significant correlations were found, however, between the dissociative score, dissociative score without the dissociative amnesia item (dissociative–DA score), and ASD-total scores and opioid analgesia received by individuals within the first 24 hours.
On the day when individuals were screened neuropsychologically, 51.6% continued to receive opioid analgesia. These individuals, however, did not appear overtly sedated at the time. Further point-biserial correlations revealed a small but significant negative correlation between CLTR scores, rpb = −.210, p = .05, and WSRT–Total Recall, rpb = −.188, p = .05 and opioid analgesia administered on the day of assessment. No significant correlations were found, however, between WSRT–Delayed Recall, verbal fluency S, written and oral SDMT, Sequential RT1 and Sequential RT2, and opioid analgesia. There was also no significant relationship revealed between depression, anxiety, stress, dissociative score, dissociative–DA score, ASD-Total, and the administration of opioid analgesia at the time of assessment.
DISCUSSION
To further understand the relationship of psychological and cognitive factors in the development of the early PCS, the association between PCS and neuropsychological and psychological outcome was investigated in an acutely hospitalized group of individuals. They had undergone assessment on the PCSC and neuropsychological and psychological measures approximately 5 days after mTBI. Fatigue was the most frequently endorsed symptom, followed by dizziness, anxiety, headache, and irritability. Fatigue, anxiety, and irritability have been described as late developing psychologically mediated PCS symptoms (Karzmark et al., 1995; Lidvall et al., 1974; Rutherford, 1989). The current data show these symptoms are present a few days after mTBI. Consistent with previous research (King et al., 1995; Levin et al., 1987a; Ponsford et al., 2000) this study also found early (neurological) symptoms (dizziness and headache) to be frequently endorsed (Karzmark et al., 1995; Lidvall et al., 1974; Rutherford, 1989).
Based on ICD-10, subjective symptom complaints of PCS, 48% of participants were classified as having a current PCS. Few studies have examined the incidence of PCS, using ICD-10 symptom criteria, acutely following mTBI. Mittenberg and Strauman (2000) reported 38% of individuals met ICD-10 symptom categories for PCS around 6 weeks post-injury. At 6 months the incidence of persistent PCS was 28% (Mittenberg & Strauman, 2000), and in males, on average 8 years after mTBI, 22% (Luis et al., 2003). Apart from verbal fluency, participants with a PCS did not differ in their performances on neuropsychological measures, in comparison to those without a PCS. When effect sizes were calculated, they ranged from no effect on list-learning and Sequential Reaction Time 2, to small effect sizes for total- recall and delayed-recall scores of the WSRT, auditory and visual-motor response speed of the SDMT, and Sequential Reaction Time 1. A medium effect size was found on verbal fluency; however the shared overlap on this test indicated that a number of participants in both groups obtained similar scores. In contrast to the current findings, large effect sizes have been reported on composite measures of cognitive function (reaction time, memory) according to whether or not high-school athletes were experiencing a single PCS symptom, headache (Collins et al., 2003), and fogginess (Iverson et al., 2004), approximately 7 days after a concussion.
Individuals classified with a PCS reported significantly more symptoms of depression, anxiety, and stress. They also endorsed significantly more symptoms of ASD. The large effect sizes present on all psychological measures show that the difference between participants with a PCS and without was greater on psychological than on neuropsychological measures. The largest effect sizes were found on the dissociative cluster and cumulative score of the reexperiencing, avoidance, and arousal clusters of the ASDS. The relatively small shared overlap on the ASD symptoms indicate that a large number of participants classified as having a PCS, obtained high ASD scores. In mTBI individuals, 14% are diagnosed with ASD. At 6 months, 25% meet criteria for PTSD (Bryant & Harvey, 1999).
The number of participants classified with a PCS may be inflated because some PCS symptoms overlap with psychological symptoms including those of ASD (anxiety, poor concentration, and irritability) (Harvey & Bryant, 1998). Nevertheless, a number of participants reported PCS symptoms that cannot be readily attributed to psychological distress. For instance, 39% of participants reported frequent dizziness and 30% reported headache. Because PTA overlaps directly with dissociative amnesia (Bryant & Harvey, 1999) analyses were performed with the dissociative amnesia item from the dissociative cluster of the ASDS included and without. Omitting this item did not reduce the effect size on this cluster. Whether the remaining four symptoms (numbing, a reduction in awareness of surroundings, depersonalization, and derealization), or individual symptoms of the dissociative cluster (e.g., numbing, depersonalization), overlap with PTA/TBI requires investigation (Grigsby & Kaye, 1993; Jones et al., 2005). Further research is needed to determine whether dissociative symptoms are related to the severity and localization of brain trauma.
Small but significant associations found between opioid analgesia and depression, anxiety and stress may reflect emotional arousal because of the sensory experience of pain (Jensen & Karoly, 2001). Assessing pain intensity would allow the relationship between pain, emotional arousal, and PCS symptoms to be further explored (Iverson & McCracken, 1997; Jensen & Karoly, 2001). The small but significant correlation evident between opioid analgesia and list-learning and total recall are consistent with previous report of reduced learning due to opioids (Zacny, 1995). There was no association found between delayed recall and opioids. If opioid dosages had been converted to morphine based equivalents rather than recording whether they were present or absent, they may have been a more sensitive measure of memory effects. Documenting whether sedatives and anaesthesia were administered may also be important to control for secondary cognitive and psychological effects (O'Donnell et al., 2003; Kaoua et al., 2002).
It is important to acknowledge the limitations of the study. Participants were clinic referrals, and many suffered extensive orthopedic and other non-central nervous system injuries; therefore they may have been more symptomatic. There was no comparison group of non-brain injured acutely hospitalized individuals to control for the effect of admission and trauma on self report of PCS symptoms (Dikmen et al., 2001; McCauley et al., 2001a). Symptoms associated with a PCS are not specific to mTBI and high base rates have been found in the general population and in other clinical groups (Iverson & McCracken, 1997; Luis et al. 2003; Kashluba et al., 2006). Including a measure of injury severity and other information such as the presence of skull fracture, may have been found to be related to the reported frequency of PCS symptoms (Carroll et al., 2004b; Williams et al., 1990). Because of privacy restrictions alcohol levels at the time of injury were not able to be recorded; therefore any effects of alcohol on the estimated duration of PTA are unknown (Forrester et al., 1994).
Finally, although these results require replication, the findings suggest that psychological factors may contribute at an earlier stage than has been considered in the development of the PCS. Cognitive function during the acute stage of mTBI bore little relationship to subjective dysfunction measured according to whether participants had a PCS or not. The constellation of symptoms may change when the individual returns to work and daily life. The expression of cognitive symptoms may increase when environmental demands surpass the individual's cognitive capacity (Binder, 1986; Rutherford 1989; van Zomeran & van den Burg, 1985). Future research may further investigate whether estimates of neurological function such as light and sound tolerance (Bohnen et al., 1991; Waddell & Gronwall, 1984) may have a relationship in the development of the PCS. Whether psychological factors contribute to or are able to predict those who develop a persistent PCS requires further study. These questions will be addressed with data from a prospective study that is in progress.
ACKNOWLEDGMENTS
The information contained in this manuscript and the manuscript itself is new and original and is not currently under review by any other publication. This information has never been published either electronically or in print. This work was supported by a Motor Accidents Authority Grant Ref: 04/247. The authors have no financial or other relationships that could be regarded as a conflict of interest. The authors thank Dr Alan Taylor for his advice and assistance with statistical methods, Ms Peta Minton and Ms Susan van den Berg for assistance with data entry, the Intern Clinical Neuropsychologists from Macquarie University, whose assessments contributed to the data used in this study, and Ms Alexandra Walker for her comments on the manuscript.






