Introduction
The maturation of white matter occurs throughout childhood and adolescence, and continues into adulthood (Casey, Giedd, & Thomas, Reference Casey, Giedd and Thomas2000; Giedd et al., Reference Giedd, Blumenthal, Jeffries, Castellanos, Liu, Zijdenbos and Rapoport1999; Hasan et al., Reference Hasan, Kamali, Iftikhar, Kramer, Papanicolaou, Fletcher and Ewing-Cobbs2009; Lebel & Beaulieu, Reference Lebel and Beaulieu2011; Lebel, Walker, Leemans, Phillips, & Beaulieu, Reference Lebel, Walker, Leemans, Phillips and Beaulieu2008; Mabbott, Rovet, Noseworthy, Smith, & Rockel, Reference Mabbott, Rovet, Noseworthy, Smith and Rockel2009; Paus et al., Reference Paus, Collins, Evans, Leonard, Pike and Zijdenbos2001; Suzuki, Matsuzawa, Kwee, & Nakada, Reference Suzuki, Matsuzawa, Kwee and Nakada2003; Verhoeven et al., Reference Verhoeven, Sage, Leemans, Van Hecke, Callaert, Peeters and Sunaert2010). This growth increases axonal signal conduction velocity and promotes efficient neural communication between cortical networks (Fields, Reference Fields2008; Helmuth, Reference Helmuth2001; Reed, Vernon, & Johnson, Reference Reed, Vernon and Johnson2004; Tsuda, Inoue, & Salter, Reference Tsuda, Inoue and Salter2005; Tsuda et al., Reference Tsuda, Shigemoto-Mogami, Koizumi, Mizokoshi, Kohsaka, Salter and Inoue2003; Ullian, Sapperstein, Christopherson, & Barres, Reference Ullian, Sapperstein, Christopherson and Barres2001). Such improvement in neural communication is considered a hallmark of brain development (Casey et al., Reference Casey, Giedd and Thomas2000). Despite its importance in neural communication, the role white matter growth may play in cognitive development has, until recently, received minimal attention. To test the functional role of white matter maturation in cognitive development we: (a) identified discrete white matter connections where age-related structural growth was evident in our sample of children and adolescents, and (b) tested whether the structure of connections mediated age-related improvements on a behavioural measure of information processing speed—visual motor reaction time.
Information processing speed is the general rate at which a person can complete cognitive operations. This core cognitive function supports higher-order functions including working memory (Ferguson & Bowey, Reference Ferguson and Bowey2005; Kail & Hall, Reference Kail and Hall2001), executive functioning (Drew, Starkey, & Isler, Reference Drew, Starkey and Isler2009; Nelson, Yoash-Gantz, Pickett, & Campbell, Reference Nelson, Yoash-Gantz, Pickett and Campbell2009), and verbal fluency (Bryan, Luszcz, & Crawford, Reference Bryan, Luszcz and Crawford1997; Luszcz, Bryan, & Kent, Reference Luszcz, Bryan and Kent1997). Age-related increases in information processing speed during childhood are robust, and predict intellectual development (Kail, Reference Kail2000; Kail & Park, Reference Kail and Park1994; Luciano et al., Reference Luciano, Wright, Geffen, Geffen, Smith and Martin2004). Reaction time paradigms measure the interval between presentation of a stimulus and onset of a voluntary response, and have successfully been used to investigate age-related changes in information processing speed (Collins & Long, Reference Collins and Long1996; Konrad, Vucurevic, Musso, Stoeter, & Winterer, Reference Konrad, Vucurevic, Musso, Stoeter and Winterer2009; Western & Long, Reference Western and Long1996). In adults, greater structural organization of white matter predicts improved speed of information processing (Konrad et al., Reference Konrad, Vucurevic, Musso, Stoeter and Winterer2009; Madden et al., Reference Madden, Whiting, Huettel, White, MacFall and Provenzale2004; Tuch et al., Reference Tuch, Salat, Wisco, Zaleta, Hevelone and Rosas2005; Turken et al., Reference Turken, Whitfield-Gabrieli, Bammer, Baldo, Dronkers and Gabrieli2008). Few studies have examined developmental relations in children. We have previously found that white matter maturation in a relatively large hemispheric area plays an important role in the development of visual information processing speed. Specifically, our findings implicated the right fronto-parietal region in performance of school-aged children on visual-motor tasks including pencil/paper visual search tasks (Mabbott, Noseworthy, Bouffet, Laughlin, & Rockel, Reference Mabbott, Noseworthy, Bouffet, Laughlin and Rockel2006) and a simple, visually cued finger movement (Dockstader, Gaetz, Rockel, & Mabbott, Reference Dockstader, Gaetz, Rockel and Mabbott2012). In recent studies, Tract-Based Spatial Statistics has been used to evaluate tissue microstructure, and show a relationship between white matter measures and variability in performance on reaction time tasks in typically developing children (Madsen et al., Reference Madsen, Baaré, Skimminge, Vestergaard, Siebner and Jernigan2011; Tamnes, Fjell, Westlye, Østby, & Walhovd, Reference Tamnes, Fjell, Westlye, Østby and Walhovd2012).
Our present goal is to identify discrete white matter connections related to the development of information processing speed in children and adolescents. Because discrete white matter tracts and pathways connect functional cortical regions, a focus on these structures is essential to advance our understanding of the role white matter maturation plays in cognitive development. Unlike TBSS, diffusion tensor imaging (DTI) tractography assembles consecutive estimates of fiber orientation to generate a continuous reconstruction of white matter connections. Hence, tractography is ideal to identify pathways linking functional cortical regions. We used tractography in a cohort of typically developing, school-aged children and adolescents to define four white matter tracts and/or pathways previously implicated in visual-motor function. Specifically, we conducted tractography of the optic radiations, inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, and the cortico-spinal tracts. We administered a reaction time task to both the dominant and non-dominant hands of study participants. Information processing speed reflected the time elapsed between presentation of visual stimulus (visual input) and finger abduction (motor output).
DTI generates quantitative metrics that index tissue structure based on water molecule displacement: in white matter, axon membranes and myelin act as barriers to random water diffusion. In the tensor model, each DTI metric is derived from vectors across three planes of directionality (λ1, λ2, λ3). Fractional anisotropy (FA) is a ratio ranging from 0 to 1 that is derived from λ1, λ2, and λ3 and indexes the primary direction of water diffusion: FA likely reflects axonal and myelin structure (Beaulieu, Reference Beaulieu2002; Song et al., Reference Song, Yoshino, Le, Lin, Sun, Cross and Armstrong2005). Mean diffusivity (MD) indexes the magnitude of water diffusion and is calculated as ([λ1 + λ2 + λ3]/3): MD is influenced by the volume of extracellular space, fiber packing and/or myelination (Beaulieu, Reference Beaulieu2002). Axial diffusivity (AD; also measured as λ1) and radial diffusivity (RD; calculated as ([λ2 + λ3]/2), reflect diffusion parallel and perpendicular to white matter pathways, respectively. AD is influenced by axonal membrane architecture and RD is considered to reflect myelin structure (Basser, Reference Basser1995; Song et al., Reference Song, Sun, Ramsbottom, Chang, Russell and Cross2002, Reference Song, Yoshino, Le, Lin, Sun, Cross and Armstrong2005). DTI tractography can be used to delineate specific white matter tracts/pathways from diffusion data. We define a tract as a discrete white matter connection where non-imaging anatomic evidence supports the existence of a bundle of axons having a common origin and termination. We define a pathway as a discrete white matter connection where non-imaging anatomic evidence of a bundle of axons having a common origin and termination is equivocal.
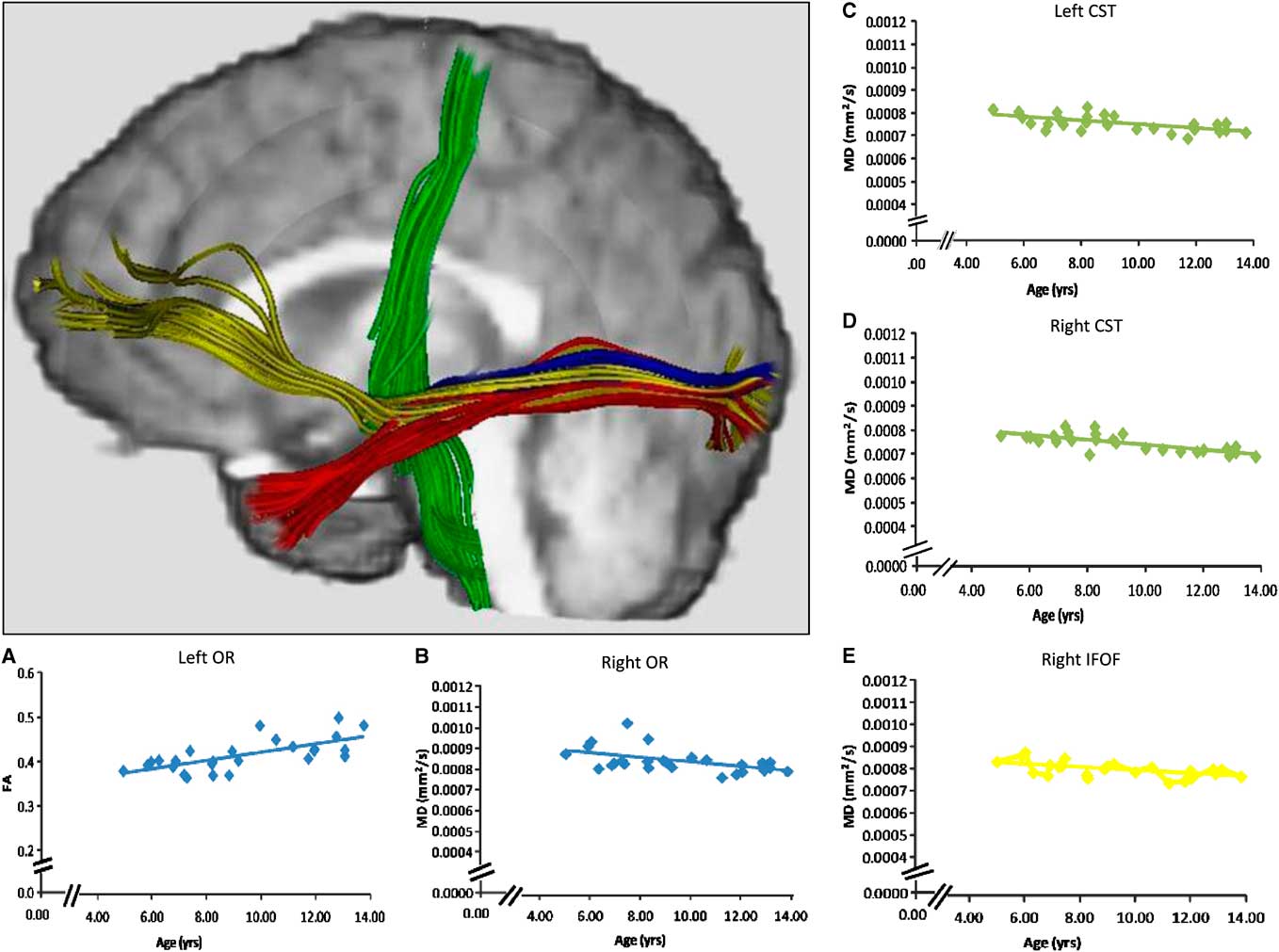
We examined the optic radiations because visual inputs are sent via this tract to visual cortex and hence it is important in visual motor responding (Figure 1). Imaging studies have described a pathway running between the ventro-medial occipital and orbito-polar frontal cortex as the inferior fronto-occipital fasciculus (Catani, Howard, Pajevic, & Jones, Reference Catani, Howard, Pajevic and Jones2002; Forkel et al., Reference Forkel, Thiebaut de Schotten, Kawadler, Dell'Acqua, Danek and Catani2012), and a tract connecting the occipital and the anterior temporal lobes as the inferior longitudinal fasciculus (Figure 1) (Catani, Jones, Donato, & Ffytche, Reference Catani, Jones, Donato and Ffytche2003). We examined the inferior fronto-occipital fasciculus and the inferior longitudinal fasciculus as they both project from the occipital lobe to other brain regions, and have been implicated in two functional paths—the dorsal and ventral streams (Loenneker et al., Reference Loenneker, Klaver, Bucher, Lichtensteiger, Imfeld and Martin2011; Urbanski et al., Reference Urbanski, Thiebaut de Schotten, Rodrigo, Catani, Oppenheim, Touze and Bartolomeo2008). Finally, because a motor response reflected completion of the reaction time task, we also examined the primary efferent tracts for voluntary motor function: the cortico-spinal tracts (Figure 1). We recognize that these white matter connections are not exhaustive in terms of visual-motor processing, but believe that they provide a solid foundation for examining relevant brain networks underlying information processing speed.

Fig. 1 Tractography of the optic radiations (blue), the inferior fronto-occipital fasciculus (yellow), and the inferior longitudinal fasciculus (red), each seeded from anatomical landmarks. The cortico-spinal tracts (green) were seeded from MEG activations. Probabilistic tractography was used to create regions of interest to calculate DTI measures, but streamline tractography was used on a representative subject in this image for visualization purposes. Age-related changes in FA and MD were observed for the optic radiations of the left and right hemispheres, respectively (scatterplots A, B). Age-related changes in MD were also detected bilaterally in the cortico-spinal tracts (scatterplots C, D), and in the inferior fronto-occipital fasciculus of the right hemisphere (scatterplot E).
We launched tractography of the optic radiations, inferior fronto-occipital fasciculus, and inferior longitudinal fasciculus using well established anatomical landmarks. The cortico-spinal tracts can be difficult to delineate using anatomical landmarks, however (Lebel & Beaulieu, Reference Lebel and Beaulieu2011). Hence, we launched tractography of the cortico-spinal tracts using magnetoencephalography (MEG) localized motor fields obtained during performance of the reaction time task. MEG measures magnetic fields generated by ionic currents moving through the brain, and spatially and temporally locates sources of neuronal activity. With recent innovations in source localization (Cheyne, Bakhtazad, & Gaetz, Reference Cheyne, Bakhtazad and Gaetz2006; Cheyne, Bostan, Gaetz, & Pang, Reference Cheyne, Bostan, Gaetz and Pang2007) MEG functional mapping is highly sensitive in localizing neural activation of the motor fields (Cheyne et al., Reference Cheyne, Bakhtazad and Gaetz2006; Gaetz & Cheyne, Reference Gaetz and Cheyne2006; Gaetz et al., Reference Gaetz, Scantlebury, Widjaja, Rutka, Bouffet, Rockel and Mabbott2010).
To account for the effects of development in examining brain/behavior relations, researchers often control for age when examining relations between DTI measures of white matter and behavioral performance. However, because white matter structure and age are correlated, simply controlling for age does not provide information on the specific contributions of a DTI measure in predicting development of performance. Age is a surrogate index of maturation and experience. A more robust approach is to test the mediating impact of DTI measures of white matter in predicting age-related changes in cognitive performance (Mabbott et al., Reference Mabbott, Noseworthy, Bouffet, Laughlin and Rockel2006). First, we used higher-order regression to identify specific connections showing age-related changes in DTI measures. We then used a second set of higher-order regression analyses to identify those where DTI measures also predicted reaction time. Only connections that showed age-related change and were related to reaction time were carried forward to primary analyses. In our primary analyses, we performed hierarchical regressions to predict reaction time. We compared the relative increase in the variance accounted for by the model when age was entered first, to that detected when DTI indices were entered first. If white matter maturation of discrete white matter connections involved in visual-motor function mediates the development of reaction time, then DTI indices from the connections selected through our prior analyses should reduce the contributions of age in predicting reaction time on our visual-motor task.
Experimental Methods
Participants
Twenty-seven typically developing children (14 males) ranging in age from 4.93 to 13.75 years old (mean age ± SD = 9.28 ± 2.63) were included. All were right-handed and underwent MEG mapping of motor cortex in combination with DTI. Subjects were recruited through community newspapers and parent networks. In addition to parent report, a review of participant medical records was performed to verify that participants were free from neurodevelopmental disabilities or injury, psychological disorders. The Research Ethics Board of The Hospital for Sick Children discourages the collection of sensitive personal health information that could result in identification of the participant if the data are not critical to the outcome of the study. Hence, race and socioeconomic status of the participants were not collected. Informed consent was obtained from parents before participation, as was verbal assent from the participants. This study conformed to the Tri-Council Ethics Principles, and was approved by the Research Ethics board of The Hospital for Sick Children.
MEG Recording and Task Parameters
Neuromagnetic activity was recorded using a whole-head 151 channel CTF MEG system (VSM MedTech, Ltd., Vancouver, Canada) in a magnetically shielded room. Before MEG data acquisition, each subject was fitted with three fiducial localization coils placed at the nasion and preauricular points to localize the position of the subject's head relative to MEG sensors. MEG data were collected continuously at a sample rate of 625 samples/sec and bandpassed from 0.3 to 200 Hz. Electromyograph (EMG) electrodes for each index finger were positioned using bi-polar electrodes placed on the first dorsal interosseous muscle with the reference to the associated muscle tendon. MEG recordings were performed with subjects lying supine on an adjustable bed with their eyes open and fixated on a semi-transparent screen placed at a distance of 55 cm from the participant's eyes. Participants were instructed to attend to the cross on the LCD screen and to abduct their right index finger as quickly as possible in response to a color change (from a white “+” to green, 200 ms duration) which occurred randomly every 3.5 to 4.5 s (see Figure 2). Approximately 100 visual responses and movements were captured for each MEG recording (approximately 400 s in total). Reaction time was defined as the latency between the appearance of the visual target and an abduction movement of left or right index finger by the participant, as measured by electromyogram (EMG) which recorded electric activity emanating from muscular contractions of the first dorsal interosseus of the right hand (EMGs localized at muscle belly and tendon). Triggers providing EMG onset markers were inserted into the raw MEG data during neuromagnetic recordings. Head movement was monitored for each data recording. Data were visually inspected and contaminated trials were removed before analysis. Less than 1% of trials were removed due to head movement artifacts. Upon completion of all MEG data collection, the MEG fiducial coils were replaced with MRI visible fiducial markers.
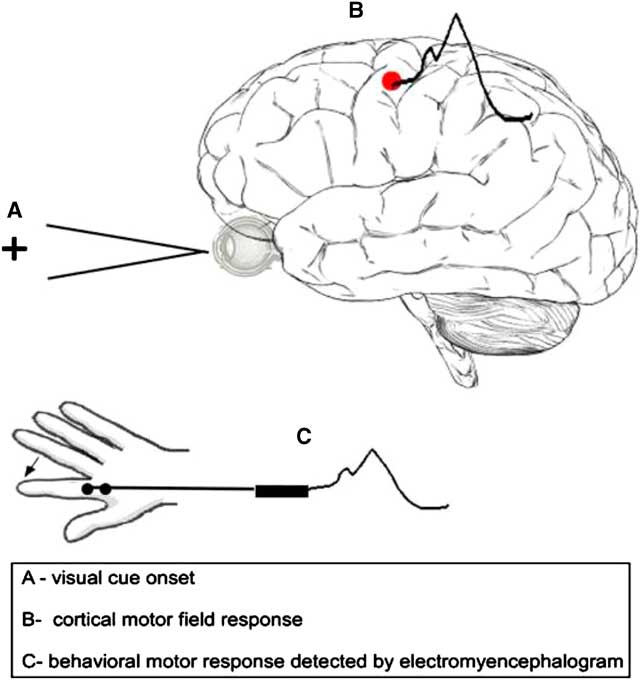
Fig. 2 The schematic depicts the reaction time task and corresponding motor measurements (cortical motor field response and EMG onset).
The event-related beamformer (ERB) (Cheyne et al., Reference Cheyne, Bostan, Gaetz and Pang2007) was used to localize the cortical responses known to accompany the transient finger movements, based on scalar beamformer algorithm (Cheyne et al., Reference Cheyne, Bakhtazad and Gaetz2006; Pang et al., Reference Pang, Drake, Otsubo, Martineau, Strantzas, Cheyne and Gaetz2008). The inner skull surface was used to create the multiple spherical head model. EMG onset was marked manually off-line and data re-epoched to −1.5 to 1 s with EMG onset as time = 0 ms after bandpass filtering from 0 to 30 Hz. Event-related images were computed with 2 mm sampling resolution, using time steps of 5 ms increments preceding and following movement onset (−0.1 s to 0.1 s) and scanned manually to observe peak activity. The motor field (MF) location was defined as the location of motor cortex activity observed at the time-point of maximum power around the time of finger abduction and marked on each subject's raw T1 volume for further DTI analysis. T1-weighted MR images were co-registered to MEG head coils sensors for spatial localization.
MRI Acquisition and Processing
MRI images were acquired with a GE LX 1.5 Tesla (T) scanner and eight-channel head coil. A T1-weighted image was collected using three-dimensional FSPGR (repetition time/echo time [TR/TE] = 8.6/4.2 ms; 122 contiguous axial slices; 1.5 mm thick; 256 × 192 matrix). Axial DTI images were acquired using a single shot spin-echo echo planar imaging DTI sequence (15 directions; b = 1000 s/mm2; TE/TR = 80/15,000 ms; 50 contiguous axial slices; 2.5 mm isotropic; 128 × 128 matrix; field of view = 32 cm; rbw = 250 kHz; number of excitations [NEX] = 2). Sequences including 15 directions at 2 NEX may display some MR signal variation and physiological noise over time, but ultimately have high test–retest reliability across various white matter measures and structures (Wang, Abdi, Bakhadirov, Diaz-Arrastia, & Devous, Reference Wang, Abdi, Bakhadirov, Diaz-Arrastia and Devous2012). To overlay MEG information on the MRI of the subject's brain, three fiducial points (two pre-auricular; one nasion) were identified on MRI images and co-registered with fiducial information acquired during the MEG procedure. Processing included eddy current correction (FMRIB's Diffusion Toolbox – FDT v2.0) to resolve spatial distortion. All images were visually inspected for motion artifact: Less than 1% of images were removed due to head movement artifacts.
Tractography
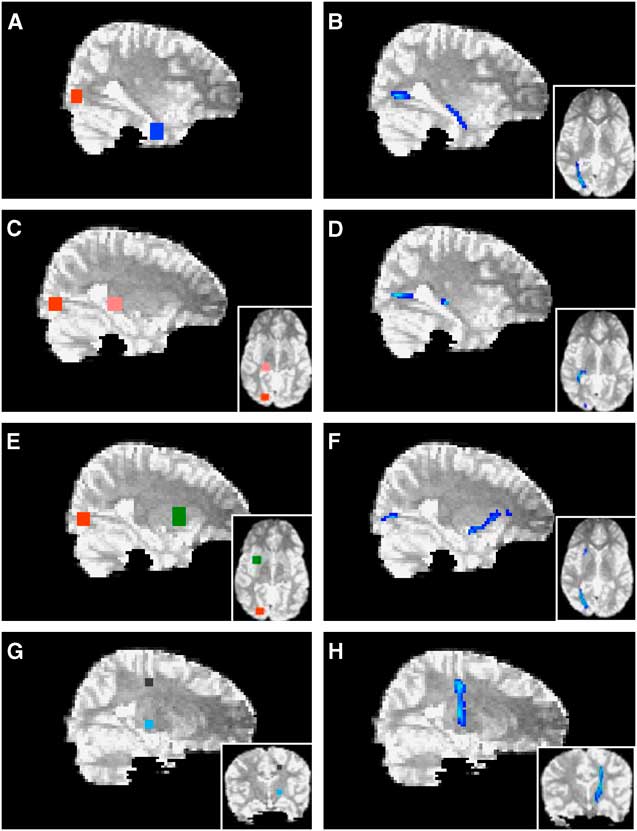
Probabilistic tractography was performed using Probtrackx (Behrens et al., Reference Behrens, Johansen-Berg, Woolrich, Smith, Wheeler-Kingshott, Boulby and Matthews2003) to delineate tracts and pathways. The cortico-spinal tracts were seeded from a region of interest (ROI) determined by MEG localization of the motor field. Represented as a 9-voxel cluster, peak activity of finger movement from MEG was localized on the T1 image (0.9375 mm slices) and served as a reference for seeding cortico-spinal tracts. This cluster was expanded across four slices in the Z-axis to create a volume large enough to accommodate reconstruction following registration to the lower resolution DTI space (2.5 mm slices). As MEG activations were localized within cortical grey matter, representative ROIs for tract-seeding were drawn in the nearest adjacent white matter orthogonal to the main axis of the respective gyrus. The volume was then non-linearly registered to the b = 0 image of the DTI sequence (Woods, Grafton, Watson, Sicotte, & Mazziotta, Reference Woods, Grafton, Watson, Sicotte and Mazziotta1998). The cortico-spinal tracts were then launched from a seed comprised of 27 voxels across two slices and tracked via the posterior arm of the interior capsule. Anatomical landmarks were used as seeds and waypoints for all other tracts and pathways (Figure 3). ROIs were drawn directly on the b = 0 image as 6 × 6-voxel clusters across five slices (total volume = 395 mm3). The lateral geniculate nucleus was used as a seed to delineate the optic radiations with a termination seed at the calcarine fissure. The calcarine fissure was used as a seed to delineate both the inferior fronto-occipital fasciculus and the inferior longitudinal fasciculus via the external capsule and temporal lobe white matter, respectively. All tracts and pathways were thresholded to eliminate non-robust probabilities by removing voxels within the lowest 10%. Quantitative measures (FA, MD, AD, and RD) were then extracted across each delineated tract or pathway.
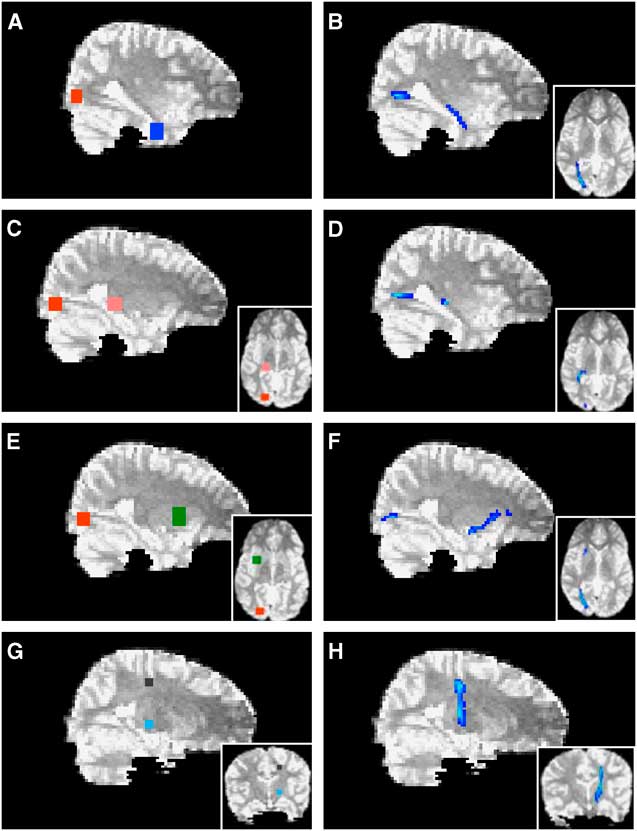
Fig. 3 Probabilistic tractography. The calcarine fissure (red in A, E) was used as a seed to delineate both the inferior longitudinal fasciculus (B) and the inferior fronto-occipital fasciculus (F) via temporal lobe white matter (blue in A) and the external capsule (green in E), respectively. The lateral geniculate nucleus (pink in C) was used as a seed to delineate the optic radiations (D) with a termination seed at the calcarine fissure (red in C). The cortico-spinal tracts (H) were launched from an MEG-derived seed (black in G) and tracked via the posterior arm of the interior capsule (light blue in G). Axial views are depicted in insets.
Statistics
We took several steps to reduce potential Type 1 error from multiple comparisons. First, we used a multi-step analytic approach where relevant measures were identified from an initial set of higher order regression analyses, and only these measures were carried forward to our primary hierarchical regression analyses. Second, within the initial regression analyses, only DTI metrics with significant age effects were carried forward to subsequent analysis thus reducing the number of comparisons. Furthermore, in all initial analyses, RD and AD were only investigated if the critical p value for relations of the same tract was less than .01 (as per Lebel and Beaulieu, Reference Lebel and Beaulieu2011). Finally, for our initial analyses (total of 58 comparisons) we enforced a standard false discovery rate (FDR) correction for multiple statistical comparisons, using the conventionally accepted false-positive rate of 5% (q ⩽ 0.05) (Benjamini & Hochberg, Reference Benjamini and Hochberg1995; Bennett, Wolford, & Miller, Reference Bennett, Wolford and Miller2009).
Initial analyses
Higher-order regression analyses were conducted to predict individual differences in: (a) reaction time as a function of age, (b) DTI measures for each connection bilaterally as a function of age, and (c) reaction time as a function of DTI measures. Both linear and quadratic terms were fit to the model. If the quadratic term did not add significant unique variance to the model, only the linear term was interpreted. To confirm that reaction time was changing as expected in our cohort, age and reaction time data for each hand were included in the equation Reaction Time = β0 + β1Age + β2Age2 + ε. Second, age and FA/MD for each white matter connection were evaluated in the equation FA (or MD) = β0 + β1Age + β2Age2 + ε. As noted above, only connections where a significant relationship with age was present in either FA or MD were carried forward to the next regression analyses. Reaction time for each hand and FA/MD were then included in the equation Reaction Time = β0 + β1FA + β2FA2 + ε (or MD).
Primary analysis
Only those white matter connections that showed (1) age-related change, and (2) a significant relationship with reaction time, were carried forward into the primary analyses. Multiple hierarchical regression analyses were used to predict reaction time for each hand separately. Specifically, to test the mediating impact of white matter maturation on the development of processing speed, we compared the relative increase in the variance accounted for by the model when age was entered first, versus when DTI indices were entered first. If multiple DTI measures selected from our initial analyses were related to reaction time, then these indices were entered together in a stepwise manner in the model(s).
Results
Motor Field Localization
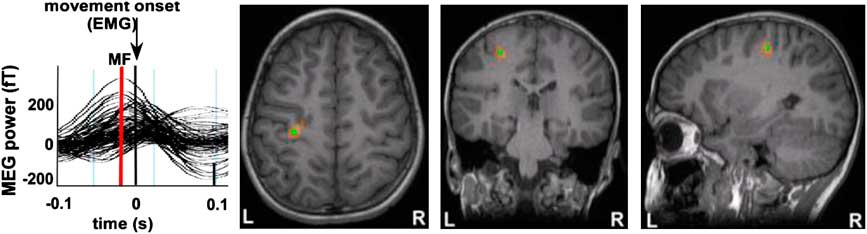
All participants showed activation in the hemisphere contralateral to the side of movement. Activations were localized to the region of the anatomically defined hand area or “knob” (Yousry et al., Reference Yousry, Schmid, Alkadhi, Schmidt, Peraud, Buettner and Winkler1997) (Figure 4). Activity reached maximal amplitude in the pre-central gyrus and occurred just before movement onset (between 20 and 80 ms). This point of maximal amplitude was defined as the motor field peak. In all participants, there was also significant activity localized to the motor knob of the ipsilateral pre-central gyrus for both right and left finger movements. Contralateral and ipsilateral source localizations were used as launch points for DTI tractography analyses.

Fig. 4 Motor Field (MF) responses are shown for a representative participant. Source wave forms from peak MF locations were observed following right index finger abduction movements for each individual and show an increase in source power (MF, red line) before EMG onset at time = 0 s. Source localization of these MF peak latencies were superimposed on each subject's MRI and were clearly situated in the contralateral pre-central gyrus (motor “knob”) for each individual. Using these methods, MF locations from primary motor cortex were used as seed points for DTI tractography for each participant in our study.
Initial Analyses
Age and reaction time
Mean reaction time across all subjects was 315 ± 121 ms for the right hand and 357 ± 177 ms for the left hand. There was a linear decrease in reaction time with increasing age for the right hand (R 2 = 0.22; F = 6.473; Critical p = .018; FDR q = 0.05). The quadratic term did not contribute a significant amount of additional variance (p = .36). There was a curvilinear decrease in reaction time of the left hand with increasing age (R 2 = 0.44; F = 8.86; Critical p = .003; FDR q = 0.02). The outcome of both regression models suggested that, as expected, reaction time for each hand decreased with age in our cohort.
Age and white matter change
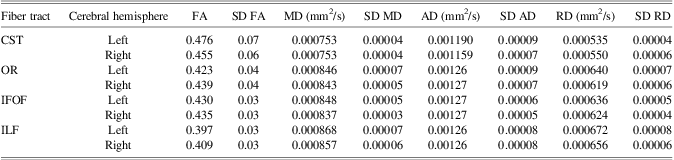
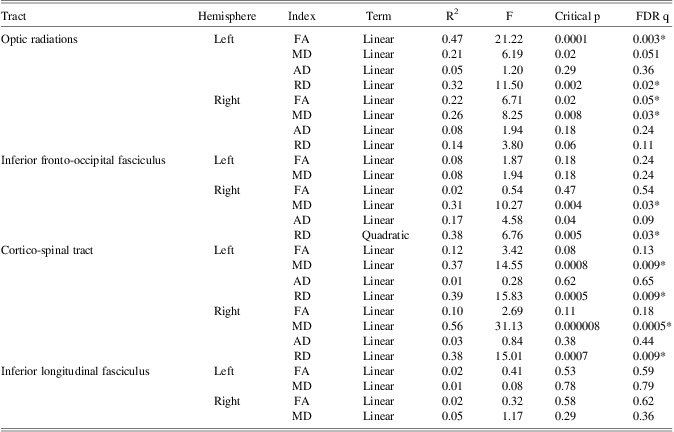
Mean and standard deviations for FA, MD, RD, and AD of each white matter connection are reported in Table 1. FA and RD of the optic radiation in the left hemisphere, and FA and MD of the optic radiation in the right hemisphere changed significantly with age in this cohort (Table 2 and Figure 1). Furthermore, MD and RD decreased with age in the inferior fronto-occipital fasciculus of the right hemisphere, and bilaterally in the cortico-spinal tracts (Table 2 and Figure 1). The quadratic term for the RD of the inferior fronto-occipital fasciculus of the right hemisphere was the only variable that added unique variance beyond that of the linear term. As no age-related changes were apparent in the inferior fronto-occipital fasciculus of the left hemisphere and bilaterally in the inferior longitudinal fasciculus, these connections were not considered in further analyses.
Table 1 Mean DTI measures for each tract as a function of tract and hemisphere.

IFOF = inferior fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus; OR = optic radiation; CST = cortico-spinal tract; SD = standard deviation
Table 2 Higher-order modeling: Age-related changes in DTI measures of the tracts

*q ⩽ .05.
Reaction time and white matter change
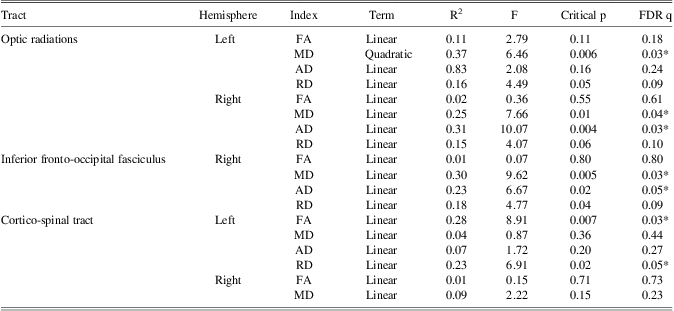
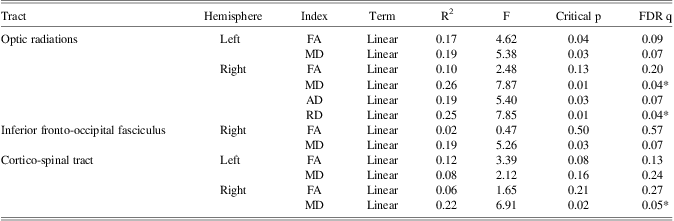
FA and RD of the cortico-spinal tracts in the left hemisphere and MD and AD of the inferior fronto-occipital fasciculus of the right hemisphere showed a significant relationship with reaction time of the right hand (Table 3a). Furthermore, AD and MD of the optic radiations in the right hemisphere, and MD of the optic radiations in the left hemisphere were also significantly related to reaction time of the right hand (Table 3a). Only the quadratic term for MD of the optic radiation in the left hemisphere added unique variance beyond the linear term. Finally, MD and RD of the optic radiations in the right hemisphere and MD of the right cortico-spinal tract showed a significant linear relationship with reaction time of the left hand (Table 3b).
Table 3a Higher-order modeling: DTI measures of age-related tracts in relation to reaction time of the right hand

*q ⩽ .05.
Table 3b Higher-order modeling: DTI measures of age-related tracts in relation to reaction time of the left hand

*q ⩽ .05.
Primary analyses of the effects of age and white matter structure on reaction time
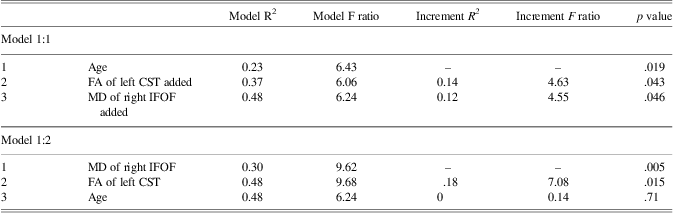
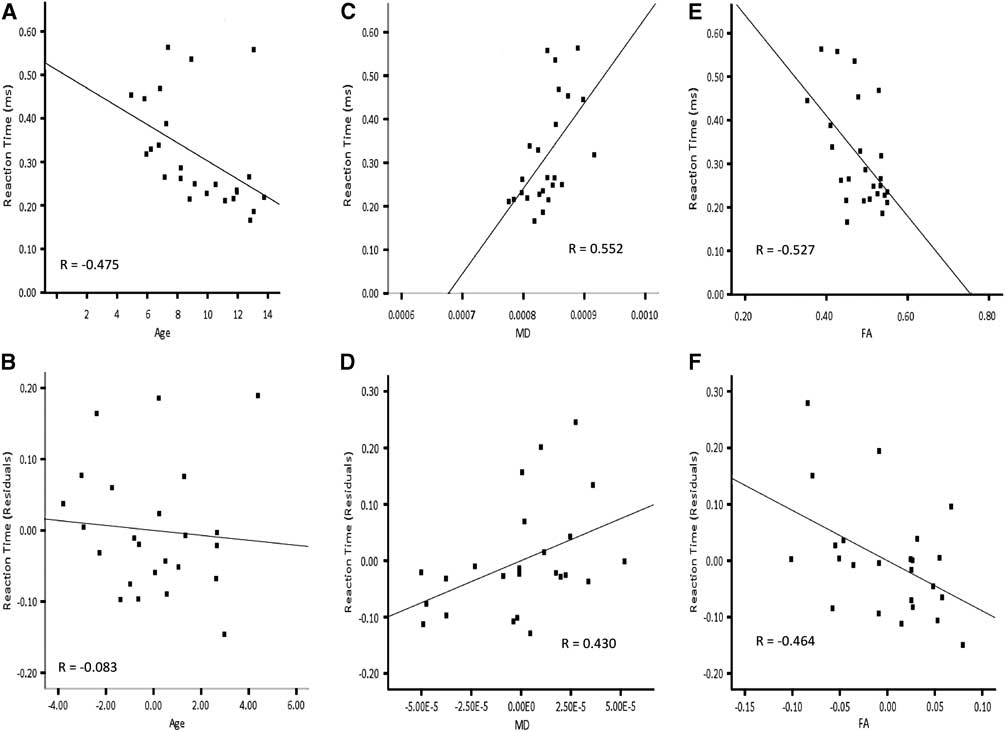
Based on higher-order regression analysis, the structure of the cortico-spinal tracts in the left hemisphere, the inferior fronto-occipital fasciculus in the right hemisphere and the optic radiations bilaterally showed age-related changes, and also predicted reaction time. Hence, these connections were included in the final model predicting right hand reaction time. The linear term for age was included in the hierarchical model. The linear terms for FA of the cortico-spinal tract of the left hemisphere, MD of the inferior fronto-occipital fasciculus of the right hemisphere, MD of the optic radiations in the right hemisphere and the quadratic term for MD of the optic radiations in the left hemisphere were included together in a stepwise manner. AD of the optic radiation in the right hemisphere was not carried forward to the primary analyses to avoid collinearity with MD. Age accounted for a significant amount of the variance observed for reaction time of the right hand when entered into the regression model first (Table 4a; Model 1:1). Adding FA of the cortico-spinal tract in the left hemisphere and MD of the inferior fronto-occipital fasciculus of the right hemisphere to the model in a stepwise manner significantly increased the amount of predicted variance in reaction time by approximately 26%. Conversely, there was no significant increment in predicted variance when age was entered into the model after FA of the cortico-spinal tract in the left hemisphere and MD of the inferior fronto-occipital fasciculus of the right hemisphere (Table 4a; Model 1:2). The striking relation between age and reaction time of the right hand was no longer apparent after accounting for FA of the cortico-spinal tract in the left hemisphere and MD of the inferior fronto-occipital fasciculus in the right hemisphere (Figure 5). On the other hand, the relation between reaction time of the right hand and FA of the cortico-spinal tract in the left hemisphere remained evident after accounting for age and MD of the inferior fronto-occipital fasciculus in the right hemisphere. Moreover, the correlation between reaction time of the right hand and MD of the inferior fronto-occipital fasciculus in the right hemisphere was still apparent after accounting for age and FA of the cortico-spinal tract in the left hemisphere. DTI indices from the optic radiations did not contribute unique variance and were not maintained in either model.
Table 4a Hierarchical regression modeling EMG reaction time for the right hand with age and DTI measures of the right inferior fronto-occipital fasciculus and the left cortico-spinal tract

IFOF = inferior fronto-occipital fasciculus; CST = cortico-spinal tract.
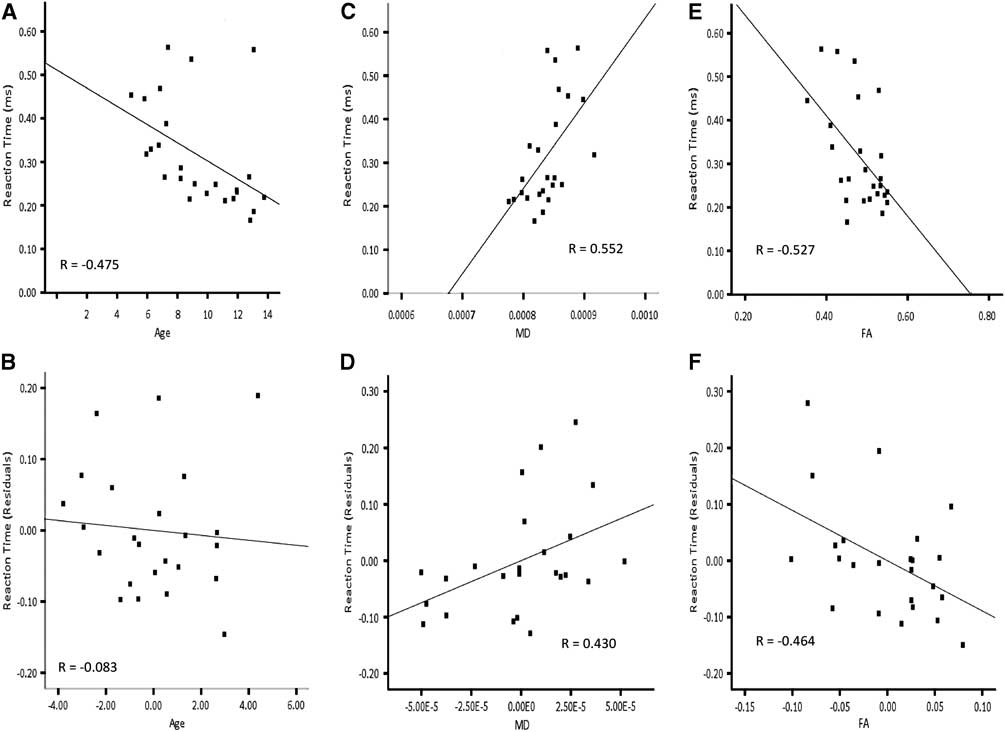
Fig. 5 Correlations for right hand reaction time and residuals as a function of age, MD of the right inferior fronto-occipital fasciculus and FA of the left cortico-spinal tract. Note that the correlation between age and reaction time (A) is not apparent in after accounting for MD of the right inferior fronto-occipital fasciculus and FA of the left cortico-spinal tract (B). The correlation between MD of the right inferior fronto-occipital fasciculus and reaction time (C) remains after accounting for age and FA of the left cortico-spinal tract (D). The correlation between FA of the left cortico-spinal tract and reaction time (E) is still evident after accounting for age and MD of the right inferior fronto-occipital fasciculus (F).
Because of the curvilinear relation between age and reaction time of the left hand, the quadratic term for age was included in the final model predicting left hand reaction time. Furthermore, MD of the optic radiations and CST in the right hemisphere were included in a stepwise manner, considering the age-related changes in these tracts and that they predicted left hand reaction time. RD of the optic radiation in the right hemisphere was not included in the primary analyses to avoid collinearity with MD. Neither age nor MD of the optic radiation accounted for unique variance of left hand reaction time when the other was included in the model (Table 4b; Model 2). The fact that age and MD together account for so much variability in reaction time, but so little unique variability, suggests that the impact of these two variables on reaction time is difficult to separate statistically. MD of the right CST did not contribute unique variance and was not maintained in either model.
Table 4b Hierarchical regression modeling EMG reaction time for the left hand with age and MD of the right optic radiation

OR = optic radiation.
Discussion
We observed linear and non-linear age-related increases in the structural organization of the optic radiations, cortico-spinal tracts, and the inferior fronto-occipital consistent with the literature (Clayden et al., Reference Clayden, Jentschke, Muñoz, Cooper, Chadwick, Banks and Vargha-Khadem2012; Eluvathingal, Hasan, Kramer, Fletcher, & Ewing-Cobbs, Reference Eluvathingal, Hasan, Kramer, Fletcher and Ewing-Cobbs2007; Hasan et al., Reference Hasan, Kamali, Abid, Kramer, Fletcher and Ewing-Cobbs2010; Lebel & Beaulieu, Reference Lebel and Beaulieu2011; Lebel et al., Reference Lebel, Walker, Leemans, Phillips and Beaulieu2008; Paus et al., Reference Paus, Zijdenbos, Worsley, Collins, Blumenthal, Giedd and Evans1999; Schmithorst, Wilke, Dardzinski, & Holland, Reference Schmithorst, Wilke, Dardzinski and Holland2002; Taki et al., Reference Taki, Thyreau, Hashizume, Sassa, Takeuchi, Wu and Kawashima2012). Furthermore, we replicated the expected decrease in reaction time on a visual motor task with increasing age (Rose, Feldman, Jankowski, & Caro, Reference Rose, Feldman, Jankowski and Caro2002; Welford, Reference Welford1977). Few studies have investigated the mediating effects of white matter maturation in the development of information processing speed (Mabbott et al., Reference Mabbott, Noseworthy, Bouffet, Laughlin and Rockel2006; Madsen et al., Reference Madsen, Baaré, Skimminge, Vestergaard, Siebner and Jernigan2011; Tamnes et al., Reference Tamnes, Fjell, Westlye, Østby and Walhovd2012). We present novel findings that maturation of discrete white matter connections predicts the development of information processing speed beyond the contributions of age.
First, linear relations were detected between age and white matter measures for the cortico-spinal tracts bilaterally (MD, RD), the optic radiation in the right hemisphere (FA, MD), the optic radiation in the left hemisphere (FA, RD), and the inferior fronto-occipital fasciculus in the right hemisphere (MD, AD). Such linear relations are consistent with ongoing maturation of these connections across the modeled time period. Only RD of the right inferior fronto-occipital fasciculus showed evidence of a quadratic relationship with age. This finding suggests that changes in myelination of the right inferior fronto-occipital fasciculus may plateau after a given time point reflecting a deceleration in myelin change. That we observed both linear and quadratic relations indicates that different connections may show different patterns of age-related change. We do note the limitations of our sample size in detecting more complex age-related functions, however. Furthermore, we did not observe age-related changes in the inferior longitudinal fasciculus as have been documented with cohorts including a broader age range (Lebel & Beaulieu, Reference Lebel and Beaulieu2011). Without data collected from older adolescents, we were unable to investigate later stages of white matter maturation. If age-related changes in the inferior longitudinal fasciculus are more protracted, it is plausible that we would not detect these effects with our sample.
Second, linear relations were detected between reaction time and white matter measures for the cortico-spinal tract bilaterally (FA, MD, RD), the optic radiations in the right hemisphere (MD, AD, RD) and the inferior fronto-occipital fasciculus in the right hemisphere (MD, AD). There is generally a one to one relationship between white matter architecture of the connections we examined and reaction time. Only MD of the left optic radiation showed evidence of a quadratic relationship with reaction time, suggesting that reaction time plateaus at some point, even if the structure of the left optic radiation continues to change.
We found that in some cases, different white matter metrics were differentially related to task performance. As all of the DTI metrics are derived from three vectors and are highly related, caution must be used not to over-interpret differential findings across the metrics. With that caveat in mind, however, we make the following observations. FA of the cortico-spinal tract was related to right finger reaction time but MD of the same tract was not. It is plausible that individual differences in reaction time reflect subtle change in AD and RD of the cortico-spinal tract (as captured by FA) that are associated with relatively small increases in fiber coherence with age (Burzynska et al., Reference Burzynska, Preuschhof, Backman, Nyberg, Li, Lindenberger and Heekeren2010). In contrast, MD of the inferior fronto-occipital fasciculus was related to right finger reaction time but FA of the same tract was not. Here, it appears that individual reaction time is better predicted by the decrease in membrane permeability, creation of intracellular compartments and fiber re-organization that reduce diffusivity within the inferior fronto-occipital fasciculus with age than by structural differences that would yield differences in directionality of water displacement. Together, these findings suggest that while reaction time is associated with white matter maturation, it is related to various structural differences that vary across different white matter pathways.
Finally, FA of the cortico-spinal tract in the left hemisphere and MD of the inferior fronto-occipital fasciculus of the right hemisphere contributed uniquely beyond the effect of age in accounting for reaction time of the right (dominant) hand. Furthermore, age did not contribute significantly to reaction time when these connections were included in the model first. Identification of the cortico-spinal tract as a unique mediator of reaction time was not surprising given that a motor task was used in the study. Moreover, involvement of the left hemisphere is concordant with decussation of the motor tract before arrival at the spinal cord. Reaction time of the non-dominant left hand was related to white matter organization of the right cortico-spinal tract.
Based on human tractography studies (Catani et al., Reference Catani, Howard, Pajevic and Jones2002; Forkel et al., Reference Forkel, Thiebaut de Schotten, Kawadler, Dell'Acqua, Danek and Catani2012; Thiebaut de Schotten, Dell'Acqua F., Valabregue R., & Catani, Reference Thiebaut de Schotten, Dell'Acqua, Valabregue and Catani2012), the inferior fronto-occipital fasciculus projects posteriorly from the inferior and dorso-lateral frontal cortex to the temporal stem, and is thought to terminate in the inferior and medial occipital lobe and medial parietal lobes (Catani et al., Reference Catani, Howard, Pajevic and Jones2002; Thiebaut de Schotten et al., Reference Thiebaut de Schotten, Dell'Acqua, Valabregue and Catani2012). While evidence from a recent post-mortem human study supports the trajectory of the inferior fronto-occipital fasciculus as described in imaging analyses (Martino, Vergani, Robles, & Duffau, Reference Martino, Vergani, Robles and Duffau2010), tracing studies in the monkey brain have failed to replicate its posterior projections (Schmahmann & Pandya, Reference Schmahmann and Pandya2007, Reference Schmahmann and Pandya2009). It remains in question whether the inferior fronto-occipital fasciculus described in tractography reports represents a tract that is exclusive to humans or is perhaps a pathway consisting of multiple connected tracts. The disparity likely arises from differences in techniques used in human imaging versus animal tracing studies to define the inferior fronto-occipital fasciculus. More detailed analyses must be undertaken to address this issue conclusively (Forkel et al., Reference Forkel, Thiebaut de Schotten, Kawadler, Dell'Acqua, Danek and Catani2012). We acknowledge that the inferior fronto-occipital fasciculus as delineated in our study may consist of either a single tract or several segments of axons connecting different brain areas that communicate. Despite this uncertainly, it is interesting to note that the fronto-occipital fasciculus has been documented as a long association system of the dorsal visual stream (Schmahmann & Pandya, Reference Schmahmann and Pandya2009) and we show MD of the inferior fronto-occipital fasciculus in the right hemisphere contributed to variance in a visually based reaction time task beyond that accounted for by age. This finding refines previous work in demonstrating that reaction time may depend on white matter structure (Dockstader et al., Reference Dockstader, Gaetz, Rockel and Mabbott2012; Mabbott et al., Reference Mabbott, Noseworthy, Bouffet, Laughlin and Rockel2006), indicating that the efficiency of transfer of visual information along the inferior fronto-occipital fasciculus is particularly important.
Some limitations must be noted in our study. As motor output defined processing speed in our study, we used MEG to collect motor field activations and delineate the primary motor output pathway, the cortico-spinal tracts. Although we acknowledge that contributions to the response from other pathways exist, it was not feasible to collect all the activation points required to delineate those tracts from a single paradigm. As such, future studies will benefit from paradigms that can be used to collect activation points for visual input, motor preparation, and performance and information processing. Second, while the inferior fronto-occipital fasciculus and inferior longitudinal fasciculus have been implicated in visual processing and represent excellent candidates to influence reaction time, we are aware that other white matter tracts/pathways exist that may also contribute to reaction time. For instance, the superior longitudinal fasciculus may be involved in focusing attention in visual space (Makris et al., Reference Makris, Papadimitriou, Sorg, Kennedy, Caviness and Pandya2007). Our sample was a relatively small cohort of right-handed participants and our findings must be interpreted in light of this limitation. Furthermore, the white matter-reaction time associations we documented in these children may not generalize to left-handed participants. Finally, images captured on a 3T scanner would have increased the signal-to-noise ratio and spatial resolution relative to those captured on the 1.5T scanner. Quantitative measures of motion during scanning would have provided added objectivity during image processing. Accordingly, an increase in field strength and the use of quantitative measures of motion should be considered in future studies.
Considering that we found that the structural organization of the cortical-spinal tract in the left hemisphere and the inferior fronto-occipital fasciculus of the right hemisphere increased with age and decreasing right hand reaction time, our findings are consistent with the role of white matter growth as mediating age-related changes in this critical cognitive function. Our findings support white matter maturation as a mechanism for the development of basic cognitive functions with age (Mabbott et al., Reference Mabbott, Noseworthy, Bouffet, Laughlin and Rockel2006), and identify the cortico-spinal tracts and right inferior fronto-occipital fasciculus as neurobiological predictors of development in information processing speed.
Acknowledgments
We have no conflicts of interest to declare. This research was supported by grants from the National Cancer Institute of Canada, the Pediatric Oncology Group of Ontario and the Garron Family Cancer Center.