INTRODUCTION
With the development of interventions for dementia, it is becoming increasingly important in clinical practice to distinguish benign memory changes associated with normal aging from early manifestations of neurodegenerative disease. Although individuals with amnestic mild cognitive impairment (aMCI) have preserved general cognition as well as intact or minimal impairment of activities of daily living, they do show memory difficulties that are greater than those occurring with normal aging (Gauthier et al., Reference Gauthier, Reisberg, Zaudig, Petersen, Ritchie, Broich, Belleville, Brodaty, Bennett, Chertkow, Cummings, de Leon, Feldman, Ganguli, Hampel, Scheltens, Tierney, Whitehouse and Winblad2006; Petersen et al., Reference Petersen, Smith, Waring, Ivnik, Kokmen and Tangelos1997). Importantly, the conversion rate from aMCI to dementia is considerable (e.g., 80% over 6 years, Petersen et al., Reference Petersen, Smith, Waring, Ivnik, Tangalos and Kokmen1999), with the majority developing Alzheimer disease (AD) (Petersen, Reference Petersen2004).
It is possible that cognitive processes known to be impaired by early AD may also be affected even earlier, in individuals with aMCI. AD patients demonstrate rapid forgetting rates and impaired delayed recall, which are often evident early in disease progression (Locasio et al., Reference Locascio, Growdon and Corkin1995; Welsh et al., Reference Welsch, Butters, Hughes, Mohs and Heyman1992). Given that an increased susceptibility to memory interference has been identified as one cognitive mechanism underlying the memory difficulties in early AD (e.g., Cushman et al., Reference Cushman, Como, Booth and Caine1988; Davis et al., Reference Davis, Price, Kaplan and Libon2002, but see Multhaup et al., Reference Multhaup, Balota and Faust2003), individuals with aMCI may also be prone to memory interference. Proactive interference (PI) is evident when learning one set of materials interferes with the later learning of another set of materials, and retroactive interference (RI) is evident when memory for the original set of materials is disturbed by having learned the new set of materials.
From a clinical perspective, the California Verbal Learning Test (CVLT; Delis et al., Reference Delis, Kramer, Kaplan and Ober1987) is frequently used to quantify episodic memory ability, and it allows assessment of PI and RI. The CVLT involves learning two lists of 16 words (see Figure 1). The first list, List A, is presented and recalled repeatedly over five trials, and then the second list, List B, is presented and recalled once. Both lists are composed of four categories of four words, and share two categories (spices and herbs, fruits) and have two non-shared categories (List A: tools, clothing; List B: fish, kitchen utensils). In addition, there are free and cued recall trials of List A following both a short and a long delay. Finally, a long delayed recognition trial of List A includes the target List A words, List B lures from shared and non-shared categories, and new words not previously presented.

Fig. 1. Depiction of the CVLT-II task.
Despite the wide use of the CVLT in clinical practice, memory interference measures from this test have not been studied in aMCI. PI can manifest as: (1) intrusions of List A words into recall of List B, (2) reduced recall on List B compared with recall on Trial 1 of List A, as well as (3) reduced recall of List B words that share a category with List A compared with recall of List B that do not share a category with List A. RI can manifest as: (1) intrusions of List B words into the delayed recall trials or recognition trial of List A, (2) reduced recall of List A short delayed free recall compared with Trial 5 of List A, as well as (3) reduced recall of the delayed recall or recognition trials for List A of words that share a category with List B compared to words that do not share a category with List B. It is anticipated that recall of non-shared category words would be better than shared category words because of the increased semantic interference similar to the effect seen in release from PI paradigms (e.g., Butters & Cermak, Reference Butters and Cermak1980).
From an experimental perspective, one classic memory interference paradigm is the AB-AC task. This task involves learning two lists of word pairs in which the first word (stimulus word) is the same on both lists but the second word (response word) differs between lists. In typical administration, the AB (first) list is administered for a set number of trials, usually three or four times, and following a delay of 20 to 30 min the AC (second) list is presented for the same number of trials. Immediately following presentation of the AC list, delayed recall of the AB list is required. Decreased recall and increased intrusion errors on the AC task compared with the AB task reflects PI while decreased recall and increased intrusions on delayed AB task (relative to the last recall trial of the AB task) reflects RI.
Early research investigating PI and RI using a standard AB-AC task found that healthy, community dwelling older adults perform similarly to younger adults, while institutionalized older adults demonstrated difficulties with PI similar to brain-damaged amnesic patients (Winocur & Moscovitch, Reference Winocur and Moscovitch1983). Although the institutionalized older adults did not meet criteria for dementia, this research was conducted before aMCI was recognized as a diagnostic classification and the institutionalized older adult group may have included individuals with aMCI (Winocur, personal communication, October, 2007).
Loewenstein et al. (Reference Loewenstein, Acevedo, Luis, Crum, Barker and Duara2004) developed Semantic Interference Test (SIT), which involves presentation of 10 common objects over three learning and recall trials followed by the presentation of 10 new but semantically related objects and later a delayed recall of the first 10 objects. Loewenstein et al. found that there was an incremental increase in PI susceptibility from older adults to MCI to mild AD, while MCI and mild AD both demonstrated increased difficulties with RI compared with healthy, older adults. More recently, Loewenstein et al. (Reference Loewenstein, Acevedo, Agron and Duara2007) have demonstrated that susceptibility to PI on the SIT predicts progression from MCI to dementia.
While comparing results across clinical measures, standard administration of the AB-AC task, or tasks such as the SIT is difficult given that they vary in stimulus type (category exemplars vs. associated word pairs) and retrieval test type (free recall, cued recall, recognition), an important limitation of each of these measures is that differences in learning is not controlled between groups. The literature exploring memory interference in AD is fraught with floor effects that may mask differences in interference between individuals AD and healthy controls (e.g., Binetti et al., Reference Binetti, Magni, Padovani, Cappa, Bianchetti and Trabucchi1995; Woodard et al., Reference Woodard, Dunlosky and Salthouse1999). Individuals with aMCI may also be susceptible to floor effects on difficult memory tasks, thus reducing the opportunities for this group to demonstrate PI and/or RI. In addition, other studies do not include a younger adult group, which would allow investigation of the effects of aMCI on performance, over and above those of aging.
The Present Research
Our goal was to explore PI and RI in young adults, healthy older adults, and older adults with aMCI using the CVLT as well as an AB-AC task. We used a modified AB-AC task that required individuals to learn lists of word pairs to a set criterion (i.e., 100% correct), thereby eliminating group differences in episodic memory performance that could otherwise influence measures of PI and RI. We predicted that healthy older adults and especially older adults with aMCI would demonstrate increased vulnerability to PI and RI compared with young adults.
METHODS
Research Participants
Younger and older adults were recruited via a volunteer research participant pool at Baycrest, as well as flyers and community talks targeting older adults with concerns about their memory. Inclusion criteria were: native English speakers or had learned English before starting primary school, right-handed, and minimum high school education (12 years). In addition, participants were free of previous or present neurological disorders, major medical disorders (e.g., heart disease, diabetes mellitus; hypertension; hypercholesterolemia; hypo- or hyperthyroidism), and psychiatric disorders. None was taking medications affecting cognition (e.g., neuroleptics, anti-convulsants, benzodiazepines, or cognitive enhancing agents). Those with a history of alcohol or substance abuse were excluded. The study was approved by the research ethics boards at Baycrest and the University of Toronto. All subjects provided informed consent and were paid a small honorarium for their participation ($25 for younger adults and $40 for older adults).
Data reported here are from 27 younger (age, 18–30 years), and 59 older adults (age, 65–90 years). Based on psychometric performance (see the Results section), 44 of the older adults performed in the normal range for their age and background on all measures and 15 older adults met criteria for aMCI.
Materials and Procedure
The study involved two sessions. The first session consisted of a 2.5 hr neuropsychological assessment for the older adults, and an abbreviated 1 hr assessment for the younger adults. For both age groups, the assessment consisted of Digit Symbol from the Wechsler Adult Intelligence Scale, 3rd Edition (WAIS-III, Wechsler, Reference Wechsler1997), the Mill Hill vocabulary test (Raven, Reference Raven1965), as well as the California Verbal Learning Test (Delis et al., Reference Delis, Kramer, Kaplan and Ober1987), Logical Memory, Visual Paired Associates, and Verbal Paired Associates from the Wechsler Memory Scale-Revised (WMS-R, Wechsler, Reference Wechsler1987). The older adults also received the Mini-Mental State Examination (Folstein et al., Reference Folstein, Folstein and McHugh1975), the Dementia Rating Scale (DRS, Mattis, Reference Mattis1988), Lawton and Brody’s (Reference Lawton and Brody1969) Activities of Daily Living scale, Matrix Reasoning and Block Design from the Wechsler Abbreviated Scale of Intelligence (WASI, Wechsler, Reference Wechsler1999), the Trail Making Test, Digit Span from the WAIS-III, and phonemic (FAS) and semantic (Animal) fluency, and the Hospital Anxiety and Depression Scale (Zigmund & Snaith, Reference Zigmund and Snaith1983).
Administration of the CVLT followed standard procedures. Namely, Lists A and B were read at a rate of 18- to 20-s per list. The short-delayed free and cued recall tests occurred immediately after participants recalled List B. Participants were not forewarned about the long-delayed memory tests, which occurred 20 min after the short-delayed memory tests.
The second session lasted 1.5–2 hr, and included the AB-AC task (30 min), followed by additional experimental tasks that are reported in part by Anderson et al. (Reference Anderson, Ebert, Jennings, Grady, Cabeza and Graham2008). Materials used in the AB-AC task were selected from Jacoby (Reference Jacoby1996), and consisted of 12 AB-AC related word pairs in which for each stimulus word, the two response words completed the same word fragment (e.g., for knee – b_n_, the response words were bone and bend).
The task began with the AB list (see Figure 2). On each trial participants were presented with a stimulus word and a word fragment (e.g., knee – b_n_). Participants had 5 s to produce the response word (e.g., bend), after which the full word pair was displayed for 2 s. Each learning cycle consisted of 12 trials, corresponding to the 12 different stimulus words. Learning cycles repeated, with the AB word pairs presented in a different random order in each cycle, until the participant recalled all response words correctly. Participants were not forewarned about the AC list, which occurred after a 20-min delay filled with a nonverbal memory task and questionnaires. For the first cycle of the AC list, participants were presented the 12 complete word pairs for 2 s each, in which the stimulus words were the same as in the AB list but the response words differed (e.g., knee – bone; see Figure 2). For the remaining cycles, on each trial participants were presented with a stimulus word and word fragment and had 5 s to produce the correct response word for the AC list (i.e., bone), after which the correct response word was displayed for 2 s. Again, learning cycles were repeated until all response words were recalled correctly. Immediately following the AC task, a delayed recall of the first list (AB) was presented. Participants were presented with the same 12 cue words one at a time and were asked to recall the related response word from the first list (i.e., bend). Correct responses were not presented for the delayed task.

Fig. 2. Depiction of the Modified AB-AC task.
A second version of stimuli (e.g, anchor- s_ _ p: ship vs. stop) was introduced near the end of the current study to accommodate participants enrolled in a memory training study in which the AB-AC task is administered before and after completing training. For participants receiving this second version (n = 16) the present analyses include data only from the pretraining administration of the AB-AC task. For both versions, the set of AB-AC pairs was constructed such that the probability of completing the word fragments with the B or C response word was equated, based on previous norms (Jacoby, Reference Jacoby1996). Materials were counterbalanced across participants so that the two response words served equally often in the AB and AC lists. E-Prime software (version 1.1, Service Pack 3; Psychological Software Tools, Inc.) was used to control stimuli presentation and response collection.
RESULTS
Demographics and Neuropsychological Results
Demographic and neuropsychological test data are presented in Table 1. The Old and aMCI groups did not differ in age, t(57) < 1, and all three groups had comparable levels of education, F(2,83) = 1.01, ns. Group differences in performance on the neuropsychological tests were explored using independent sample t tests. Differences between Young and Old were explored to examine age-related effects. Diagnosis of aMCI was made when performance on one or more memory measure was 1.5 SD or more below their general cognitive functioning and fluid intellectual abilities (as assessed by the DRS and Matrix Reasoning), and their performance on all nonmemory measures was within the normal range. Therefore, the pattern of significant and nonsignificant differences between the Old and aMCI groups is reported to confirm that diagnosis of aMCI was consistent with published criteria (Winblad et al., Reference Winblad, Palmer, Kivipelto, Jelic, Fratiglioni, Wahlund, Nordberg, Bäckman, Albert, Almkvist, Arai, Basun, Blennow, De Leon, DeCarli, Erkinjuntti, Giacobini, Graff, Hardy, Jack, Jorm, Ritchie, Van Duijn, Visser and Petersen2004). All group differences in neuropsychological performance were significant at an adjusted p < .001 to correct for multiple comparisons.
Table 1. Demographic and neuropsychological data for the Young, Old, and aMCI groups (SD in parentheses)

Notes.
CVLT = California Verbal Learning Test; CVLT Recognition values are hit rates minus false alarm rates; WMS-R = Wechsler Memory Scale-Revised; WAIS-III = Wechsler Adult Intelligence Scale-III; WASI = Wechsler Abbreviated Scale of Intelligence; HADS = Hospital Anxiety and Depression Scale. Means are total scores unless otherwise noted.
a Age effect (Young ≠ Old, p < .001).
m MCI effect (Old ≠ MCI, p < .001).
Similar to previous research comparing young and old adults, the Old outperformed the Young on a vocabulary (i.e., Mill Hill) test, but had significantly slower psychomotor speed (i.e., Digit Symbol). The Young also performed better than the Old on each of the CVLT measures reported in Table 1, Visual Paired Associates I, and Verbal Paired Associates I. There were no age-related differences on Logical Memory I, Verbal Paired Associates II, or Visual Paired Associates II, likely due to ceiling effects in both groups on the last two sub-tests.
The aMCI demonstrated significant episodic memory deficits relative to the Old on every memory measure except Digit Span and Visual Paired Associates I. On nonmemory measures, with one exception there were no differences between Old and aMCI, including MMSE, DRS, Digit Symbol, Matrix Reasoning, Block Design, Trail Making, and phonemic and semantic fluency. The exception was the superior performance of the Old on the Mill Hill vocabulary test, where the aMCI performed similarly to the young adults. It should be noted that participants with impaired verbal fluency were excluded from the study as such deficits would not be consistent with a diagnosis of single-domain aMCI. The Mill Hill vocabulary test was not used for diagnostic purposes because normative data were not available. Perhaps the difficult nature of the vocabulary test (e.g., “nugatory” and “minatory”) revealed early semantic deficits in aMCI, despite equivalent performance on the fluency measures. Finally, neither the Old nor aMCI groups endorsed significant levels of depressive or anxious symptoms.
Memory Interference on the CVLT
Although the CVLT recall scores were used to make the diagnosis of aMCI, intrusion errors and interference scores from the CVLT were not considered for diagnostic purposes, but instead were explored to determine whether the CVLT is sensitive to heightened memory interference in aMCI relative to healthy older adults. We first describe measures addressing PI and then describe measures exploring RI. Where suitable, appropriate learning values were included as covariates (i.e., List A learning when exploring PI on List B, List B learning when exploring RI on delayed recall of List A) to control for individual differences in learning rates on interference. Mill Hill vocabulary scores were not included as an additional covariate, as these scores correlated with only one of all the dependent measures from the CVLT and AB-AC tasks (CVLT List B words that shared a category with List A, p = .02). Finally, for the analysis of the CVLT and AB-AC data, significant group differences were explored further using Sidak post hoc tests.
Proactive interference on the CVLT
The first analysis of PI on the CVLT explored group differences in intrusions of List A words into List B recall. These intrusions were rare (see Table 2), and an one-way analysis of covariance (ANCOVA) with total correct recall across Trials 1–5 of List A as a covariate did not reveal significant differences between groups, F(2,82) < 1. The second analysis compared List B correct recall to correct recall on Trial 1 of List A. In a 3 × 2 mixed ANCOVA, with Group as a between-subjects variable, List as a within-subjects variable, and total correct recall across Trials 1–5 of List A as a covariate, none of the effects were significant, Fs < 1.86. Finally, recall of List B words from categories shared with List A was compared with recall of List B words from categories non-shared with List A. This was explored in a 3 × 2 mixed ANCOVA, with Group as a between-subjects variable, Shared versus Non-Shared List B words as a within-subjects variable, and total correct recall on Trials 1–5 of List A as a covariate. Although neither main effect was significant, Fs < 1, there was a significant interaction, F(2,82) = 5.89; p = .004; ![]() = .12. This interaction reflected a greater suppression of Shared compared with Non-Shared List B words (reflecting greater PI) for the Old and aMCI groups relative to the Young, but this effect was comparable in the two older adult groups. In summary, examination of PI on the CVLT was not helpful in distinguishing individuals with aMCI from their age-matched counterparts.
= .12. This interaction reflected a greater suppression of Shared compared with Non-Shared List B words (reflecting greater PI) for the Old and aMCI groups relative to the Young, but this effect was comparable in the two older adult groups. In summary, examination of PI on the CVLT was not helpful in distinguishing individuals with aMCI from their age-matched counterparts.
Table 2. Proactive and retroactive interference on the CVLT in Young, Old, and aMCI groups (SE in parentheses)

a Proactive interference data are adjusted for the covariate List A correct recall across Trials 1–5.
b Retroactive interference data are adjusted for the covariate List B correct recall.
Retroactive interference on the CVLT
The first set of analyses of RI on the CVLT explored intrusions of List B words into the delayed recall trials, as well as false alarms to List B words in the recognition trial. The intrusions were rare (see Table 2). Group differences, analyzed in separate one-way ANCOVAs, with total correct recall on List B as a covariate, were marginally significant for short delay free recall, F(2,82) = 2.74; p = .07; ![]() = .06, significant for short delay cued recall, F(2,82) = 7.94; p = .001;
= .06, significant for short delay cued recall, F(2,82) = 7.94; p = .001; ![]() = .16, not significant for long delay free recall, F(2,82) = 1.05, p = .36, significant for long delay cued recall, F(2,82) = 6.31; p = .003;
= .16, not significant for long delay free recall, F(2,82) = 1.05, p = .36, significant for long delay cued recall, F(2,82) = 6.31; p = .003; ![]() = .13, and significant for recognition false alarms to List B words, F(2,82) = 4.26; p = .02;
= .13, and significant for recognition false alarms to List B words, F(2,82) = 4.26; p = .02; ![]() = .09. Only for recognition did the aMCI group exceed their healthy counterparts in terms of false endorsement of List B words. Two secondary analyses were conducted to explore whether this latter difference reflected heightened RI in the aMCI group. False alarms to List B words from Shared versus Not-Shared categories was analyzed in a 3 × 2 mixed ANCOVA, with Group as a between-subjects variable, Shared versus Not-Shared as a within-subjects variable, and total correct recall on List B as a covariate. The only reliable finding was that Shared List B words were more often falsely endorsed than Non-Shared List B words were, F(1,82) = 7.21; p = .009;
= .09. Only for recognition did the aMCI group exceed their healthy counterparts in terms of false endorsement of List B words. Two secondary analyses were conducted to explore whether this latter difference reflected heightened RI in the aMCI group. False alarms to List B words from Shared versus Not-Shared categories was analyzed in a 3 × 2 mixed ANCOVA, with Group as a between-subjects variable, Shared versus Not-Shared as a within-subjects variable, and total correct recall on List B as a covariate. The only reliable finding was that Shared List B words were more often falsely endorsed than Non-Shared List B words were, F(1,82) = 7.21; p = .009; ![]() = .08, an effect that was equally true for all three groups. Moreover, older adults, especially those with aMCI, were more likely to intrude and make false alarms to new words (neither List A nor List B words), across both delays of free and cued recall and the recognition trial, F(2,83) = 4.51 to 20.83; p = .01 to < .001;
= .08, an effect that was equally true for all three groups. Moreover, older adults, especially those with aMCI, were more likely to intrude and make false alarms to new words (neither List A nor List B words), across both delays of free and cued recall and the recognition trial, F(2,83) = 4.51 to 20.83; p = .01 to < .001; ![]() = .10 to .33. Together, these results suggest the aMCI group’s greater tendency to falsely endorse List B words does not reflect a greater tendency toward RI, but simply a general tendency toward greater false recognition, regardless of the source of the new words.
= .10 to .33. Together, these results suggest the aMCI group’s greater tendency to falsely endorse List B words does not reflect a greater tendency toward RI, but simply a general tendency toward greater false recognition, regardless of the source of the new words.
The second analysis of RI involved comparing correct recall on Trial 5 of List A with correct recall on the short-delay free recall trial of List A. The assumption was that in addition to simple forgetting of List A, learning and recalling List B may interfere with retention of List A. This was explored in a 3 × 2 mixed ANCOVA, with Group as a between-subjects variable, List (Trial 5 vs. short delay free recall) as a within-subjects variable, and total correct recall on List B as a covariate. There was a significant difference in recall between groups (Young > Old > aMCI), F(2,82) = 43.13; p < .001; ![]() = .51, better free recall at Trial 5 than at the short delay, F(1,82) = 13.24; p < .001;
= .51, better free recall at Trial 5 than at the short delay, F(1,82) = 13.24; p < .001; ![]() = .14, as well as an interaction between Group and List, F(2,82) = 3.63; p = .03;
= .14, as well as an interaction between Group and List, F(2,82) = 3.63; p = .03; ![]() = .08. Follow-up ANCOVAs revealed that the decrease in recall of List A at the short delay relative to Trial 5, after accounting for List B learning, was smaller in the Young than aMCI group, but did not differ reliably between the two older adult groups.
= .08. Follow-up ANCOVAs revealed that the decrease in recall of List A at the short delay relative to Trial 5, after accounting for List B learning, was smaller in the Young than aMCI group, but did not differ reliably between the two older adult groups.
The final set of analyses to explore RI on the CVLT involved comparing correct delayed recall and correct recognition of List A words that shared or did not share a category with List B. These data were analyzed separate in 3 × 2 ANCOVAs, with Group as a between-subjects variable, Shared versus Not-Shared List A words as a within-subjects variable, and total correct on List B as a covariate. There was a significant difference between groups in all analyses, Fs(2,82) = 3.63 to 38.32; p = .03 to < .001; ![]() = .08 to .48. Relative to Non-Shared List A words, Shared List A words were better recognized, F(1,82) = 11.52; p = .001;
= .08 to .48. Relative to Non-Shared List A words, Shared List A words were better recognized, F(1,82) = 11.52; p = .001; ![]() = .12, but not better recalled, all F(1,82) < 1. Importantly, in none of these analysis did the difference between memory for Shared and Not-Shared List A words vary across groups, all Fs(2,82) < 1, except for long delay cued recall, F(2,82) = 2.88; p = .06. This latter marginal effect was due to the fact that whereas Younger adults recalled more Shared than Non-Shared words (Ms = 6.8 vs. 6.5, respectively), the Old and aMCI groups recalled fewer Shared than Non-Shared words (Ms = 5.5 and 5.7, respectively, for the Old, and Ms = 3.2 and 4.3, respectively, for the aMCI). In summary, the CVLT is not sensitive to differences in RI between individuals with aMCI and their age-matched peers.
= .12, but not better recalled, all F(1,82) < 1. Importantly, in none of these analysis did the difference between memory for Shared and Not-Shared List A words vary across groups, all Fs(2,82) < 1, except for long delay cued recall, F(2,82) = 2.88; p = .06. This latter marginal effect was due to the fact that whereas Younger adults recalled more Shared than Non-Shared words (Ms = 6.8 vs. 6.5, respectively), the Old and aMCI groups recalled fewer Shared than Non-Shared words (Ms = 5.5 and 5.7, respectively, for the Old, and Ms = 3.2 and 4.3, respectively, for the aMCI). In summary, the CVLT is not sensitive to differences in RI between individuals with aMCI and their age-matched peers.
Memory Interference on the AB-AC Task
Version and response analyses
The first 12 trials of the AB task involved presentation of a cue word, to which participants guessed the response word, after which the correct response word was displayed (see Figure 2). This procedure allows an assessment of the probability of guessing the B and C response words. Despite the fact that the word lists were selected on the basis of published norms, participants were more likely to guess one set of response words than the other for Version 1. Therefore, the Version by Response combination was used as a covariate in all subsequent analyses.
Proactive Interference on the AB-AC Task
We explored evidence of PI by comparing performance on the AB and AC lists in terms of learning cycles required, proportion correct performance, and proportion of other-list intrusions errors. Learning cycles required was defined as the number of cycles (sets of 12 trials) needed before participants reached criterion (100% recall accuracy), after guessing response words in cycle 1 of the AB list, and after presentation of the full word pairs in cycle 1 of the AC list. These data were analyzed in a 3 × 2 mixed ANCOVA with Group as a between-subjects variable, Task (AB, AC) as a within-subjects variable, and Version by Response combination as a covariate (see Figure 3). The Young required fewer learning cycles (M = 1.8) than did the Old (M = 3.1), who in turn required fewer learning cycles than did the aMCI (M = 4.7), F(2,82) = 25.90; p < .001; ![]() = 0.39. Participants required marginally more cycles to learn the AC (M = 3.7) list than AB (M = 2.7) list, F(1,82) = 3.26; p = .07;
= 0.39. Participants required marginally more cycles to learn the AC (M = 3.7) list than AB (M = 2.7) list, F(1,82) = 3.26; p = .07; ![]() = 0.04. These main effects were qualified by a significant group by task interaction, F(2,82) = 9.05; p < .001,
= 0.04. These main effects were qualified by a significant group by task interaction, F(2,82) = 9.05; p < .001, ![]() = 0.18, due to the fact that the aMCI group demonstrated greater PI than the Old, who in turn had more PI than the Young (M number of more cycles to learn AC than AB = 2.1, 0.9, and 0.0, respectively).
= 0.18, due to the fact that the aMCI group demonstrated greater PI than the Old, who in turn had more PI than the Young (M number of more cycles to learn AC than AB = 2.1, 0.9, and 0.0, respectively).
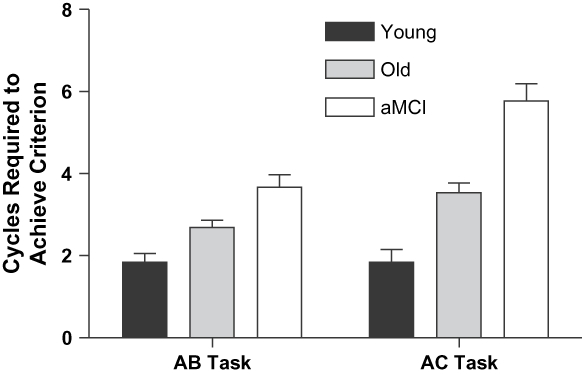
Fig. 3. Number of learning cycles required to meet criteria on the AB and AC lists, with Version by Response combination covaried.
The proportion of correct responses on the first recall trial was analyzed in a 3 × 2 mixed ANCOVA, with Group as a between-subjects variable, List (AB, AC) as a within-subjects variable, and Version by Response combination as well as AB learning cycles as covariates (see Figure 4). The number of learning cycles to meet criterion on the AB task was included as a covariate to account for differences in learning ability and exposure to the first list. The Young (M = 0.84) outperformed the Old (M = 0.74), who in turn outperformed the aMCI (M = 0.64), F(2,82) = 21.53; p < .001; ![]() = .34. More correct responses were provided for the AB (M = 0.80) list than the AC (M = 0.69) list, F(1,81) = 5.97; p = .02;
= .34. More correct responses were provided for the AB (M = 0.80) list than the AC (M = 0.69) list, F(1,81) = 5.97; p = .02; ![]() = .07, but this difference varied across groups, F(2,81) = 6.45; p = .003;
= .07, but this difference varied across groups, F(2,81) = 6.45; p = .003; ![]() = .14. While recall of the AB list was equivalent across groups, F(2,81) = 1.27; p = .29, on the AC list the Young (M = .86) outperformed the Old (M = .67), who in turn outperformed the aMCI (M = .52). This pattern is consistent with greater PI among older than younger adults, especially among those with aMCI. The same results were obtained when correct recall across all learning cycles was examined.
= .14. While recall of the AB list was equivalent across groups, F(2,81) = 1.27; p = .29, on the AC list the Young (M = .86) outperformed the Old (M = .67), who in turn outperformed the aMCI (M = .52). This pattern is consistent with greater PI among older than younger adults, especially among those with aMCI. The same results were obtained when correct recall across all learning cycles was examined.

Fig. 4. Proportion correctly recalled on first recall trial of the AB and AC lists, with Version by Response combination and AB learning cycles covaried.
The proportion of other-list intrusion errors on the first recall trial was analyzed in a 3 × 2 mixed ANCOVA with Group as a between-subjects variable, List (AB, AC) as a within-subjects variable, and Version by Response combination as well as AB learning cycles as covariates (see Figure 5). The Young (M = .06) and Old (M = .09) made comparable proportions of other-list intrusion errors, while the aMCI (M = .17) made significantly more, F(2,81) = 8.54; p < .001; ![]() = .17. Other-list intrusion errors did not differ overall between the AB (M = 0.06) and the AC (M = 0.16) lists, F(1,81) = 1.6, but List interacted with Group, F(2,81) = 6.21; p = .003;
= .17. Other-list intrusion errors did not differ overall between the AB (M = 0.06) and the AC (M = 0.16) lists, F(1,81) = 1.6, but List interacted with Group, F(2,81) = 6.21; p = .003; ![]() = .13. While the groups did not differ in the proportion of C intrusions into the first recall trial of the AB List, F(2,81) < 1, the proportion of B intrusions into the first recall trial of the AC list was smallest in the Young (M = .07), larger in the Old (M = .13), and larger still in the aMCI (M = .28), F(2,81) = 10.12; p < .001;
= .13. While the groups did not differ in the proportion of C intrusions into the first recall trial of the AB List, F(2,81) < 1, the proportion of B intrusions into the first recall trial of the AC list was smallest in the Young (M = .07), larger in the Old (M = .13), and larger still in the aMCI (M = .28), F(2,81) = 10.12; p < .001; ![]() = .20. Similar results were obtained when the proportion of other-list intrusion errors across all learning cycles were examined. Together, these modified AB-AC results identify both an age-related and aMCI-related increase in susceptibility to PI.
= .20. Similar results were obtained when the proportion of other-list intrusion errors across all learning cycles were examined. Together, these modified AB-AC results identify both an age-related and aMCI-related increase in susceptibility to PI.

Fig. 5. Proportion of other-list intrusion errors on the first recall trial of the AB and AC lists, with Version by Response combination and AB learning cycles covaried.
Retroactive interference on the AB-AC Task
We explored evidence of RI by examining performance on the AB delayed list in terms of proportion correct performance and proportion of other-list intrusions errors (see Figure 6). The proportion of correct responses on the AB delayed task was analyzed in a one-way ANCOVA, with Group as a between-subjects variable, and Version by Response combination and AC learning cycles as covariates. The Young (M = 0.90) had better AB delayed recall than both the Old (M = 0.78) and aMCI (M = 0.76), who performed similarly, F(2,81) = 3.67; p = .03; ![]() = .08.
= .08.

Fig. 6. Proportion correctly recalled and proportion of C intrusions in the AB delayed list, with Version by Response combination and AC learning cycles covaried.
The proportion of C intrusions into delayed recall of the AB list (see Figure 6) was examined in a one-way ANCOVA with Group as a between-subjects variable, and Version by Response combination and AC learning cycles as covariates. There were no group differences in these intrusion rates, F(2,81) = 1.31; p = .28. In summary, while older adults with and without aMCI appear more prone to forgetting, there is no evidence from this AB-AC task that individuals with aMCI are preferentially susceptible to RI.
DISCUSSION
This study used the CVLT and an AB-AC task to investigate proactive interference (PI) and retroactive interference (RI) in young adults, healthy older adults, and older adults with aMCI. On the CVLT, although both groups of older adults were more susceptible to PI and RI compared with the younger adults, memory interference did not differ between healthy older adults and older adults with aMCI despite significant differences in episodic memory performance. Older adults with aMCI made more intrusions in general (e.g., recall and recognition) compared with healthy older adults (see also Davis et al., Reference Davis, Price, Kaplan and Libon2002; Greenaway et al., Reference Greenaway, Lacritz, Binegar, Weiner, Lipton and Munro Cullum2006); however, these errors were not specific to memory interference. These results suggest that the CVLT is not sensitive to group differences in memory interference, most likely because learning is not equated across groups on this task. The poorer memory performance in the older adult group and especially the aMCI group relative to the young group meant that they had less opportunity for interference.
On the AB-AC task, participants learned two lists of semantically related word pairs, in which the cue words were the same between lists while the response words differed. The effects of group differences in learning were eliminated by requiring participants learn the word lists to 100% accuracy. The results clearly indicate that while healthy aging is associated with an increased susceptibility to PI, this increase is exaggerated in individuals with aMCI. Both aMCI and healthy older adult groups demonstrated greater RI than young adults, but RI did not differ between the two older adult groups.
Our study found that healthy older adults in general demonstrated greater susceptibility to memory interference than younger adults, replicating previous findings of increased vulnerability to memory interference with aging (e.g., Hartman & Hasher, Reference Hartman and Hasher1991; Shimamura & Jurica, Reference Shimamura and Jurica1994; Winocur & Moscovitch, Reference Winocur and Moscovitch1983). Importantly, older adults with aMCI were particularly vulnerable to PI compared with healthy older adults. This finding suggests that the increased vulnerability to PI in early AD (Cushman et al., Reference Cushman, Como, Booth and Caine1988; Davis et al., Reference Davis, Price, Kaplan and Libon2002) is evident before individuals meet criteria for dementia (see also Loewenstein et al., Reference Loewenstein, Acevedo, Luis, Crum, Barker and Duara2004).
Explanations that have been offered for the increased susceptibility to interference seen in AD may apply to aMCI. Some researchers argue that increased susceptibility to memory interference in AD is related to a breakdown in semantic memory networks (e.g., Adlam et al., Reference Adlam, Bozeat, Arnold, Watson and Hodges2006; Balota, Watson, Duchek, & Ferraro, Reference Balota, Watson, Duchek and Ferraro1999; Bayles et al., Reference Bayles, Tomoeda and Cruz1999). Our finding of poorer vocabulary in the aMCI than healthy older adult group, despite equivalent years of education and general cognitive abilities, is consistent with this interpretation, as are other reports of semantic memory difficulties in aMCI (e.g., Ahmed et al., Reference Ahmed, Arnold, Thompson, Graham and Hodges2008; Vogel et al., Reference Vogel, Gade, Stokholm and Waldemar2005).
Others argue that the semantic network remains relatively intact while intentional retrieval breaks down early in AD, forcing increased reliance on automatic memory systems such as familiarity (e.g., Balota & Duchek, Reference Balota and Duchek1991; Balota et al., Reference Balota and Duchek1999; Bell et al., Reference Bell, Chenery and Ingram2000; Shenaut & Ober, Reference Shenaut and Ober1996). Indeed, PI can be related to difficulties applying important contextual or source information to discriminate between lists (Hogge et al., Reference Hogge, Adam and Collette2008). Using data from these same individuals, we have recently shown selective impairments in intentional retrieval (recollection) but sparing of familiarity in aMCI relative to healthy aging when source discrimination is required (Anderson et al., Reference Anderson, Ebert, Jennings, Grady, Cabeza and Graham2008). The current data are not sufficient to determine the relative contributions of these two potential sources of increased PI in aMCI, but this could be addressed in future studies that include indices of semantic memory and recollection/familiarity in the investigation of PI.
The 1970s and 1980s literature placed considerable focus on the heightened interference among individuals with Korsakoff’s syndrome (see Butters & Cermak, Reference Butters and Cermak1980) with the general conclusion that frontal lobe dysfunction was responsible. Modern neuroimaging tools have indeed identified frontal lobe structures, particularly the left ventrolateral frontal lobe, as critically involved in resolution of PI (for a review, see Jonides & Nee, Reference Jonides and Nee2006). Although aMCI has been considered primarily a deficit of episodic memory due to disruption of medial temporal regions such as the hippocampus and entorhinal cortex (de Leon et al., Reference de Leon, Mosconi, Blennow, DeSanti, Zinkowski, Mehta, Pratico, Tsui, Saint Louis, Sobanska, Brys, Li, Rich, Rinne and Rusinek2007), more recent evidence has shown subtle decline on other tasks implicating subtle change in executive functioning in aMCI (Kramer et al., Reference Kramer, Nelson, Johnson, Yaffe, Glenn, Rosen and Miller2006; Traykov et al., Reference Traykov, Raoux, Latour, Gallo, Hanon, Baudic, Bayle, Wenisch, Remy and Rigaud2007). Hence, heightened sensitivity to PI in aMCI may be related to early change in frontal functioning, causing increased source confusion. We are currently using a more extensive neuropsychological battery to explore the relationships between executive functioning and interference.
We did not find differences between healthy older adults and older adults with aMCI on the AB-AC measures of RI. This is in contrast to previous findings of elevated RI in older adults with aMCI relative to healthy older adults (Loewenstein et al., Reference Loewenstein, Acevedo, Luis, Crum, Barker and Duara2004). Our aMCI sample was composed of community dwelling older adults in rather exceptional health for their age who had not been previously diagnosed with aMCI, whereas Loewenstein et al. studied adults who were already diagnosed with aMCI. It may be that our sample was at the very earliest stage of aMCI and not yet manifesting increased RI vulnerability. In addition, group differences in encoding were not controlled in Loewenstein et al.’s paradigm. We required participants to learn words lists to 100% accuracy thus equalizing encoding of word lists. It may be that equalizing encoding performance between groups eliminated group differences in RI.
The CVLT, SIT, and our modified AB-AC task differ ways other than the imposition of a strict learning criterion that may also be responsible for the differences between the current results and those of Loewenstein et al. (Reference Loewenstein, Acevedo, Luis, Crum, Barker and Duara2004). The CVLT and SIT involve category exemplars, whereas our modified AB-AC task involved associated word pairs. In addition, the CVLT involves free recall, cued recall, and recognition, the SIT involves only free recall, and the AB-AC task reported here involves only cued recall. Sensitivity to PI or RI may vary as a function of stimulus type or retrieval demands. In addition, encoding features, such as the degree to which item or source distinctiveness is encouraged, may affect interference susceptibility. Future research that systematically manipulates the presence or absence of a learning criterion, stimulus type, retrieval demands, and encoding conditions would help to identify specific parameters that help minimize memory interference in aMCI.
The results from this study have important clinical implications. The addition of measures of PI may be helpful in distinguishing aMCI from normal aging. Due to sample size limitations we were unable to determine the sensitivity or specificity of our PI measures in distinguishing among the two older adult groups, but this is one goal of future studies. Nevertheless, the current results highlight the fact that traditional clinical measures are not sensitive enough to detect heightened sensitivity to PI in aMCI, most likely because learning is not equated between groups. However, a paradigm that allows individuals adequate opportunity to learn the information is sensitive to heightened PI in aMCI. Clinical interventions aimed at reducing memory interference by emphasizing the use of controlled processes necessary for source discrimination may improve memory performance in this group.
ACKNOWLEDGMENTS
We thank Fern Jaspers-Fayer, Samy Arita, Magdelena Lysenko, Vlad Kushnir, Sandra Priselac, and Mira Rizkalla for their assistance with this project. This research was supported by operating grants from the Canadian Institutes of Health Research (67015) and the Alzheimer Society of Canada awarded to NDA. Mira Rizkalla’s co-op term was supported by an operating grant from the Natural Sciences and Engineering Research Council of Canada awarded to NDA.










