INTRODUCTION
The prevalence of cognitive impairment up to three months after stroke is reported to be as high as 83% (Jokinen et al., Reference Jokinen, Melkas, Ylikoski, Pohjasvaara, Kaste, Erkinjuntti and Hietanen2015). Impaired cognition is thought to interfere with post-stroke rehabilitation (Robertson et al., Reference Robertson, Ridgeway, Greenfield and Parr1997; Skidmore et al., Reference Skidmore, Whyte, Holm, Becker, Butters, Dew and Lenze2010) and is associated with reduced functioning following hospital discharge (Desmond et al., Reference Desmond, Moroney, Paik, Sano, Mohr, Aboumatar and Stern2000; Leys et al., Reference Leys, Henon, Mackowiak-Cordoliani and Pasquier2005). The severity of long-term post-stroke cognitive impairment and its potential for recovery or further decline may be influenced by the level of impairment apparent in the acute period following stroke (Pendlebury & Rothwell, Reference Pendlebury and Rothwell2009). Consequently, it is important to establish risk factors for early post-stroke cognitive impairment to better understand the mechanism of development of post-stroke cognitive problems and identify targets for potential intervention.
Frailty is a condition of emerging interest in medicine; its prevalence ranges from 4–59% depending on the population studied (Soong et al., Reference Soong, Poots, Scott, Donald, Woodcock, Lovett and Bell2015). Frailty has been associated with development of cognitive impairment (Robertson et al., Reference Robertson, Savva and Kenny2013), delirium (Quinlan et al., Reference Quinlan, Marcantonio, Inouye, Gill, Kamholz and Rudolph2011), and vascular dementia (Solfrizzi et al., Reference Solfrizzi, Scafato, Frisardi, Seripa, Logroscino, Maggi and Panza2013). The Rockwood ‘accumulated deficits’ concept proposes that frailty arises from the increasing burden of age-related medical conditions that accrue over the lifetime (Rockwood et al., Reference Rockwood, Mitnitski and Macknight2002). This accumulation may lead to a state of physiological exhaustion and impaired repair mechanisms, thus limiting the body and brain’s ability to respond to and minimize the damage of further stressors (Song et al., Reference Song, Mitnitski and Rockwood2014). Based on this theory, a frailty index can be quantified based on the number of age-related conditions present.
As stroke is a cognitive stressor, the state of vulnerability induced by frailty may heighten the cognitive consequences experienced by patients in the aftermath of the event. We therefore sought to assess the relationship between pre-stroke frailty and post-stroke cognition in the acute period following stroke. It is possible that any association between frailty and post-stroke cognition may be accounted for by differences in confounders such as age, pre-stroke cognitive impairment, and onset of delirium. We aimed to investigate if an association between frailty and post-stroke cognition was independent of these and other variables that are typically associated with post-stroke cognitive impairment.
We hypothesised that pre-stroke frailty would be significantly associated with lower post-stroke cognition and that this association would be independent of other well-established moderators of post-stroke cognitive impairment.
METHOD
We conducted a cross-sectional study, using the Glasgow Stroke Research Database (GSRD). The GSRD allows a collection of anonymized patient level data and has full research ethics approval (ws/16/0001). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
We followed guidelines proposed by Riley et al. (Reference Riley, Sauerbrei and Altman2009) regarding prognostic factor research related to the design, conduct, and analysis of this study. We followed STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidance for reporting (von Elm et al., Reference von Elm, Altman, Egger, Pocock, Gotzsche and Vandenbroucke2007). The protocol for this study was registered at Research Registry (UIN: researchregistry2712).
Setting and Population
We recruited consecutive admissions to the acute stroke unit of an urban teaching hospital. The unit admits suspected and confirmed stroke or transient ischemic attack (TIA) patients. Recruitment occurred in three waves: wave 1 was from May 2016 to Feb 2017; wave 2 from April to June 2017; wave 3 from October to December 2017.
To be included in this study, patients required a stroke diagnosis confirmed by a stroke clinician and a cognitive assessment within seven days post-stroke. We did not exclude any patients based upon age or presence of aphasia, dysarthria, previous cognitive impairment, or physical or sensory disability. However, patients were excluded from analyses if they had a TIA or non-stroke diagnosis or were unable to fully complete the cognitive assessment for any reason.
Clinical and Demographic Information
Clinical and demographic information was collated for each patient by trained researchers via patient self-report and medical records.
Stroke diagnosis and stroke-type [using Oxford Community Stroke Project (OCSP)] (Bamford et al., Reference Bamford, Sandercock, Dennis, Burn and Warlow1991) was confirmed by a stroke consultant. Stroke severity was assessed via retrospective review of medical charts to generate a National Institutes for Health Stroke Scale (NIHSS) (Brott et al., Reference Brott, Adams, Olinger, Marler, Barsan, Biller and Hertzberg1989) score for each patient. Retrospective review of stroke severity has been established to be a valid, reliable means of assessment (Kasner et al., Reference Kasner, Chalela, Luciano, Cucchiara, Raps, McGarvey and Conroy1999; Williams et al., Reference Williams, Yilmaz and Lopez-Yunez2000).
Delirium was assessed via the ‘4A Test’ screening tool 4AT (www.the4AT.com); a cut-off of ≥4 was used to define a positive screen for delirium. Pre-stroke cognition was determined via medical history (prior diagnosis of mild cognitive impairment or dementia) and, where possible, informant assessment via the informant section of the Geriatric Practitioner Assessment of Cognition (GPCOG) (Brodaty et al., Reference Brodaty, Pond, Kemp, Luscombe, Harding, Berman and Huppert2002) using a cut-off score of ≥3 (out of 6) as indicative of previous cognitive impairment.
Cognitive Assessment
All patients admitted to the acute stroke unit underwent a short cognitive assessment using a mini-Montreal Cognitive Assessment (MoCA) to generate a total score out of 12 points (Nasreddine et al., Reference Nasreddine, Phillips, Bédirian, Charbonneau, Whitehead, Collin and Chertkow2005). Higher scores indicate better cognition. The assessment covered the cognitive domains of episodic memory (5-word recall), visuospatial and executive functioning (clock draw), language (verbal fluency test), and orientation (date).
Pre-Stroke Frailty Assessment
We used patient medical information up to the date of the stroke to generate a Frailty Index score for each patient, based upon recommended guidelines (Searle et al., Reference Searle, Mitniski, Gahbauer, Gill and Rockwood2008). The Frailty Index produces a frailty score ranging from 0 to 100, with higher scores indicating increased levels of frailty (see supplementary materials). Each of the 33 conditions on the Frailty Index identified to be present via clinical notes and medical history were added up to give a total score out of 33 (e.g., 5 conditions present out of 33). The total number of conditions present was then divided by the total number of conditions on the scale (e.g., 5/33) and multiplied by 100 to generate the Frailty Index score (0–100). If multiple instances of a medical condition were present (e.g., multiple falls, multiple diagnoses of cancer), these would be scored once and once only (i.e., a patient with a medical history consisting of 1 previous fracture and 2 previous diagnoses of cancer would generate a score of 2/33, not 3/33).
For clarity of presentation in our descriptive statistics, patients were dichotomized at a recommended cut-off point of >24 into ‘frail’ and ‘not frail’ categories according to Frailty Index scores (Rockwood et al., Reference Rockwood, Andrew and Mitniski2007).
Statistical Analysis
We used bespoke software (G-Power, version 3.1; Faul et al., Reference Faul, Erdfelder, Lang and Buchner2007) to inform a sample size calculation for multiple linear regression. Based on inclusion of 8 important independent variables, a clinically useful, moderate, effect size of F2= 0.10, power of 0.80 and a statistical significance level of 0.05, we required a sample size of 159 participants for analysis.
We performed a univariate linear regression analysis, with ‘score on cognitive testing’ as the dependent variable and pre-stroke Frailty Index score (continuous) as the independent variable. Linear multiple regression analysis was then conducted, adjusting for important covariates that were deemed likely to vary by frailty status and/or impact upon post-stroke cognition (Pendelbury et al., Reference Pendlebury and Rothwell2009; Robertson et al., Reference Robertson, Savva and Kenny2013; Sun et al., Reference Sun, Tan and Yu2014; Verloo et al., Reference Verloo, Goulet, Morin and von Gunten2016). Included covariates were age, sex, stroke severity, stroke-type (lacunar/non-lacunar), previous stroke/TIA, delirium, and previous cognitive impairment. Stroke-type was categorized as a nominal variable with 2 levels (lacunar/non-lacunar) on the basis that lacunar strokes and cortical strokes may differentially impact cognition (Makin et al., Reference Makin, Turpin, Dennis and Wardlaw2013). Each covariate was forced into the model regardless of significance in univariate analysis.
Constant variance, linearity, independence of observations, normality of residuals, and error-free (CLINE) values assumptions were checked for each model. Multicollinearity between continuous variables was also assessed (see supplementary materials). All models were created using SPSS statistics for Windows, version 24.0, Armonk, NY, IBM Corp.
RESULTS
Patient Characteristics
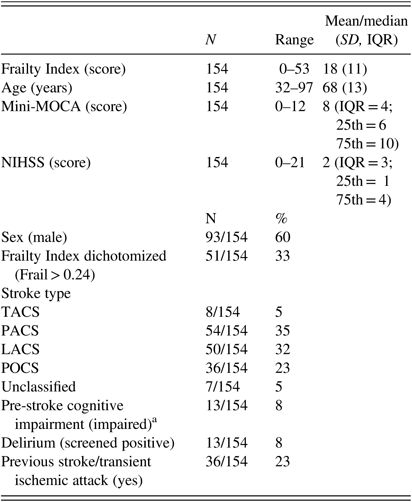
Of 262 confirmed stroke patients admitted during recruitment waves between May 2016 and Dec 2017, full data were available for 154 patients (59%). Ninety-four (36%) patients were excluded due to being untestable on cognitive assessment, and 14 (5%) were excluded due to not completing a cognitive assessment within 7 days following admission. Mean Frailty Index score for the population was 18 (SD= 11; range= 0–53); mean age of included participants was 68 years (SD=13; range=32–97); median score on cognitive assessment was 8 (IQR=4; range= 0–12); median stroke severity was 2 (IQR=3; range=0–21); 92/154 (60%) were male; frailty prevalence based upon Frailty Index dichotomization at a Frailty Index score of >24 was 51/154 (33%); 54/154 (35%) of stroke types were partial anterior circulation strokes (PACS); 36/154 (23%) had a previous stroke/TIA; 13/154 (8%) were cognitively impaired before the stroke (informant assessment data were available for 72/154; 47%); 13/154 (8%) screened positive for post-stroke delirium. Full demographic and clinical data for patients can be seen in Table 1.
Table 1 Descriptive statistics
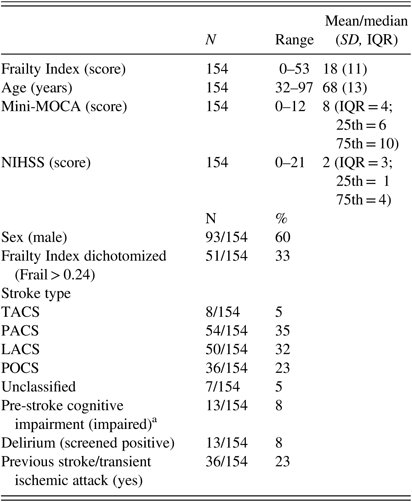
LACS=lacunar stroke; Mini-MOCA=mini-Montreal Cognitive Assessment; NIHSS=National Institutes of Health Stroke Scale; PACS=partial anterior circulation stroke; POCS=posterior circulation stroke; TACS=total anterior circulation stroke.
a Prior diagnosis of Mild Cognitive Impairment or Dementia or ≥3/6 on General Practitioner Cognitive Assessment—informant section questionnaire.
Associations With Cognitive Status After Stroke
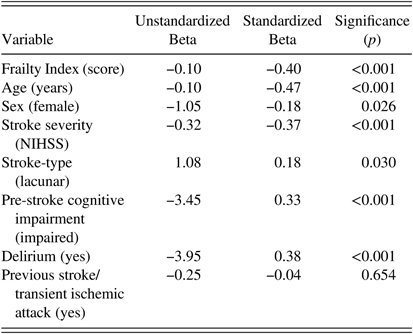
Results of the univariate and multiple linear regression analyses are presented in Tables 2 and 3. Pre-stroke frailty was significantly associated with post-stroke cognition (Standardized-Beta= −0.40, p<0.001) based on univariate linear regression. As Frailty Index scores increased, cognitive scores declined. Age, sex, NIHSS, delirium, pre-stroke cognition, and stroke-type were all associated with post-stroke cognitive score based on univariate analysis (all p<0.05). Previous stroke/TIA was not (p=0.65).
Table 2 Univariate analysis results of selected variables association with cognition
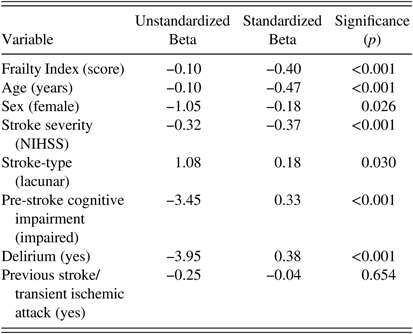
Dependent variable=Montreal Cognitive Assessment score. Stroke-type was categorized as a nominal variable with two levels (lacunar/non-lacunar). Frailty Index, age, and stroke severity were entered as continuous variables.
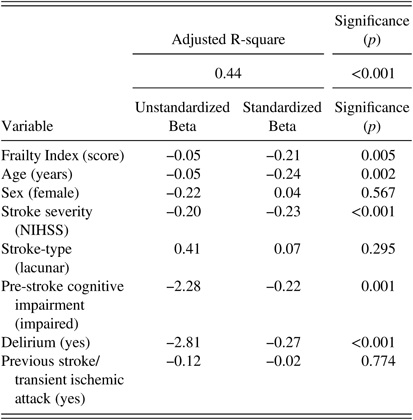
Table 3 Multiple Linear regression output of selected variables’ associations with post-stroke cognition
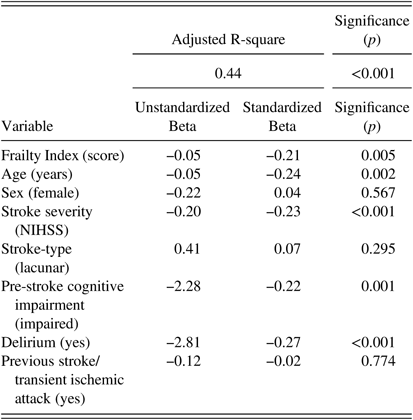
Dependent variable=Montreal Cognitive Assessment score. All variables were forced into the model. Stroke-type was categorized as a nominal variable with two levels (lacunar/non-lacunar). Frailty Index, age, and stroke severity were entered as continuous variables.
After adjusting for covariates, the association between frailty and post-stroke cognition remained significant (Unstandardized Beta=−0.05; Standardized beta=−0.21; p=0.005). Additional independent variables associated with post-stroke cognition were age (Unstandardized Beta=−0.05; Standardized beta =0.24; p=0.002), pre-stroke cognitive disorder (Unstandardized Beta=−2.28; Standardized beta =−0.22; p=0.001), delirium (Unstandardized-Beta=−2.81; Standardized-Beta=−0.27; p<0.001), and stroke severity (NIHSS) (Unstandardized Beta=−0.19; Standardized beta =−0.22; p=0.001). Stroke-type (p=0.30), previous stroke/TIA (p=0.77), and sex (p=0.57) were not significantly associated with post-stroke cognition. The combined model explained 43.5% of the variance in cognitive scores (adjusted R-square=0.435) at p<0.001.
Tests of CLINE assumptions for the model revealed collinearity between Frailty Index scores and age. However, multicollinearity was not a problem in the model according to variance inflation factor (VIF) scores (all <2). All other assumptions were satisfied (see supplementary materials).
DISCUSSION
Frailty-Cognition Association
We investigated the association between pre-stroke frailty and cognition in the acute period after stroke. Pre-stroke frailty was hypothesized to be independently associated with lower post-stroke cognition. Our findings support this hypothesis.
Those patients who had higher levels of frailty before stroke demonstrated significantly lower cognitive scores than those with comparatively lower frailty. Moreover, the association was apparent even after adjusting for other well-established risk factors for post-stroke cognitive impairment, including those that often co-occur with frailty.
This frailty-cognition association is consistent with findings from non-stroke populations suggesting a potentially important clinical relationship between frailty and cognitive impairment (Kojima et al., Reference Kojima, Taniguchi, Iliffe and Walters2016; Robertson et al., Reference Robertson, Savva and Kenny2013). It must be highlighted, however, that the observed effect size for pre-stroke frailty was relatively small, and less than half of the overall variance in post-stroke cognition was explained by our model despite inclusion of eight predictors. This emphasizes that a number of variables contribute in combination to the post-stroke cognitive state and no single variable is paramount to its outcome.
Mechanisms
There are several plausible mechanisms by which the observed association between pre-stroke frailty and poorer post-stroke cognition may have arisen. Frail patients may differ in pre-stroke brain reserve or levels of pre-stroke neuroinflammation—each of which could conceivably contribute to lower post-stroke cognitive performance (Jefferson et al., Reference Jefferson, Massaro, Wolf, Seshadri, Au, Vasan and DeCarli2007; Stern, Reference Stern2002).
Alternatively, the observed association may not be attributable to a few select mechanisms. Central to the accumulated deficits concept of frailty is that the ‘system’ of the body and brain is broken down due to an overabundance of problems. As the accumulation of deficits increases, physiological redundancy is reduced, leading to exhaustion or impairment of the brain’s repair mechanisms, thus, prohibiting ability to prevent or minimize further damage. The specific health deficits present matter a little; more relevant is the overall number of deficits (Rockwood et al., Reference Rockwood and Mitnitski2012). In this sense, when a plethora of general age-related health deficits combine, the brain may be left in a state of increased vulnerability to the cognitive consequences of a stroke (Song et al., Reference Song, Mitnitski and Rockwood2014). This ‘brain frailty’ may in part explain frail patients’ predisposition to delirium; however, it is noteworthy that patients with greater frailty in our study were still found to have lower post-stroke cognition even after controlling for presence of delirium post-stroke, suggesting that the association with lower post-stroke cognition itself cannot be attributed solely to delirium.
Strengths and Limitations
We have conducted an inclusive, exploratory study with an adequate sample size to investigate the association between frailty and post-stroke cognition, controlling for multiple relevant covariates. However, there are some important limitations worth mentioning. First, there was no blinding to cognitive scores when generating pre-stroke frailty ratings for each patient, creating a potential risk of bias. In addition, it was not possible to control for some potentially important covariates that could influence the observed associations with post-stroke cognition, such as premorbid IQ. Moreover, our assessment of pre-stroke cognition was limited by missing data and as such the observed frailty-cognition association could be contributed to by the pre-stroke cognitive state. Finally, while the generalizability of included patients is heightened by our very limited exclusion criteria, there were few severe strokes included in our analysis, and patients unable to complete cognitive assessment may often have been the more severely cognitively impaired. Greater inclusion of such patients may influence the associations and effect sizes that we report.
Clinical Implications and Future Directions
Our findings emphasize that not all variables that predict post-stroke cognition are classically ‘psychological.’ It is therefore important that clinicians are aware of the potential influence of frailty on post-stroke cognition and should consider incorporating the measurement of frailty into typical clinical assessment.
At present, our understanding of the frailty relationship with post-stroke cognition is limited by the cross-sectional nature of our study. Frail patients typically show less improvement or stabilization in cognition over time compared to non-frail patients in general older-adult populations (Mitnitski et al., Reference Mitnitski, Fallah and Rockwood2011). In this regard, the initial cognitive status combined with the frailty status of the patient could matter to future cognitive trajectories following stroke. The long-term effects of frailty on post-stroke cognition should therefore be investigated.
It would also be beneficial to determine if other concepts of frailty, for example, the frailty phenotype (Fried et al., Reference Fried, Tangen, Walston, Newman, Hirsch and Gottdiener2001), are also associated with post-stroke cognition. The frailty phenotype defines frailty as a biological syndrome characterized by specific physical conditions (weight loss, exhaustion, slow walking speed, limited physical activity, and weakness). While many patients defined as frail according to the accumulated deficits concept will also be frail in a form consistent with the phenotype, agreement between these concepts is far from perfect (Theou et al., Reference Theou, Brothers, Mitniski and Rockwood2013); hence there may be differences in post-stroke cognitive risk based on the frailty definition adopted.
Finally, replications of our results are important in order to establish if frailty is indeed associated with post-stroke cognition, independent of already established risk factors. If our findings are supported, future studies should assess and control for frailty when investigating post-stroke cognition as a failure to do so could confound results.
CONCLUSION
There is evidence that frailty is associated with lower post-stroke cognition, independent of factors that have previously been associated with post-stroke cognitive impairment. When assessing post-stroke cognition, clinicians and researchers should be aware of this potential relationship. Future studies should investigate the influence of frailty on longer term trajectories of post-stroke cognition.
SUPPLEMENTARY MATERIALS
To view supplementary material for this article, please visit https://doi.org/10.1017/S1355617719000092
ACKNOWLEDGMENTS
We gratefully acknowledge the supporters of our study. MT is partially funded by Chest Heart & Stroke Scotland; this work is part of the APPLE program of work funded by the Stroke Association and Chief Scientist Office Scotland. TQ is supported by a joint Stroke Association/Chief Scientist Office Senior Clinical Lectureship award.