Introduction
Schizophrenia Spectrum Disorders
Schizophrenia spectrum disorders (SSD) are debilitating psychiatric illnesses that primarily comprise positive symptoms, negative symptoms, and cognitive impairment. While positive symptoms encompass the psychotic components of SSD, negative symptoms reflect loss of function, such as affective flattening, social withdrawal, and avolition (Marder & Galderisi, Reference Marder and Galderisi2017). Furthermore, a diverse range of enduring deficits are prevalent amongst the SSD population, which exist for most non-social and social cognitive domains (Carruthers, Van Rheenen, Gurvich, Sumner, & Rossell, Reference Carruthers, Van Rheenen, Gurvich, Sumner and Rossell2019; Fett et al., Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam2011; Keefe & Harvey, Reference Keefe, Harvey, Geyer and Gross2012; Tan & Rossell, Reference Tan and Rossell2014; Van Rheenen et al., Reference Van Rheenen, Lewandowski, Tan, Ospina, Ongur, Neill and Burdick2017).
Research has shown that negative symptoms are related to impaired social functioning, while global cognition (comprising both social and non-social) has been found to predict the severity of deficits in everyday functioning (Strassnig et al., Reference Strassnig, Raykov, O’Gorman, Bowie, Sabbag, Durand and Harvey2015; Tan, Thomas, & Rossell, Reference Tan, Thomas and Rossell2014). Despite the substantial impact of negative and global cognitive symptoms on real-world outcomes (Fett et al., Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam2011; Halverson et al., Reference Halverson, Orleans-Pobee, Merritt, Sheeran, Fett and Penn2019; Harvey & Strassnig, Reference Harvey and Strassnig2012; Rabinowitz et al., Reference Rabinowitz, Levine, Garibaldi, Bugarski-Kirola, Berardo and Kapur2012), there are limited efficacious treatment options (Choi, Wykes, & Kurtz, Reference Choi, Wykes and Kurtz2013; Fusar-Poli et al., Reference Fusar-Poli, Papanastasiou, Stahl, Rocchetti, Carpenter, Shergill and McGuire2015). While antipsychotic medications are reasonably effective for the reduction of positive symptoms, both social and non-social cognitive deficits and negative symptoms often persist (Bobes, Arango, Garcia-Garcia, Rejas, & Group, Reference Bobes, Arango, Garcia-Garcia, Rejas and Group2010). Additionally, cognitive remediation therapy (CRT) is reliably effective in ameliorating global cognitive deficits amongst some individuals with SSD; however, a large proportion of SSD participants fail to realise significant cognitive benefits (e.g. Bryce et al., Reference Bryce, Rossell, Lee, Lawrence, Tan, Carruthers and Ponsford2018; Reser, Slikboer, & Rossell, Reference Reser, Slikboer and Rossell2019). Novel adjunct treatments, including antioxidant N-acetylcysteine (NAC; Yolland, Hanratty et al., Reference Yolland, Hanratty, Neill, Rossell, Berk, Dean and Siskind2019; Yolland, Phillipou et al., Reference Yolland, Phillipou, Castle, Neill, Hughes, Galletly and Rossell2018) and mindfulness (Khoury, Lecomte, Gaudiano, & Paquin, Reference Khoury, Lecomte, Gaudiano and Paquin2013; Louise, Fitzpatrick, Strauss, Rossell, & Thomas, Reference Louise, Fitzpatrick, Strauss, Rossell and Thomas2018), are under investigation as potential avenues for the treatment of negative symptoms (Dean, Giorlando, & Berk, Reference Dean, Giorlando and Berk2011; Rossell et al., Reference Rossell, Francis, Galletly, Harris, Siskind, Berk and Castle2016); however, these targeted treatments are in their infancy. Improved understanding of both social and non-social cognition and negative symptoms, and their relationships, will aid the development of targeted and efficacious treatment options.
Global Cognition and Negative Symptoms
There has been extensive debate as to whether negative symptoms and global cognition are interrelated in SSD (Addington, Addington, & Maticka-Tyndale, Reference Addington, Addington and Maticka-Tyndale1991; Harvey, Koren, Reichenberg, & Bowie, Reference Harvey, Koren, Reichenberg and Bowie2006; Hughes et al., Reference Hughes, Kumari, Soni, Das, Binneman, Sonia and Sharma2002). Harvey et al. (Reference Harvey, Koren, Reichenberg and Bowie2006) conducted an early review which concluded that overall, negative symptoms and global cognitive deficits are associated on a cross-sectional basis but are most likely separable domains of the illness.
Several studies have found no observable relationship between negative symptoms and global cognitive function, highlighting the possibility that they may be truly orthogonal domains of SSD (Altamura et al., Reference Altamura, Caletti, Paoli, Cigliobianco, Zugno, Grillo and Zago2015; Bagney et al., Reference Bagney, Dompablo, Santabarbara, Moreno-Ortega, Lobo, Jimenez-Arriero and Rodriguez-Jimenez2015; Bismark et al., Reference Bismark, Thomas, Tarasenko, Shiluk, Rackelmann, Young and Light2018; Teigset et al., Reference Teigset, Mohn, Brunborg, Juuhl-Langseth, Holmen and Rund2018). In contrast, a meta-analysis by Ventura, Hellemann, Thames, Koellner, and Nuechterlein (Reference Ventura, Hellemann, Thames, Koellner and Nuechterlein2009) and a systematic review conducted by Dominguez, Viechtbauer, Simons, van Os, and Krabbendam (Reference Dominguez, Viechtbauer, Simons, van Os and Krabbendam2009) concluded that negative symptoms had a moderate negative association with global cognition and non-social cognitive domains [i.e. attention (ATT), processing speed (PS), reasoning and problem solving (RaPS), verbal learning and memory (VerbL), visual learning and memory (VisL), and working memory (WM)]. There are also a number of individual studies that have found significant associations between negative symptoms and cognitive function in SSD (Couture, Granholm, & Fish, Reference Couture, Granholm and Fish2011; Dibben, Rice, Laws, & McKenna, Reference Dibben, Rice, Laws and McKenna2009; Gonzalez-Ortega et al., Reference Gonzalez-Ortega, de Los Mozos, Echeburua, Mezo, Besga, Ruiz de Azua and Gonzalez-Pinto2013; Huang et al., Reference Huang, Huang, Yu, Hu, Chen, Jin and Xu2016; Tanaka et al., Reference Tanaka, Tomotake, Ueoka, Kaneda, Taniguchi, Nakataki and Ohmori2012).
Social Cognition
A notable consideration here is the diversity of global cognitive function and the potential differences between social and non-social cognition in terms of their relationships to negative symptoms. Social cognition refers to a broad construct involving how people process information within social contexts and encompasses the domains of emotion perception, theory of mind (TOM), attribution style, and social processing (Penn, Sanna, & Roberts, Reference Penn, Sanna and Roberts2008; Van Rheenen, Ganella, Bauer, & Bartholomeusz, Reference Van Rheenen, Ganella, Bauer, Bartholomeusz, Lewandowski and Moustafa2019). Previous research suggests that social and non-social cognition are somewhat independent cognitive domains within SSD (Fanning, Bell, & Fiszdon, Reference Fanning, Bell and Fiszdon2012; Fett et al., Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam2011; Mehta et al., Reference Mehta, Thirthalli, Subbakrishna, Gangadhar, Eack and Keshavan2013; Sergi et al., Reference Sergi, Rassovsky, Widmark, Reist, Erhart, Braff and Green2007; van Hooren et al., Reference van Hooren, Versmissen, Janssen, Myin-Germeys, a Campo, Mengelers and Krabbendam2008). However, some studies have suggested a correlation between social and non-social cognition (Ventura, Wood, & Hellemann, Reference Ventura, Wood and Hellemann2013). In relation to negative symptoms, a further meta-analysis conducted by Ventura et al. (Reference Ventura, Wood and Hellemann2013) found that negative symptoms were detrimental to social cognition. Bell, Corbera, Johannesen, Fiszdon, and Wexler (Reference Bell, Corbera, Johannesen, Fiszdon and Wexler2013) found that negative symptoms correlated with some aspects of social cognition, namely TOM, but not others.
Negative Symptoms Sub-Domains
Past research has most commonly investigated the relationships between global negative symptoms and both social and non-social cognitive domains. However, literature suggests that within negative symptoms there may be two broad sub-domains; expressive deficits (ED), which consist of restricted affect and alogia items, and avolition-apathy (AA), comprising avolition and anhedonia-asociality (Strauss et al., Reference Strauss, Horan, Kirkpatrick, Fischer, Keller, Miski and Carpenter2013). In addition, Liemburg et al. (Reference Liemburg, Castelein, Stewart, van der Gaag, Aleman and Knegtering2013) had parallel findings, resulting in a two-factor structure of the negative symptoms of schizophrenia, encompassing an ED sub-domain and a social amotivation sub-domain, akin to AA. Strauss et al. (Reference Strauss, Horan, Kirkpatrick, Fischer, Keller, Miski and Carpenter2013) observed that SSD participants with high AA had significantly poorer social cognition than the SSD participants with high ED, whereas, Konstantakopoulos et al. (Reference Konstantakopoulos, Ploumpidis, Oulis, Patrikelis, Soumani, Papadimitriou and Politis2011) found that overall apathy score was associated with poorer executive function. Together, these findings suggest that specific facets of negative symptoms may have associations with both social and non-social cognition, although to our best knowledge, past studies have not investigated this in a single sample. Past research has most frequently adopted the global negative symptom subscale of the Positive and Negative Syndrome Scale (PANSS; Kay, Fiszbein, & Opler, Reference Kay, Fiszbein and Opler1987) to determine total negative symptoms. As such, previous literature has commonly treated negative symptoms as a single and uniform domain, whereas important distinctions may exist within this symptom cluster. Understanding the potential differences between the sub-domains of negative symptoms has important clinical implications when considering potential treatment and classification. A more in-depth exploration of specific negative symptom sub-domains would be possible through use of the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, Reference Andreasen1984a).
Having a greater understanding of the potential relationship between negative and social and non-social cognitive symptoms would clarify whether a shared underlying aetiology exists, or if they are orthogonal characteristics of the illness. This would improve classification of the symptom clusters in SSD and guide more specific treatment targets as novel interventions continue to evolve. Given the contradictory findings thus far, the nature of this relationship remains unclear. Past literature has commonly adopted one of two approaches to examine the relationship between global cognition and symptoms. The first involves defining clinical subgroups based on clinical cut-off scores (e.g. high vs. low, negative or non-negative) and investigating subgroup differences on measures of social and non-social cognition (Gold, Reference Gold2004; Greenwood, Landau, & Wykes, Reference Greenwood, Landau and Wykes2005; Greenwood, Morris, Sigmundsson, Landau, & Wykes, Reference Greenwood, Morris, Sigmundsson, Landau and Wykes2008). This method fails to take into account the true heterogeneity of clinical subgroups within SSD. Adopting a data-driven cluster analytic method to group closely related SSD participants on clinical symptoms would address this issue, as it allows for a more accurate representation of the symptom profiles within the specific sample. Additionally, cluster analysis would provide a clinically meaningful representation of the complexity of SSD symptom profiles, by simultaneously grouping participants on all clinical symptom domains (e.g. positive and negative subscales). The second, more common method, has been to explore the relationship between global negative symptoms and both social and non-social cognition, without consideration of potential differences between individual negative symptoms (Ahmed, Strauss, Buchanan, Kirkpatrick, & Carpenter, Reference Ahmed, Strauss, Buchanan, Kirkpatrick and Carpenter2018; Altamura et al., Reference Altamura, Caletti, Paoli, Cigliobianco, Zugno, Grillo and Zago2015; Dibben et al., Reference Dibben, Rice, Laws and McKenna2009; Tanaka et al., Reference Tanaka, Tomotake, Ueoka, Kaneda, Taniguchi, Nakataki and Ohmori2012; Ventura et al., Reference Ventura, Hellemann, Thames, Koellner and Nuechterlein2009).
Aims and Hypotheses
The present study had two aims. First, we sought to identify unique clinical subgroups within our sample through a data-driven sub-grouping technique. We aimed to investigate whether the individuals in our sample with the most prominent negative symptoms differed from those with more prominent positive symptoms on measures of both social and non-social cognition. Meta-analytic investigation has revealed that emotion processing is a key domain of social cognition that is markedly impaired in SSD, and one that requires substantially more research (Savla, Vella, Armstrong, Penn, & Twamley, Reference Savla, Vella, Armstrong, Penn and Twamley2013). Emotion management falls within emotion processing and requires further investigation. As such, social cognition was operationalised in the present study through an emotional intelligence and decision-making measure to assess emotion management, the Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT): managing emotions (Mayer, Salovey, Caruso, & Sitarenios, Reference Mayer, Salovey, Caruso and Sitarenios2003).
Exploratory cluster analysis has commonly been used to characterise the considerable cognitive variability within the SSD population by identifying homogenous subgroups of overlapping ability (see Carruthers, Gurvich et al., Reference Carruthers, Gurvich, Meyer, Bousman, Everall and Rossell2019; Van Rheenen et al., Reference Van Rheenen, Lewandowski, Tan, Ospina, Ongur, Neill and Burdick2017). Adopting this technique, it was predicted that distinct clinical symptom subgroups would emerge and enable a more accurate examination of the associations between negative symptoms and both emotion management and non-social cognition, when compared with predominantly positive symptoms, in contrast to the typically applied and less specific group-averaged approach. Past research that has utilised cluster analysis to identify clinical subgroups within SSD has resulted in somewhat varying cluster solutions, but reasonably consistently one severely negative subgroup has appeared, a predominantly positive subgroup, and often a mixed subgroup or subgroups (Dollfus et al., Reference Dollfus, Everitt, Ribeyre, Assouly-Besse, Sharp and Petit1996; Mohr et al., Reference Mohr, Cheng, Claxton, Conley, Feldman, Hargreaves and Neumann2004). In line with these past studies, it was hypothesised that a subgroup would emerge with most prominent negative symptoms. Given findings from Altamura et al. (Reference Altamura, Caletti, Paoli, Cigliobianco, Zugno, Grillo and Zago2015), Bell et al. (Reference Bell, Corbera, Johannesen, Fiszdon and Wexler2013), Sergi et al. (Reference Sergi, Rassovsky, Widmark, Reist, Erhart, Braff and Green2007), and Ventura et al. (Reference Ventura, Wood and Hellemann2013), it was predicted this subgroup would have lower emotion management than the other subgroups. Based on Dominguez et al. (Reference Dominguez, Viechtbauer, Simons, van Os and Krabbendam2009) and Ventura et al. (Reference Ventura, Hellemann, Thames, Koellner and Nuechterlein2009), it was also predicted that the most severe negative symptom subgroup would have poorer outcomes on other non-social cognitive domains. The design of the present study in utilising cluster analysis is advantageous as it allows for a clinically meaningful investigation of clinical subgroups which will accurately reflect the SSD sample.
Recent network analysis has suggested that differing negative symptom domains are more central to SSD (Strauss, Esfahlani et al., Reference Strauss, Esfahlani, Kirkpatrick, Allen, Gold, Visser and Sayama2019). There is currently minimal literature investigating the relationship between specific negative sub-domains with social and non-social cognition, as most have measured negative symptoms as a single construct. As such, the second aim was to conduct an exploratory investigation of these relationships across our sample. Given findings from Strauss et al. (Reference Strauss, Horan, Kirkpatrick, Fischer, Keller, Miski and Carpenter2013) and Konstantakopoulos et al. (Reference Konstantakopoulos, Ploumpidis, Oulis, Patrikelis, Soumani, Papadimitriou and Politis2011), it was predicted that the negative symptoms associated with AA, anhedonia-asociality, and avolition-apathy, would negatively correlate with emotion management and non-social cognition.
Methodology
Participants
Data from 87 schizophrenia and 43 schizoaffective disorder patients were obtained from the Cognitive and Genetic Explanations of Mental Illnesses (CAGEMIS) bio-databank. All participants were recruited from metropolitan-based outpatient and community clinics and had given prior informed consent for the analysis of their stored data. Clinical diagnosis was confirmed through the MINI-International Neuropsychiatric Interview for Schizophrenia and Psychotic Disorder Studies English Version 5.0.0. (Sheehan et al., Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller and Dunbar1998) or the Structured Clinical Interview for DSM-IV (SCID-IV; First, Spitzer, Gibbon, & Williams, Reference First, Spitzer, Gibbon and Williams1995). Inclusion criteria were the same for the bio-databank and the present study. All participants were fluent in English, between the ages of 18 and 63 years old, with an estimated premorbid intelligence quotient (IQ) >70 as scored by the Wechsler Test of Adult Reading (WTAR; Wechsler, Reference Wechsler2001). Participants with significant physical, visual, or verbal impairments, a known neurological disorder, or current substance abuse or dependence were excluded. All participants were on stable doses of antipsychotic medication for at least 8 weeks. All procedures contributing to this work complied with ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration (1975, as revised in 2008). Ethics approval was granted from The Swinburne Human Research Ethics Committee. Demographic and basic clinical information can be found in Table 1.
Table 1. Demographic summary.
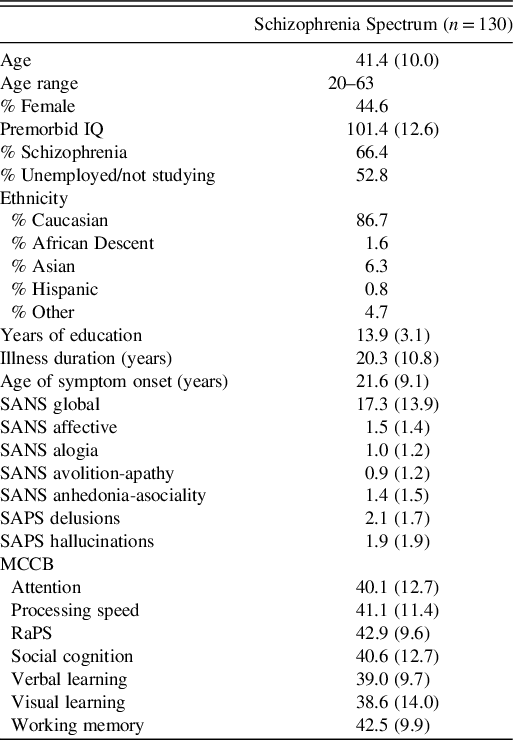
Note. Data were reported as mean (SD) unless stated otherwise; SANS: Scale for the Assessment of Negative Symptoms; SAPS: Scale for the Assessment of Positive Symptoms; MCCB: MATRICS Consensus Cognitive Battery; RaPS: Reasoning and Problem Solving
Materials
Clinical Symptoms
Clinical symptoms were assessed using the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, Reference Andreasen1984a) and the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, Reference Andreasen1984b). Given the nature of the research questions proposed, we elected to use the SANS and SAPS as they provide comprehensive and detailed symptom assessment for positive and negative symptoms. The SANS evaluates five major negative symptoms domains: alogia, affective blunting, anhedonia, avolition, and attentional impairment. The SAPS assesses five major positive symptoms: delusions, hallucinations, positive formal thought disorder, bizarre behaviour, and inappropriate affect. For the SANS and SAPS higher scores indicate greater symptom severity, ranging from 0 = “Not at all” to 5 = “Severe”.
Social and Non-social Cognition
The MATRICS Consensus Cognitive Battery (MCCB; Nuechterlein et al., Reference Nuechterlein, Green, Kern, Baade, Barch, Cohen and Marder2008) is a seven-domain cognitive battery that has been constructed to examine the major cognitive impairments reported in psychosis. It assesses ATT, PS, RaPS, VerbL, VisL, WM, and social cognition. The MCCB is a sensitive and valid measure of global cognition in schizophrenia and is relevant in terms of functional outcome (August, Kiwanuka, McMahon, & Gold, Reference August, Kiwanuka, McMahon and Gold2012; Nuechterlein et al., Reference Nuechterlein, Green, Kern, Baade, Barch, Cohen and Marder2008). Raw MCCB test scores were converted to age- and gender-corrected domain t-scores (μ = 50, SD = 10) that were derived from a normative sample of 300 healthy controls (Kern et al., Reference Kern, Nuechterlein, Green, Baade, Fenton, Gold and Marder2008). The MCCB assesses social cognition through a single emotional intelligent test, the MSCEIT: managing emotions. This is a paper-and-pencil multiple choice test which assesses participants’ emotion management in response to fictional scenarios. This emotion management test aims to assess 1) regulation of one’s own emotions in decision-making (self-management) and 2) incorporation of one’s own emotions with the emotions of others in decision-making that impacts others (social management; Mayer, Salovey, & Caruso, Reference Mayer, Salovey and Caruso2002). Non-social global cognition scores (GCS) were derived by calculating the mean t-scores for all non-social cognitive domains. Higher scores indicate better cognitive performance.
Statistical Analyses
Cluster analysis
Hierarchical cluster analysis (HCA) groups subjects with similar features into clusters, aiming to classify samples into more homogenous groups based on the variables of interest (Zhang, Murtagh, Van Poucke, Lin, & Lan, Reference Zhang, Murtagh, Van Poucke, Lin and Lan2017). Agglomerative clustering is a bottom–up approach, where individual cases start as single clusters and in a step-by-step process the most similar clusters are joined together, eventually resulting in one cluster which contains all cases (Clatworthy, Buick, Hankins, Weinman, & Horne, Reference Clatworthy, Buick, Hankins, Weinman and Horne2005). Several exploratory HCAs were performed in SPSS (v.26) using the SAPS and SANS to identify homogenous subgroups within the sample. The following global ratings were entered into the final subgrouping analysis from the SANS: affective flattening, alogia, anhedonia-asociality, and avolition-apathy; and the SAPS: delusions and hallucinations. Discriminant analysis determined that attentional Impairment (SANS), positive thought disorder (SAPS), and bizarre behaviour (SAPS) did not contribute significantly to the cluster analysis model and these variables were therefore removed hierarchically. Similarity between participants was calculated using hierarchical agglomerative clustering, with Ward’s minimum variance method and Squared Euclidean distance. Inspection of the agglomeration schedule (Supplementary Figure 1) and dendrogram (Supplementary Figure 2) was conducted independently by authors C.Y. and S.P.C. and checked for consistency, to establish the appropriate numbers of clusters to be retained and confirmed by discriminant function analysis. This method of visual inspection of the agglomeration schedule and dendrogram to determine total number of cluster groups is the conventional process adopted in HCA (Chan, Reference Chan2005; Schonlau, Reference Schonlau2004).
Next, a k-means iterative partitioning technique was conducted to optimise the retained clusters, with initial partitions in the k-means solution defined using the cluster means obtained from the initial cluster solution. The stability of the final cluster solution was assessed through split-sample and alternate method replication via Cohen’s κ analysis, with high agreement over multiple design iterations required to validate the final clustering solution obtained (κ > 0.8; Landis & Koch, Reference Landis and Koch1977). This method has previously been used to internally validate cluster solutions in SSD (Carruthers, Gurvich et al., Reference Carruthers, Gurvich, Meyer, Bousman, Everall and Rossell2019).
Analysis of variance
Emergent clusters were compared on demographic and clinical variables, and MCCB domain scores using analysis of variance (ANOVA). Past research has determined that IQ does not meet criteria for a covariate for neurodevelopmental disorders, except in cases where the group IQ is noticeably deviant from clinical expectations, and as such was not included in the present analysis (Dennis et al., Reference Dennis, Francis, Cirino, Schachar, Barnes and Fletcher2009). Brown-Forsyth F-ratio was used as a conservative approach to manage violations to tests of homogeneity of variances. Post-hoc p-values were Games-Howell corrected for unequal sample sizes/variances (Lee & Lee, Reference Lee and Lee2018), and Cohen’s d was used to establish effect size for pairwise comparisons (large effect, d > 0.8; Cohen, Reference Cohen1988).
Spearman’s correlations
The variables were not normally distributed and thus non-parametric Spearman’s correlation analyses were conducted to investigate the potential relationships between individual negative symptoms as measured by the SANS and social and global non-social cognition across the whole sample. Initial α was set at 0.05. With eight comparisons under investigation, we performed a Bonferroni correction for multiple comparisons, and the adjusted α was 0.006 (i.e. 0.05 divided by 8).
Results
Cluster Analysis
Four distinct clusters emerged from the exploratory HCA and were retained for k-means optimisation. Following this process, emergent clusters were determined to represent the following clinical subgroups: 1. “high hallucinations”; 2. low negative symptoms of affect and alogia, high everything else, including hallucinations, delusions, avolition-apathy, and anhedonia-asociality (“Mixed”); 3. “high negative symptoms”; and 4. “relatively asymptomatic” (see Supplementary Table 1; Supple-mentary Figures S1, S2, and S3). Supplementary Table 1 presents the SAPS and SANS scores between cluster subgroups. Table 2 presents the demographic characteristics of the four emergent subgroups. There were no significant differences between subgroups on demographic variables. The means plots of subgroup scores for SANS/SAPS can be found in Supplementary materials (S4, S5, S6, S7, S8, and S9).
Table 2. Demographic characteristics of emergent cluster subgroups.

Note. Data were reported as mean (SD) unless otherwise stated. aBrown-Forsythe F-ratio reported. bRemaining participants had a diagnosis of schizoaffective disorder. cYears since first symptom onset.
Analysis of Variance
Table 3 outlines the comparison of the MCCB domain t-scores across subgroups. There was a significant difference between clinical subgroups for emotion management at the p < 0.01 level. Overall, 10.7% of the variance in emotion management score was accounted for by clinical subgroup membership. Games-Howell post-hoc analysis revealed that the high negative subgroup scored significantly lower for emotion management than both the high hallucinations and relatively asymptomatic subgroups, but not the mixed subgroup (see Table 4). There were no further significant differences between subgroups for the non-social cognitive domains. Supplementary Table 1 presents comparisons of the emergent cluster subgroups on clinical symptom measures.
Table 3. Comparison of MCCB t-scores across emergent cluster subgroups.

Note. Data were reported as mean (SD) unless otherwise stated.
PS, processing speed; ATT, attention; WM, working memory; VerL, verbal learning; VisL, visual learning; RaPS, reasoning and problem solving; SC, social cognition.
Table 4. Post-hoc comparison of emergent cluster subgroups for social cognition.

Note. Group 1: high hallucinations; Group 2: mixed; Group 3: high negative; Group 4: relatively asymptomatic; Games-Howell p-values significant at the <0.05 level in bold.
Correlations
As can be seen in Table 5, significant negative correlations were observed between emotion management and SANS anhedonia-asociality and avolition-apathy, but not alogia or affective flattening. There were no significant associations between the SANS symptoms and global non-social cognition.
Table 5. Spearman’s correlations between SANS negative symptoms and cognition.

Note. Significant correlations in bold; SANS: Scale for the Assessment of Negative Symptoms; GCS: Global Composite Score of MCCB without social cognition; rs: Spearman’s rank order correlation coefficient. Differences in n due to missing data.
Discussion
The present study aimed to investigate the potential relationships between negative symptoms and both social and non-social cognitive function in SSD. Using exploratory cluster analysis, four clinical subgroups were identified. As hypothesised, the high negative subgroup (Cluster 3) scored significantly lower on emotion management than two other subgroups, which included high hallucination (Cluster 1) and relatively asymptomatic subgroups (Cluster 4). This is in line with past findings that have identified a relationship between negative symptoms and social cognition (Altamura et al., Reference Altamura, Caletti, Paoli, Cigliobianco, Zugno, Grillo and Zago2015; Lin et al., Reference Lin, Huang, Chang, Chen, Lin, Tsai and Lane2013; Strassnig et al., Reference Strassnig, Bowie, Pinkham, Penn, Twamley, Patterson and Harvey2018; Tan & Rossell, Reference Tan and Rossell2017; Ventura et al., Reference Ventura, Wood and Hellemann2013). However, there was no significant difference between the negative subgroup and the mixed subgroup (Cluster 2). The mixed subgroup had a complex collection of positive and negative symptoms. In particular, the high negative and mixed subgroups were matched in regard to high levels of anhedonia-asociality and avolition-apathy. The similarity between these two subgroups on key measures of negative symptoms may have contributed to the non-significant difference between the two subgroups in emotion management, suggesting that anhedonia-asociality and avolition-apathy may be associated with social cognitive function. This suggests that different clinical profiles may result in similar outcomes regarding social cognition, dependent on the presence of anhedonia-asociality and avolition-apathy components of negative symptoms, potentially regardless of the severity of other symptoms.
In contrast to our hypotheses, there were no significant differences between the subgroups on any of the non-social cognitive domains. Our results contrasted with the findings of Dominguez et al. (Reference Dominguez, Viechtbauer, Simons, van Os and Krabbendam2009) and Ventura et al. (Reference Ventura, Hellemann, Thames, Koellner and Nuechterlein2009), who found that negative symptoms were associated with impaired non-social cognition. However, the current findings are aligned with more recent studies reporting no significant relationships between negative symptoms and ATT, PS, RaPS, VerbL, VisL, or WM (Altamura et al., Reference Altamura, Caletti, Paoli, Cigliobianco, Zugno, Grillo and Zago2015; Bagney et al., Reference Bagney, Dompablo, Santabarbara, Moreno-Ortega, Lobo, Jimenez-Arriero and Rodriguez-Jimenez2015; Tan & Rossell, Reference Tan and Rossell2017). The disparity in the previous literature may arise from the fact that earlier studies have generally adopted the PANSS negative subscale as the primary measure of negative symptoms (Kay et al., Reference Kay, Fiszbein and Opler1987). It has been suggested that this subscale may include items that are closer to global cognitive dysfunction than negative symptoms, thereby inflating the observed relationship between negative symptoms and global cognitive impairment (Bagney et al., Reference Bagney, Dompablo, Santabarbara, Moreno-Ortega, Lobo, Jimenez-Arriero and Rodriguez-Jimenez2015). More recent studies have adopted the consistently validated five-factor model of the PANSS to identify negative symptom severity (Lehoux, Gobeil, Lefèbvre, Maziade, & Roy, Reference Lehoux, Gobeil, Lefèbvre, Maziade and Roy2009; Rodriguez-Jimenez et al., Reference Rodriguez-Jimenez, Bagney, Mezquita, Martinez-Gras, Sanchez-Morla and Mesa2013; van der Gaag et al., Reference van der Gaag, Cuijpers, Hoffman, Remijsen, Hijman, de Haan and Wiersma2006). It may be that the recent trend towards more advanced classification of negative symptoms has contributed to these varying findings.
The use of cluster analytic investigation has allowed the current study to identify clinical subgroups that are reflective of the prevailing symptoms in the present sample, in order to explore how individuals with predominantly negative symptoms differ from other SSD clinical presentations in terms of social and non-social cognition. The four distinct subgroups were reasonably clear in symptom presentation, with the exception of Cluster 2, which exhibited mixed symptoms across the negative and positive domains. While this makes interpretation of the clinical subgroups slightly more challenging, it accurately reflects the recognised heterogeneity of SSD, and the idea that individuals may not be easily classified as having either predominantly positive or negative symptoms. The present findings support the notion that social cognition, but not other non-social cognitive domains, may be related to negative symptoms in SSD. These findings suggest that social cognition is empirically separable from non-social cognitive domains, in terms of their relationship with negative symptoms (Fett et al., Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam2011; Sergi et al., Reference Sergi, Rassovsky, Widmark, Reist, Erhart, Braff and Green2007; van Hooren et al., Reference van Hooren, Versmissen, Janssen, Myin-Germeys, a Campo, Mengelers and Krabbendam2008).
These results are particularly pertinent when considering a potential hierarchy of global cognition. For example, Fanning et al. (Reference Fanning, Bell and Fiszdon2012) found that a significant proportion of 119 schizophrenia participants had intact non-social cognition and impaired social cognition, while only one participant had the reverse. This suggests that intact non-social cognition may be a necessary component that contributes to the potentially higher order processing of social cognition. These findings are also important when considering schizophrenia symptoms in terms of real world functioning. Bell et al. (Reference Bell, Corbera, Johannesen, Fiszdon and Wexler2013) grouped schizophrenia on both negative symptoms and social cognition to investigate the potential differences in community functioning. This study found that community functioning appears to be reliant on both the absence of substantial negative symptoms and intact social cognition, supporting the potential interaction between these symptom clusters.
The present study also conducted a follow-up analysis designed to investigate how individual negative symptoms may relate to social cognition. Both SANS anhedonia-asociality and SANS avolition-apathy were negatively correlated with social decision-making. As such, this hypothesis was supported in that the two negative symptoms related to the AA sub-domain were associated with impaired social cognition. This suggests that loss of motivation relates to social cognition, while ED may not. These findings were in contrast to those of Bell et al. (Reference Bell, Corbera, Johannesen, Fiszdon and Wexler2013) who found that SANS symptoms correlated with TOM but not the MSCEIT social decision-making task. The items associated with the avolition-apathy subscale of the SANS suggest that there may be a link between social cognition and aspects of self-care (in relation to grooming and hygiene), poor school or employment functioning, and a tendency to remain physically inert. Additionally, the findings suggest that individuals with superior social cognition may have improved motivation in regard to social relationships and activities. This is in line with recent suggestions from Pelletier-Baldelli and Holt (Reference Pelletier-Baldelli and Holt2020), who argue that negative symptoms may be the real-world consequences of impaired social cognition. It should be noted that while there are some aspects of the SANS, specifically in the anhedonia-asociality scale, which encompass elements of social functioning, overall the scale assesses motivation in day-to-day activities, as opposed to social cognition and processing. So, while these components of behaviour and social cognition may be related (and our findings suggest they are), they are not the same underlying constructs. Taken together, these findings suggest that the relationship observed between the AA sub-domain and social cognition may translate to real-world social functioning. It is suggested that future studies could adopt a real-world social functioning measure to investigate this potential relationship.
These findings also support the notion that negative symptoms are more complex than can be summarised in a single factor. Recent network analysis has even revealed that there may be up to a five-factor structure of negative symptoms (Strauss, Ahmed, Young, & Kirkpatrick, Reference Strauss, Ahmed, Young and Kirkpatrick2019; Strauss, Esfahlani et al., Reference Strauss, Esfahlani, Galderisi, Mucci, Rossi, Bucci and Sayama2019), which may lie under the broader two dimensions of AA and ED. In summary, considering negative symptoms as a single construct may hinder treatment efforts which may be more appropriately targeted to specific symptom sub-domains (Strauss, Esfahlani et al., Reference Strauss, Esfahlani, Galderisi, Mucci, Rossi, Bucci and Sayama2019).
In contrast to the hypotheses, there was no association between the negative symptom facets and non-social cognition. This is consistent with the findings from the above cluster analysis, indicating that the independence of negative symptoms and non-social cognition is consistent between clinical subgroups and across our sample as a whole.
These findings are relevant to understanding the aetiological nature of negative and both social and non-social cognitive symptoms in SSD and provide insight for potential treatment targets. Given the present study, it would be reasonable to suggest that negative symptoms and global cognitive impairment seem somewhat independent, and therefore would benefit from distinct interventions. However, given the association between specific negative symptoms and emotion management, improvements in social processing may arise from treatments designed to address negative symptoms. This would be an interesting avenue for future research. Indeed, Rossell et al. (Reference Rossell, Francis, Galletly, Harris, Siskind, Berk and Castle2016) are currently undertaking a large-scale randomised controlled trial investigating NAC as an adjunct treatment for schizophrenia, targeting negative symptoms. Given the findings of the present study, it would be interesting to see if NAC is also effective for improvement of social cognition.
Limitations and Strengths
The findings of the present study should be considered in light of its limitations. First, the cluster solutions identified were only internally validated, with no external independent validation available. As data-driven subgrouping methods are influenced by the variables entered and characteristics of the sample, external validation of the current subgrouping methodology is recommended. However, by adopting the cluster analytic method, the present study was able to investigate differences in both social and non-social cognition between clinically meaningful emergent clusters, rather than relying solely on subscale scores or individual item scores. Early meta-analytic investigation has shown that data-driven methods for classification of clinical groups generally provide superior reliability and validity, in comparison to diagnostic grouping (Grove, Zald, Lebow, Snitz, & Nelson, Reference Grove, Zald, Lebow, Snitz and Nelson2000). This has implications for clinical decision-making in terms of classification of symptoms. While there has been a tendency to move away from traditional subtypes of schizophrenia in the classification of symptoms, these data driven techniques suggest that clinically meaningful subgroups may still exist, but we could have previously been categorising symptom characteristics incorrectly. The present study is the first to our knowledge that has adopted this clinical clustering approach to investigate social and non-social cognitive function.
Second, the MSCEIT is the social cognition measured adopted in the MCCB, which assesses a specific aspect of social cognition, namely emotional intelligence through decision-making (Mayer et al., Reference Mayer, Salovey, Caruso and Sitarenios2003). Factor analysis suggests that emotional intelligence may consist of two broad components, emotion perception and emotion management, and that these may differ in terms of association with functional outcome (Eack et al., Reference Eack, Greeno, Pogue-Geile, Newhill, Hogarty and Keshavan2010; Lin et al., Reference Lin, Huang, Chang, Chen, Lin, Tsai and Lane2013). There has been ongoing commentary regarding the strengths and limitations of current measures of emotional intelligence and the MSCEIT (e.g. MacCann, Matthews, Zeidner, & Roberts, Reference MacCann, Matthews, Zeidner and Roberts2003; MacCann & Roberts, Reference MacCann and Roberts2008; Orchard et al., Reference Orchard, MacCann, Schulze, Matthews, Zeidner, Roberts, Parker, Saklofske and Stough2009; Palmer, Gignac, Manocha, & Stough, Reference Palmer, Gignac, Manocha and Stough2005). Overall, while the current study was limited to a single measure of social cognition, this emotion management task has been shown it to be one of the more promising empirical measures of emotional intelligence, and meaningfully linked with functional outcome in SSD (DeTore, Mueser, & McGurk, Reference DeTore, Mueser and McGurk2018; Roberts, Schulze, & MacCann, Reference Roberts, Schulze and MacCann2008). Nevertheless, it would be of benefit to explore these relationships utilising other social cognitive measures. Additionally, it would have been valuable to investigate the correlational relationships between negative symptom facets and all domains of non-social cognition rather than the overall composite score, although this was decided against due to the error inflation associated with multiple comparisons.
Furthermore, the design of the correlation analyses in the present study allowed for a deeper investigation regarding the specific facets of negative symptoms. Past research has suggested that combining these facets into the general domain of “negative symptoms” may result in the loss of valuable predictive information (Strauss et al., Reference Strauss, Horan, Kirkpatrick, Fischer, Keller, Miski and Carpenter2013). A further consideration is that the clinical diagnostic assessment was conducted using DSM-IV criteria. While there were minimal changes between the DSM-IV and DSM-V criteria for schizophrenia and schizoaffective disorder, this is worth considering when interpreting the current findings. Finally, consistent with most SSD research, the participants in our study were medicated at the time of testing, and medication effects must be considered when interpreting the present findings. Participants were treated with a combination of typical and atypical antipsychotic medication, in conjunction with other psychotropic medications.
Directions for Future Research
Given the findings from the present research, it would be beneficial for future studies to explore whether specific negative symptoms of SSD are related to non-social cognitive domains, rather than treating negative symptoms as a unitary construct. Additionally, exploration of these relationships in terms of other social cognition measures is suggested, as the present study can only draw conclusions in relation to emotion management, specifically. Furthermore, when investigating interventions for negative symptoms (e.g. NAC) it would be interesting to explore potential improvements in specific negative symptoms. Finally, future research could explore whether successful interventions for these negative symptoms also results in parallel improvement in social cognition.
Conclusion
In summary, the present research investigated the relationship between negative symptoms and cognition in SSD. First, a cluster analytic revealed the following subgroups: high hallucinations, mixed, high negative, and relatively asymptomatic. The negative subgroup was found to have significantly poorer emotion management than the high hallucinations and the relatively asymptomatic subgroups. No further differences between groups on measures of non-social cognition were identified. Follow-up correlation analyses revealed that this relationship between negative symptoms and emotion management appeared to be exclusive to the sub-domains of negative symptoms associated with motivation (such as anhedonia and apathy), but not affective flattening. These results suggest that negative symptoms could be potentially comprised of two sub-domains, differing in their association with social cognition, in terms of emotion management. Clinically, the findings imply that both social and non-social cognition and negative symptoms in SSD may need to be targeted independently and that sub-domains within negative symptoms may be important when considering treatment and classification within SSD. Overall, the results of the present study suggest that specific negative symptoms related to motivation are associated with social cognition in SSD, but not non-social cognitive domains.
Acknowledgments
The authors wish to acknowledge the contributions of study participants, staff at recruitment services, staff at the Centre for Mental Health, and staff at the Monash Alfred Psychiatry Research Centre (MAPrc), including Professor Jayashri Kulkarni. All authors contributed to and have approved the final version of the manuscript.
Funding
Participants for this study were sourced, in part, through the Cognitive and Genetic Explanations of Mental Illness (CAGEMIS) bio-databank, which is supported by the Australian National Health and Medical Research Council Project Grant (GNT1060664 awarded to SLR). SLR holds an NHMRC Senior Research Fellowship (GNT1154651); EJT (GNT1142424), CG (GNT0546262) and TEVR (1088785) hold NHMRC Early Career Research Fellowships. None of the funding sources played any role in the study design; collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Conflicts of interest
The authors have nothing to disclose.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1355617720001290







