INTRODUCTION
Traumatic brain injury (TBI) is a leading cause of childhood mortality and morbidity, with estimates of 47–280 per 100,000 children affected (Dewan, Mummareddy, Wellons, & Bonfield, Reference Dewan, Mummareddy, Wellons and Bonfield2016). Child TBI is associated with cognitive, behavioral, and psychological problems and reduced quality of life (QOL; Anderson et al., Reference Anderson, Le Brocque, Iselin, Eren, Dob, Davern and Kenardy2012; Catroppa et al., Reference Catroppa, Crossley, Hearps, Yeates, Beauchamp, Rogers and Anderson2015; Catroppa, Godfrey, Rosenfeld, Hearps, & Anderson, Reference Catroppa, Godfrey, Rosenfeld, Hearps and Anderson2012; Garcia, Hungerford, & Bagner, Reference Garcia, Hungerford and Bagner2015). Fatigue is one of the most challenging and commonly reported symptoms in the early stages of recovery (Blinman, Houseknecht, Snyder, Wiebe, & Nance, Reference Blinman, Houseknecht, Snyder, Wiebe and Nance2009; Falk, Reference Falk2013). Published rates of fatigue range from 11% to 47% (Crichton et al., Reference Crichton, Babl, Oakley, Greenham, Hearps, Delzoppo and Anderson2016; Hooper et al., Reference Hooper, Alexander, Moore, Sasser, Laurent, King and Callahan2004; Limond, Dorris, & McMillan, Reference Limond, Dorris and McMillan2009). In other childhood conditions, fatigue is associated with poorer psychosocial, physical, and school participation, lower QOL, and is a significant burden on parents (Berrin et al., Reference Berrin, Malcarne, Varni, Burwinkle, Sherman, Artavia and Chambers2007; Gedaly-Duff, Lee, Nail, Nicholson, & Johnson, Reference Gedaly-Duff, Lee, Nail, Nicholson and Johnson2006; Gibson, Garnett, Richardson, Edwards, & Sepion, Reference Gibson, Garnett, Richardson, Edwards and Sepion2005; Gold, Mahrer, Yee, & Palermo, Reference Gold, Mahrer, Yee and Palermo2009; Gordijn et al., Reference Gordijn, van Litsenburg, Gemke, Huisman, Bierings, Hoogerbrugge and Kaspers2013; Meeske, Katz, Palmer, Burwinkle, & Varni, Reference Meeske, Katz, Palmer, Burwinkle and Varni2004).
Despite the high prevalence of fatigue and its negative impacts, we know little about the clinical and psychological underpinnings of these symptoms. Research examining factors underpinning fatigue post-TBI has been called for within the adult TBI literature (Mollayeva et al., Reference Mollayeva, Kendzerska, Mollayeva, Shapiro, Colantonio and Cassidy2014). In child TBI, studies are limited and further research is needed to understand the nature of fatigue and its recovery trajectories after injury.
Fatigue refers to a subjective awareness of a negative balance between available energy and the mental and physical requirements of activities (Aaronson et al., Reference Aaronson, Teel, Cassmeyer, Neuberger, Pallikkathayil, Pierce and Wingate1999). It is commonly described in neurological disorders (Chaudhuri & Behan, Reference Chaudhuri and Behan2004). Fatigue is multidimensional, although there is no consensus on the specific number and nature of fatigue dimensions, although mental and physical domains are commonly described (Chaudhuri & Behan, Reference Chaudhuri and Behan2000; Finsterer & Mahjoub, Reference Finsterer and Mahjoub2014; Kluger, Krupp, & Enoka, Reference Kluger, Krupp and Enoka2013). Assessment tools for measure multiple domains of fatigue are available for use in childhood (Crichton et al., Reference Crichton, Babl, Oakley, Greenham, Hearps, Delzoppo and Anderson2016; Crichton, Knight, Oakley, Babl, & Anderson, Reference Crichton, Knight, Oakley, Babl and Anderson2015). In particular, the Pediatric Quality of Life Inventory; Multidimensional Fatigue Scale (PedsQL MFS, Meeske et al., Reference Meeske, Katz, Palmer, Burwinkle and Varni2004; Varni, Burwinkle, Katz, Meeske, & Dickinson, Reference Varni, Burwinkle, Katz, Meeske and Dickinson2002) measures three domains (general, sleep/rest, and cognitive fatigue), supported in confirmatory factor analysis (Panepinto et al., Reference Panepinto, Torres, Bendo, McCavit, Dinu, Sherman-Bien and Varni2014; Varni, Beaujean, & Limbers, Reference Varni, Beaujean and Limbers2013).
Multiple biological causes of fatigue exist. These include neuroanatomical, functional, psychological, bio-chemical, endocrine, and sleep-related causes, occurring independently or in combination after TBI (Mollayeva et al., Reference Mollayeva, Kendzerska, Mollayeva, Shapiro, Colantonio and Cassidy2014). While biological, psychological, and social factors have all been considered to exert bidirectional influences on fatigue (Chaudhuri & Behan, Reference Chaudhuri and Behan2000; Elovic, Reference Elovic2003, Reference Elovic2004; Elovic, Dobrovic, & Fellus, Reference Elovic, Dobrovic and Fellus2007; Elovic et al., Reference Elovic, Doppalapudi, Miller, DeLuca, Masel, Fellus and Urban2006), the specific relationships are yet to be examined in child TBI. The brain basis to fatigue is poorly understood, although in adults, the basil ganglia [and hypothalamic-pituitary-adrenal (HPA) axis] are likely candidates (Chaudhuri & Behan, Reference Chaudhuri and Behan2000; for a review see Elovic et al., Reference Elovic, Dobrovic and Fellus2007). Brain injury may represent a biological cause of fatigue, although dose-response relationships between fatigue and injury severity are unclear (Gagner, Landry-Roy, Lainé, & Beauchamp, Reference Gagner, Landry-Roy, Lainé and Beauchamp2015).
Several adult studies have demonstrated postinjury fatigue associated with psychological symptoms (depression, pain, chronic stress), self-reported neurobehavioral impairment (cognitive, motor, somatic), and reduced QOL (Ashman et al., Reference Ashman, Cantor, Gordon, Spielman, Egan, Ginsberg and Flanagan2008; Cantor, Gordon, & Gumber, Reference Cantor, Gordon and Gumber2013; Mollayeva et al., Reference Mollayeva, Kendzerska, Mollayeva, Shapiro, Colantonio and Cassidy2014). Ponsford and coworkers (Ponsford, Schönberger, & Rajaratnam, Reference Ponsford, Schönberger and Rajaratnam2014; Ponsford & Sinclair, Reference Ponsford and Sinclair2014) proposed that fatigue causes anxiety, depression, and daytime sleepiness, which then further exacerbate fatigue by affecting cognitive function (inattention). Empirical data provided partial support for this relationship.
Taking a different approach, based on systematic review of the literature, Mollayeva et al. (Reference Mollayeva, Kendzerska, Mollayeva, Shapiro, Colantonio and Cassidy2014) argued that pre- and postinjury physical (neurological, non-neurological/pain/medication) psychological, and physiologic (physical deconditioning, sleep, diet) factors all contribute to TBI fatigue. Of note, meta-analysis results provided limited data to support the significance of these factors. Rather, preinjury fatigue was a robust predictor of postinjury fatigue (de Leon et al., Reference de Leon, Kirsch, Maio, Tan-Schriner, Millis, Frederiksen and Breer2009; Norrie et al., Reference Norrie, Heitger, Leathem, Anderson, Jones and Flett2010). Child-specific factors might additionally contribute to TBI-fatigue in children. Environment (family socioeconomic status, SES) and developmental factors (age at injury; Anderson et al., Reference Anderson, Catroppa, Haritou, Morse, Pentland, Rosenfeld and Stargatt2001; Dennis, Yeates, Taylor, & Fletcher, Reference Dennis, Yeates, Taylor and Fletcher2007; Spencer-Smith & Anderson, Reference Spencer-Smith and Anderson2011; Taylor, Reference Taylor2010) are potentially important determinants of outcome after child TBI.
Fatigue in other child health conditions (e.g., cancer) has been associated with older child age and female sex (Dampier et al., Reference Dampier, Lieff, Lebeau, Rhee, McMurray, Rogers and Wang2010; Gold et al., Reference Gold, Mahrer, Yee and Palermo2009; Hinds et al., Reference Hinds, Nuss, Ruccione, Withycombe, Jacobs, DeLuca and DeWalt2013; Looman, Thurmes, & O’Conner-Von, Reference Looman, Thurmes and O’Conner-Von2010; Mears, Taylor, Jordan, & Binns, Reference Mears, Taylor, Jordan and Binns2004; Sumpter, Brunklaus, McWilliam, & Dorris, Reference Sumpter, Brunklaus, McWilliam and Dorris2011; ter Wolbeek, van Doornen, Kavelaars, & Heijnen, Reference ter Wolbeek, van Doornen, Kavelaars and Heijnen2008; Viner et al., Reference Viner, Clark, Taylor, Bhui, Klineberg, Head and Stansfeld2008). Sleep-wake cycle maturation (Carskadon & Acebo, Reference Carskadon and Acebo2002; Iglowstein, Jenni, Molinari, & Largo, Reference Iglowstein, Jenni, Molinari and Largo2003) may also contribute to fatigue after child TBI.
To date, these potential causes have not been studied in the context of child TBI. Several factors identified in adult models may be relevant to fatigue in child TBI (preinjury fatigue, sociodemographic factors, mental and physical health). We postulate that other factors [central nervous system (CNS) maturation or child age at injury, family SES] may also contribute to TBI-fatigue in childhood. Following others (for example Taylor et al., Reference Taylor, Swartwout, Yeates, Walz, Stancin and Wade2008; Yeates et al., Reference Yeates, Taylor, Rusin, Bangert, Dietrich, Nuss and Jones2009) we examined the contribution of child factors (sex, age at injury, and SES) to fatigue as a TBI outcome. We adopted a multi-dimensional approach to understanding the experience of fatigue after child TBI (Crichton et al., Reference Crichton, Babl, Oakley, Greenham, Hearps, Delzoppo and Anderson2016). The relative contribution of factors identified as significant within bi-directional (unidimensional) models, might vary with respect to different aspects of fatigue relevant to multi-dimensional models.
This study examined predictors of 12-month multidimensional fatigue following child TBI. We examined preinjury and postinjury (6 month) symptoms predictive of 12-month fatigue. We evaluated whether child factors (age, sex, and history of medical/neurological illness or learning/psychological difficulties), SES (parent education), preinjury fatigue, or symptoms reported preinjury or 6 months postinjury (sleep, pain, physical/motor difficulties, mood, and inattention) were predictive of 12-month fatigue, across three domains (general, sleep/rest, and cognitive fatigue). We hypothesized that predictors of worse fatigue would include: child/family factors (younger age at injury/ female sex, significant clinical history, lower SES), preinjury symptoms (sleep, pain, motor, cognitive, and mood), and 6-month symptoms (sleep, pain, motor, cognitive, and mood), over and above injury severity. A secondary hypothesis was that the three domains of fatigue would be predicted uniquely by different factors.
METHODS
Design
The study was a substudy of a larger study, “Biomarkers in child TBI”. The larger study was a prospective longitudinal multi-site study, conducted at The Royal Children’s Hospital (RCH), Melbourne, Australia; Ste-Justine Hospital (HSJ), Montreal, Canada; Hospital for Sick Children (SickKids), Toronto, Canada.
Participants
Participants were 0–17 years of age at injury, presenting to the Emergency Department or Intensive Care Unit at participating hospitals within 24 hours of sustaining a mild, moderate or severe TBI. Of 159 children initially enrolled, 79 were included in this substudy. Of the original cohort, reasons for exclusion were: failure to return questionnaires on at least one of three time-points (n=80), and late return of 12-month questionnaire (n=5). All participants completed 12-month follow-up in addition to preinjury or 6-month follow-up. Exclusion criteria were: previous TBI requiring hospital admission, moderate to severe neurodevelopmental disorder, and inability to read or speak sufficient English (RCH, SickKids) or English/French (HSJ). Relative to the study participants, those not participating in this sub-study were more likely to be of non-white race (p<.01). Groups were otherwise comparable for demographics. The retained sample comprised more males (84%) than females (16%), which was representative of the original cohort.
Children were grouped by TBI severity using lowest in-hospital documented Glasgow Coma Score (GCS, Teasdale & Jennett, Reference Teasdale and Jennett1974) within 24 hr of admission. GCS 13–15 defined the mild TBI group (n=52), GCS 9–12 defined the moderate TBI group (n=8) and GCS <9 defined the severe TBI group (n=19). Due to small sample sizes the moderate/severe groups were combined for analysis (n=27).
Measures
Measures were chosen to be appropriate to the age range of the study participants. Age range and time point completed for each measure are summarized in Table 1.
Table 1 Measures used for analysis in hierarchical regression models, by domain

Demographics, injury variables, and preinjury functioning
At recruitment, demographic information (child age at injury, sex), socioeconomic status (number of years of education for the highest educated parent), GCS, cause of injury, medical and clinical history were collected using a clinical report form. Clinical history variables included: previous head injuries, abnormal development, diagnosed neurological/medical/psychological condition or learning difficulties, scored as present (1) or absent (0), summed for a total clinical history score (range, 0–6).
Primary outcome: fatigue
Parents rated multidimensional fatigue at 12-month follow-up using the PedsQL MFS. The PedsQL MFS includes 18 items that provide ratings across three fatigue dimensions: general fatigue (lack of physical energy, 6 items), sleep/wake fatigue (sleep/wake cycle disruption and daytime sleepiness, 6 items), and cognitive fatigue (depleted mental endurance and attention, 6 items). The PedsQL MFS is designed for use in children 2–19 years. All children were within this age range at follow up (n=79). Responses were rated on a 5-point Likert-scale (0, Never a problem to 4, Almost always a problem). Scores were reversed and transformed to a 0- to 100-point scale. Higher scores indicate fewer symptoms of fatigue. Reliability, internal consistency, and validity have been demonstrated in children with a range of health conditions (Dampier et al., Reference Dampier, Lieff, Lebeau, Rhee, McMurray, Rogers and Wang2010; MacAllister et al., Reference MacAllister, Christodoulou, Troxell, Milazzo, Block, Preston and Krupp2009; Marcus et al., Reference Marcus, Strople, Neighbors, Weissberg-Benchell, Nelson, Limbers and Alonso2009; Varni et al., Reference Varni, Burwinkle, Berrin, Sherman, Artavia, Malcarne and Chambers2006, Reference Varni, Burwinkle, Katz, Meeske and Dickinson2002; Varni, Burwinkle, & Szer, Reference Varni, Burwinkle and Szer2004; Varni, Limbers, Bryant, & Wilson, Reference Varni, Limbers, Bryant and Wilson2009, Reference Varni, Limbers, Bryant and Wilson2010) and TBI (Crichton et al., Reference Crichton, Babl, Oakley, Greenham, Hearps, Delzoppo and Anderson2016). Clinically significant fatigue was defined as 2 SDs above published controls (Panepinto et al., Reference Panepinto, Torres, Bendo, McCavit, Dinu, Sherman-Bien and Varni2014).
Upon recruitment, parents were asked to describe their child’s preinjury abilities. Preinjury fatigue was extracted from the Pediatric Injury Functional Outcome Scale (PIFOS, Ewing-Cobbs et al., Reference Ewing-Cobbs, Bloom, Prasad, Waugh, Cox and Swank2014) a 26-item parent interview used to assess multiple symptoms after child TBI. The PIFOS is designed for use in children 3–15, although items are rated relative for age, was considered appropriate for use in our sample (n=75). The fatigue item is rated: Energy and fatigue level age-appropriate=1, Mild fatigue/lack of energy with minimal effort on school or social activities=2, Significant fatigue/lack of energy with moderate impact on school, family, or social activities=3, Severe fatigue/lack of energy=4. The PIFOS has good internal consistency (α=.90), inter-rater reliability (α=.90), and correlates with TBI severity outcome (Ewing-Cobbs et al., Reference Ewing-Cobbs, Bloom, Prasad, Waugh, Cox and Swank2014).
Health
Sleep, pain, and physical/motor functioning items were extracted from the PIFOS interview at recruitment (n=75). Preinjury symptoms were rated using a 4-point Likert-scale (Age appropriate=1 to Severe difficulties/disability=4). Responses were dichotomized into presence/absence of symptoms using pre-defined disability ratings (scores >1 Ewing-Cobbs et al., Reference Ewing-Cobbs, Bloom, Prasad, Waugh, Cox and Swank2014).
The Pediatric Quality of Life Inventory (PedsQL, Varni et al., 1999), is a 23-item parent-rated questionnaire used to measure health related quality of life, completed 6 months postinjury (n=73). The PedsQL is designed for use in children 2–18; all children were within this age range at 6-month follow-up. PedsQL sleep, pain, and motor function items were extracted. Responses were rated on 5-point Likert-scale from: Never=0, to Almost Always=4. The presence/absence of symptoms was coded using criteria: 0=, never/almost never and 1, sometimes/often/almost always. Parent report has demonstrated acceptable internal consistency reliability for group comparisons (α=.88–.90), and associations with morbidity and illness burden in clinical samples (Varni, Seid, & Kurtin, Reference Varni, Seid and Kurtin2001).
Psychosocial
Adaptive function
The Adaptive Behavior Assessment System, Second Edition (ABAS-II, Harrison & Oakland, Reference Harrison and Oakland2003) provided parent ratings of children’s general adaptive ability or overall level of day to day functioning relative to normative data for children 0–18 years (Harrison & Oakland, Reference Harrison and Oakland2003). Parents rated each skill from: Is not able=0, to Always or almost always when needed=3. The ABAS-II was completed at recruitment based on preinjury function (n=79), and readministered 6 months postinjury (n=67). General Adaptive Composite (GAC) results were divided into; (i) intact: at or above age-normative expectations (scores ≥85); (ii) mild impairment: one to two standard deviations below expectations (scores 70–84); (iii) severe impairment: greater than two standard deviations below expectations (scores ≤69). Reliability studies have provided evidence of high internal consistency coefficients (>.90), good test–retest reliability and support for the theoretical structure (Harrison & Oakland, Reference Harrison and Oakland2003).
Emotional function
Infants (0- to 3-year-olds). Psychological data were collected using the Brief Infant Toddler Social Emotional Assessment (BITSEA) completed at recruitment (preinjury ratings, n=9) and 6 months postinjury (n=6). The BITSEA contains 34 parent-report items assessing the presence of emotional problems. Items were rated on a 3-point scale from: Not true/rarely=1, to Very true/often=3. Scores on the “problem” subscale were categorized into presence or absence of psychological problems at or above the 75th percentile in a normative sample. These scores show most potential in screening for psychiatric disorders (Goodman, Ford, Simmons, Gatward, & Meltzer, Reference Goodman, Ford, Simmons, Gatward and Meltzer2000; Goodman, Renfrew, & Mullick, Reference Goodman, Renfrew and Mullick2000). Internal consistency has been demonstrated to be good to excellent (α=.80), and interrater reliability was good to excellent (p=.66) in a community sample (Goodman, Reference Goodman2001; Karabekiroglu, Briggs-Gowan, Carter, Rodopman-Arman, & Akbas, Reference Karabekiroglu, Briggs-Gowan, Carter, Rodopman-Arman and Akbas2010).
Children (4- to 18-year-olds). Psychological data were collected using the Strengths and Difficulties Questionnaire (SDQ) at recruitment (retrospective preinjury ratings, n=70), and was readministered 6 months postinjury (n=61). The SDQ consists of five subscales (emotional symptoms, conduct problems, hyperactivity, peer problems, and prosocial), each containing five items rated on a 3-point scale (from Not True=0 to Certainly True=3). The SDQ has demonstrated reliability and validity across studies, with a confirmed five-factor structure (Stone, Otten, Engels, Vermulst, & Janssens, Reference Stone, Otten, Engels, Vermulst and Janssens2010).
We used cut-points provided for normal, borderline, and abnormal to dichotomize the presence (borderline/abnormal) or absence (normal) of psychological symptoms. To compare emotional function across age groups, emotional problems were rated as present/absent, across ratings of the BITSEA “problems” or SDQ “emotional symptoms.”
Attention
Parent-rated attention items from the BITSEA (0- to 3-year-olds) and SDQ (4- to 18-year-olds) provided measures of inattention at preinjury and 6-month time points (n as above). Ratings on inattention items; BITSEA item 25 (Briggs-Gowan & Carter, Reference Briggs-Gowan and Carter2006) and SDQ items 15 and 25 (Goodman, Reference Goodman2001) were dichotomized into absence/presence of difficulties. To compare inattention ratings across age groups, SDQ and BITSEA items were reverse scored, with higher scores indicating the presence of inattention difficulties across all ages.
Procedure
The study protocol was approved by the ethics review board at each institution, and research was completed in accordance with the Helsinki Declaration. Written informed consent to participate in the study was obtained during the initial hospitalization.
At recruitment and during admission, basic child demographic and family SES data were collected by trained research assistants or nurses using the clinical report form. Interviews were completed with parents in which parents were asked to rate their children’s preinjury functioning (PIFOS). More detailed information was collected via questionnaire. Parents returned completed questionnaires (ABAS-II, SDQ, or BITSEA) by mail within one week of injury to gather preinjury adaptive function and psychosocial data. Parents were contacted between 6 and 12 months after the child’s injury, and the PIFOS was readministered via phone interview. Adaptive, psychosocial, and health-related quality of life questionnaires injury (ABAS-II, SDQ, or BITSEA, PedsQL) were administered by mail at 6 months. The PedsQL MFS was administered at 12 months by mail.
Analyses
All data were analyzed using Stata IC v14 (StataCorp, 2015).
Development and injury factors
Descriptive statistics were used to examine key demographics, injury and clinical data. Fisher’s exact tests were used to examine differences in rates of impairment across health and psychosocial domains preinjury and at 6 months postinjury (Figure 1). Chi square analysis was used to examine TBI severity group differences in fatigue at 12 months postinjury.
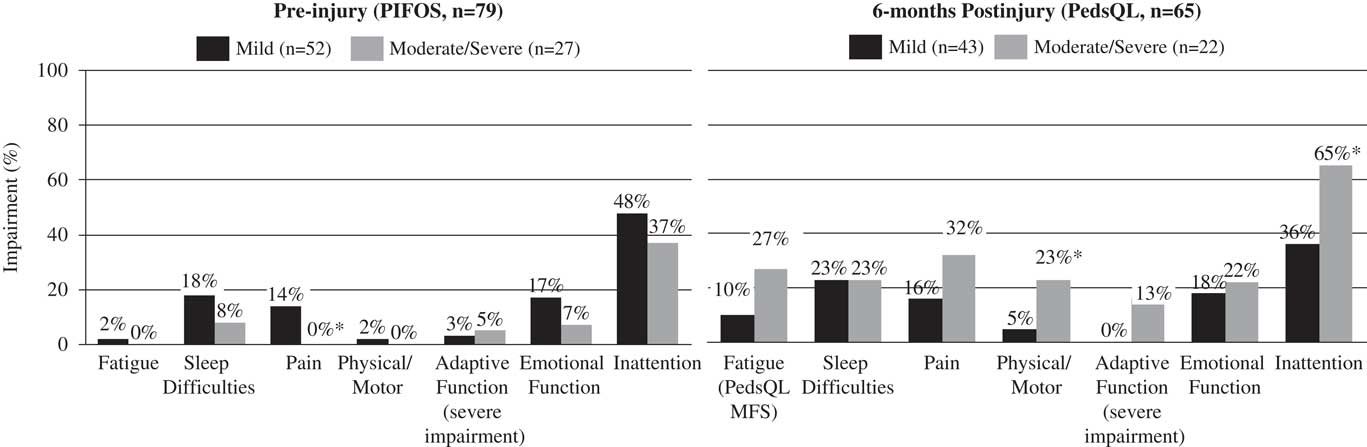
Fig. 1 Rates of impairment across fatigue, adaptive, psychosocial, and physical domains (preinjury PIFOS, 6 months postinjury PedsQL). PIFOS=Pediatric Injury Functional Outcome Scale; PedsQL=Pediatric Quality of Life Inventory; PedsQL MFS=Pediatric Quality of Life Inventory Multidimensional Fatigue Scale. *p<.05, two-tailed.
Predictors of fatigue
To investigate preinjury and postinjury predictors of fatigue, we conducted two separate, hierarchical, multiple regressions. They were conducted with each of the three fatigue domains (sleep/rest, general, cognitive). This allowed us to include maximal observations in each analysis. Child demographics (age at injury, sex, SES) were included in Block 1 of all models. Bivariate regressions were conducted to determine which additional explanatory variables were to be carried forward into hierarchical models for Block 2. For the first regression (Model 1), we explored preinjury factors predictive of 12-month fatigue. Preinjury variables included child clinical history score, PIFOS symptom classifications (sleep, fatigue, pain, and physical/motor), ABAS-II impairment classification, SDQ/BITSEA emotional function and inattention classification.
For the second regression (Model 2), we explored symptoms at 6 months as predictors of 12-month fatigue. Six-month variables were PedsQL symptom classifications (sleep, fatigue, pain, and physical/motor), ABAS-II impairment classification, SDQ/BITSEA emotional problems and inattention.
Hierarchical linear regression analyses were then conducted using variables predictive of fatigue at 12 months (at p<.10). In Block 1, child factors (child age, gender, SES) and injury severity (mild vs. moderate/severe TBI) were forced to enter. In Block 2, preinjury or 6-month variables could enter. R2 change provided the incremental variance accounted for by each of the preinjury or 6-month predictors. Descriptive analysis revealed preinjury fatigue to be reported in one participant, so we tested Block 2 models with and without preinjury fatigue using likelihood ratio tests. If the addition of preinjury fatigue to the model resulted in significant model improvement, it was retained in the final model. Tests for multicollinearity were examined, using variance inflation factor analysis.
Power analysis was conducted for the two models. Using the total number of possible preinjury variables (11), with a sample of n=75 assuming power of .80, we were able to detect an effect of η2=.212 (medium to large effects). The second set of models included a sample of n=65, and seven possible predictor variables. At a power of .80 we were able to detect an effect size of η2=.202 (medium to large). Thus, there was adequate power (i.e., power > .80) in the two series of hierarchical models at the moderate to large effect size level.
RESULTS
Demographic and Injury Factors
There was some variability in the return of 12-month fatigue data (range, 11.0–15.5 months; mean=12.73; SD=0.88). Of the study sample, sufficient data were available for inclusion in hierarchical regression models predicting 12-month fatigue from preinjury variables for 75 (of 79) participants (Model 1). Sufficient data were available for inclusion in hierarchical models predicting fatigue at 12 months postinjury from symptoms at 6 months postinjury for 65 (of 79) participants (Model 2). Demographic and injury factors for the sample, by injury severity, are described in Table 2.
Table 2 Demographic and early injury characteristics of study participants, according to severity of TBI

Note. ABAS=Adaptive Behavior Assessment System, Second Edition; ES=effect size, Cohen’s d for continuous and odds ratio for categorical.
*p<0.05.
Mean age across the included sample was 9.74 years (range, 1.12–16.90 years; median, 10.72 years). As illustrated in Table 2, the mild TBI group was slightly older at injury relative to the moderate/severe TBI group, although this did not reach significance). Severity groups were comparable for race, parental education and sex. Time from injury to 6- and 12-month assessment did not differ between groups, t(67)=−0.20, two-tailed p=.84; t(77)=0.79; p=.43, respectively). By definition, the mild TBI group had a higher mean GCS relative to the moderate/severe TBI group (M=14.6 vs. 6.1, p<.001). Injuries from motor vehicle accidents were more common in the moderate/severe TBI group (p<.001) and injury from falls was more common in the mild TBI group (p=.03).
Baseline Function
The proportion of children rated by parents as demonstrating symptoms or impairment preinjury and at 6-month follow-up are summarized by TBI group in Figure 1. Fisher’s exact tests revealed fatigue preinjury was comparable between the mild and moderate/severe groups (1% and 0%, respectively). Preinjury impairment differed between groups, with more frequent pain, emotion and inattention in mild TBI (14%, 17%, and 48%) when compared to moderate/severe TBI (0%, 7%, and 37%, respectively). Fatigue at 6 months was 27% for the moderate/severe group, and 10% in the mild group, and differences were nonsignificant. Moderate/severe TBI was more often associated with symptoms 6 months postinjury in the physical/motor domain (23%) and inattention (36%) compared to mild TBI (5% and 36%, respectively).
Predictors of Fatigue
PEDSQL MFS scores 12 months postinjury were: total (M=81.01; SD=16.91; range 22–100), general (M=82.17, SD=18.05; range, 29–100), sleep/rest (M=84.76; SD=17.298; range 33–100), cognitive fatigue (M=76.11; SD=24.41: range; 0–100). Rates of 12-month fatigue within the clinical range were 17% (mild TBI) and 19% (moderate/severe TBI; differences were nonsignificant, p=.89).
General Fatigue
Preinjury emotional function emerged as the only significant preinjury predictor of general fatigue with those with pre-existing difficulties experiencing worse 12-month general fatigue at the bivariate level (p=.03). However, in the combined model, the overall model failed to significantly predict general fatigue (p=.07).
At 6 months postinjury, several factors, including sleep, pain, physical/motor function, emotional functioning, significantly predicted fatigue at the bivariate level (all p values<.01). Physical/motor function (β=−28.62; p<.001; R2=.27) accounted for most of the unique variance, with medium effect size. Sleep (β=−11.02; p=.02; R2=.10) and emotional function (β=−10.78%; p=.03; R2=.9) also contributed significantly independently, with small and medium effect sizes, respectively. The final model evidenced significant R2 change, and explained 43% of the variance in 12-month general fatigue (p<.001; Table 4). Pain at 6 months was nonsignificant in independently predicting 12-month general fatigue in hierarchical models.
Sleep/Rest Fatigue
Baseline predictors were, preinjury fatigue (β=−9.39; p=.09) and emotional function (β=−12.94, p=.01) and these variables remained predictors in hierarchical analyses (β=−38.7; p=.02; and β=−13.9; p=.007, respectively, Table 3). Presence of preinjury fatigue or emotional dysfunction accounted for 10% and 7% of variance, respectively, in 12-month sleep/rest fatigue. The overall hierarchical model was significant (p=.02) and accounted for more variance than child factors (age, sex, SES) and injury alone (p=.004). Likelihood ratio test results showed that retaining preinjury fatigue in Block 2 resulted in a statistically significant improvement in model fit (p=.02), so it was retained in the model. Overall, preinjury factors drove 19% of the preinjury model of sleep/rest fatigue (Table 4). Effect sizes were small.
Table 3 Hierarchical regression analysis of preinjury factors predicting fatigue (general, sleep/rest, cognitive) at 12-months postinjury (n=75)

*p<.05.
**p<.01.
Table 4 Hierarchical regression analysis of symptoms at 6 months predicting fatigue (general, sleep/rest, cognitive) at 12 months postinjury (n=65)

*p<.05.
**p<.01.
***p<.001.
Problems with sleep, physical/motor function, and emotional function 6 months postinjury were predictive of sleep/rest fatigue in bivariate analyses (all p values<.01). Hierarchical analyses including these variables accounted for 35% of variance (p<.001). Sleep function at 6 months was a strong independent predictor explaining 13% (β=−13.72; p=.007) of the variance in 12-month sleep/rest fatigue, respectively, with medium effect sizes. Physical/motor and emotional function also independently contributed to sleep/rest fatigue, contributing 10% (β=−17.44; p=.02) and 9% (β=−11.68; p=.03) respectively, with medium and small effect sizes, respectively.
Cognitive Fatigue
No individual preinjury factors significantly contributed to postinjury fatigue at the bivariate level. Inclusion of Block 1 alone (child factors and injury severity) in the hierarchical model failed to significantly predict postinjury cognitive fatigue (p=.12).
Postinjury, sleep, physical/motor function, and emotional functioning were significant bivariate predictors of 12-month cognitive fatigue (all p<.05). The hierarchical model accounted for 38% of variance (p<.001). Only physical/motor symptoms 6 months postinjury predicted cognitive fatigue at 12 months postinjury (β=−35.00; p=.001), and explained 12% of variance and effect size were small/medium.
DISCUSSION
The aim of this study was to identify preinjury factors and 6 month postinjury symptoms predictive of fatigue 12 months after child TBI. Overall, preinjury or baseline models that included child demographics and preinjury functioning were poor predictors of 12-month fatigue, across all fatigue domains. In contrast, child symptoms at 6 months were predictive of 12-month fatigue. Specifically, physical/motor symptoms at 6 months postinjury predicted worse fatigue across all domains. Other variables were specifically associated with worse fatigue across three domains.
Baseline Predictors
Our data did not support any strong association between child variables at injury and fatigue post TBI. This is not consistent with the existing research conducted in adult TBI that has documented a relationship between female sex and worse fatigue. For example, Ponsford and Sinclair (2014) reviewed the available published studies to examine demographic factors associated with fatigue, and concluded that female sex is the variable most clearly linked to worse fatigue. Furthermore, younger age at injury was not predictive of fatigue, so findings are contrary to the expectation that young age represents a developmental risk for poor outcome. Older age was associated with worse sleep/rest fatigue, suggesting older children suffer greater disturbance of sleep/wake cycles or increased sleepiness than younger children. Adolescent sleep difficulties have been reported widely, further highlighting the risk for older children in the sleep/rest domain (Carskadon, Acebo, & Jenni, Reference Carskadon, Acebo and Jenni2004; Crowley, Acebo, & Carskadon, Reference Crowley, Acebo and Carskadon2007; Gregory & Sadeh, Reference Gregory and Sadeh2016; Ivanenko, Crabtree, & Gozal, Reference Ivanenko, Crabtree and Gozal2005; Lofthouse, Gilchrist, & Splaingard, Reference Lofthouse, Gilchrist and Splaingard2009; Mindell, Owens, & Carskadon, Reference Mindell, Owens and Carskadon1999). Socio-economic status was not related to child fatigue in the present study, although socio-economic status was estimated on highest parent education alone. As such, non-significant findings may relate to the insensitivity of this measure of socio-economic status.
Current data do, however, provide preliminary evidence for the role of preinjury child emotional functioning and preinjury fatigue on postinjury fatigue outcome. Child-specific research demonstrating significant bidirectional associations between mental health and sleep/rest in nonclinical pediatric samples (Alfano & Gamble, Reference Alfano and Gamble2009; Gregory & Sadeh, Reference Gregory and Sadeh2012) suggests that sleep-related fatigue might be particularly linked to emotional well-being, and this relationship might be bi-directional. Preinjury mood impacted on both postinjury general and sleep/rest fatigue, while preinjury fatigue impacted postinjury sleep/rest fatigue only. The role of preinjury mood and fatigue on postinjury fatigue has been established in adult samples (de Leon et al., Reference de Leon, Kirsch, Maio, Tan-Schriner, Millis, Frederiksen and Breer2009; Norrie et al., Reference Norrie, Heitger, Leathem, Anderson, Jones and Flett2010; Sundström et al., Reference Sundström, Nilsson, Cruts, Adolfsson, Van Broeckhoven and Nyberg2007). The current research is novel in documenting this relationship in pediatric samples, and also with respect to specific fatigue domains.
Postinjury Predictors
In general, findings confirm the complexity of fatigue after TBI (Cantor et al., Reference Cantor, Gordon and Gumber2013) and suggest that symptoms experienced postinjury (emotional/physical function, pain, and sleep disturbance) influence fatigue following child TBI (Ponsford et al., Reference Ponsford, Schönberger and Rajaratnam2014).
This study builds upon previous literature that situates fatigue in the context of other psychological symptoms after TBI. There is robust evidence to support a link between mood and post-TBI fatigue. Initially, van Zomeren, Brouwer, and Deelman (Reference van Zomeren, Brouwer and Deelman1984) proposed the “coping hypothesis” to explain fatigue as resulting from the need for compensatory effort for cognitive impairments in information processing speed and attention. Increased need for effort is proposed to result in psychological distress by creating an imbalance between perceived demands and available resources (Ponsford et al., Reference Ponsford, Schönberger and Rajaratnam2014).
Further theoretical and empirical work has explored broader psychological factors associated with fatigue (Ponsford et al., Reference Ponsford, Schönberger and Rajaratnam2014; Schönberger, Herrberg, & Ponsford, Reference Schönberger, Herrberg and Ponsford2014). Although only partially supported by empirical data, authors suggested there are bidirectional influences between postinjury fatigue and postinjury anxiety, depression, and daytime sleepiness, which in turn, exacerbate fatigue by affecting cognitive functioning (through reduced attention vigilance). Current data (in general and cognitive fatigue domains) support the role of mood (but not attention) in bi-directional models of fatigue after TBI (Ponsford et al., Reference Ponsford, Schönberger and Rajaratnam2014; Schönberger et al., Reference Schönberger, Herrberg and Ponsford2014) and suggest that psychological factors also perpetuate child TBI-fatigue.
A novel predictor of 12 months postinjury fatigue was physical symptoms 6 months after injury. Increased physical/motor symptomatology 6 months postinjury consistently predicted 12-month fatigue, across all domains. This finding concords with an adult study demonstrating fatigue 2-years postinjury was associated with worse physical/motor outcomes relative to those with decreased or stable fatigue (Bushnik, Englander, & Wright, Reference Bushnik, Englander and Wright2008). While child TBI studies are lacking, recent data from other acquired brain injuries (multiple sclerosis), reported increased fatigue associated with reduced physical activity participation (Grover et al., Reference Grover, Aubert-Broche, Fetco, Collins, Arnold, Finlayson and Yeh2015). Our study is limited in analysis of the cause of physical/motor impairment, although these preliminary data hints at physical activity as an important influence of fatigue.
Symptoms 6 months postinjury may reflect injury-related sequalae. These symptoms might in turn perpetuate fatigue symptoms. For example, physical/motor symptoms were more frequent in the moderate/severe TBI group postinjury, suggesting these symptoms may result from the initial injury.
Multi-dimensional Fatigue Symptoms
Unique to the present findings are the specificity of predictors across fatigue domains. General and sleep/rest fatigue were associated with sleep disturbance and psychological symptoms 6 months after injury in addition to physical functioning postinjury. Cognitive fatigue was associated only with general physical functioning postinjury. These data suggest that the mechanisms underpinning fatigue vary depending on the dimension(s) being assessed. Consequently, assessment of each fatigue domain is recommended.
Future Directions
Child age at injury and its relationship to fatigue requires further examination, and greater consideration to specific age groups. Influencing factors may vary within the age range examined, for example. Future studies should examine individual age groups (infant/toddler, middle childhood, adolescent) to provide a more developmentally sensitive approach. Longitudinal follow-up after 12 months is also required, as the full impact early TBI may not become apparent until later in development (Anderson, Spencer-Smith, & Wood, Reference Anderson, Spencer-Smith and Wood2011).
Evidence for fatigue intervention is lacking, yet current data highlighted several areas of child function that are implicated in perpetuating TBI-fatigue in childhood. Physical/motor symptoms predicted worse fatigue across all domains. The physical/motor domain may provide an avenue for intervention. Encouragingly, a recent systematic review of the field (Cantor et al., Reference Cantor, Ashman, Bushnik, Cai, Farrell-Carnahan, Gumber and Dijkers2014) highlighted a study that included adult patients who suffered TBI and included fitness training as part of rehabilitation (Hassett et al., Reference Hassett, Moseley, Tate, Harmer, Fairbairn and Leung2009). Although fatigue reduction was not supported by the intervention, participants did not report significant fatigue preinjury, potentially limiting the ability to detect positive effects (as noted by Mollayeva et al., Reference Mollayeva, Kendzerska, Mollayeva, Shapiro, Colantonio and Cassidy2014). Intervention studies are yet to be implemented after child TBI, and findings are pending from a current study that includes active rehabilitation and a low-intensity exercise programme (Reed et al., Reference Reed, Greenspoon, Iverson, DeMatteo, Fait, Gauvin-Lepage and Gagnon2015).
Physical activity may help mitigate an “activity intolerance” cascade, recently proposed as a mechanism for perpetuation of symptoms after mild TBI due to physical deconditioning (DiFazio, Silverberg, Kirkwood, Bernier, & Iverson, Reference DiFazio, Silverberg, Kirkwood, Bernier and Iverson2016). Further research is also needed to examine if strategies to manage sleep difficulties, using behavioral and/or pharmacotherapeutic interventions (Gordijn et al., Reference Gordijn, van Litsenburg, Gemke, Huisman, Bierings, Hoogerbrugge and Kaspers2013), or established mood interventions in child TBI (Ross, Dorris, & McMillan, Reference Ross, Dorris and McMillan2011) would ultimately mitigate persisting fatigue as a result of sleep disturbance. Difficulty sustaining attention, also known as “vigilance decrement” (Pattyn, Neyt, Henderickx, & Soetens, Reference Pattyn, Neyt, Henderickx and Soetens2008), is thought to be associated with cognitive fatigue and negatively impacts on an individual’s QOL by limiting participation in social and educational activities.
Despite the limited data relating to the significance of attention in the current study based on screening measures, and as this may reflect insensitivity of measures, further neuropsychological and neuroanatomical studies (in particular, of cortico-striatal networks) would further inform these potential causal links. Other studies have linked child fatigue decreased quality of life (Eddy & Cruz, Reference Eddy and Cruz2007) as well as reduced participation and school functioning (Carter, Edwards, Kronenberger, Michalczyk, & Marshall, Reference Carter, Edwards, Kronenberger, Michalczyk and Marshall1995; Gold et al., Reference Gold, Mahrer, Yee and Palermo2009; Meeske et al., Reference Meeske, Katz, Palmer, Burwinkle and Varni2004) and provide clues to areas of future intervention research to reduce the impact of fatigue. At the very least, findings should help inform clinical practice and identified risk factors should be areas for careful clinical history taking (particularly of physical function, sleep, and mood) and monitoring in children with TBI.
Limitations
Our current study was adequately powered to detect large effect sizes. Resultantly, caution should be taken when interpreting the nonsignificant findings. Differences between TBI severity groups preinjury might have contributed to variance in fatigue postinjury. The distribution of brain injury by sex was broadly comparable to other TBI samples, however, ratios of males to females are noted to vary across age in child TBI. Therefore, these findings are specific to the age and gender breakdown of our sample. To more accurately characterize sex based differences in fatigue, replication with a larger sample is required.
Additional limitations to the study sample include the disproportionate representation of white relative to non-white racial groups, and reflect a potential selection bias in the sample. Therefore, findings have limited generalizability to participants from non-white racial backgrounds. Socio-economic status was measured using highest parent education, and more robust methods (such as medium income) should be used in future studies. Findings are also constrained by symptom measurement, and reliance on parent-rated outcome measures. Questionnaire report was chosen as the preferred method to assess a broad range of domains while optimizing participant retention. More valid and psychometrically robust measurement methods such as objective measurement of specific symptoms would complement current findings from questionnaire report (e.g., actigraphy for sleep disturbance and activity). Areas of interest beyond the scope of the study include pre- and postinjury cardiovascular health, endocrine problems, infectious diseases, and medication effects. Interrelationships between sleep, mood, and fatigue were also not considered in this study, and further research is required to determine any relationship between these factors.
CONCLUSION
This research examined predictors of fatigue in children 12 months after TBI. We identified child factors preinjury symptoms (fatigue and psychological difficulties), and 6-month symptoms (physical/motor, sleep, and mood) related to 12-month fatigue. These factors might represent potential treatable pathways to address fatigue. Future research would benefit from examining interactions between factors that perpetuate fatigue after child TBI.
ACKNOWLEDGMENTS
This work was supported by a jointly awarded grant from The Ontario Neurotrauma Foundation, Toronto, Canada and The Victorian Neurotrauma Initiative, Melbourne, Australia (V.A. & J.H.); the Victorian Government’s Infrastructure Support Program, Melbourne, Australia (V.A., M.H.B, J.S.H); the National Health and Medical Research Council (A.C., Postgraduate Scholarship 1075048), (E.O. & F.E.B., Centre of Research Excellence Grant); Moving Ahead (A.C., seed grant); Neurosciences Victoria (A.C., Brain and Mind Scholarship); and the Royal Children’s Hospital Foundation (F.E.B). We thank the parents and children for participating in this study. Thank you to Brenton Ward and Carmel Delzoppo (RCH) for assisting in recruitment of participants at RCH. We acknowledge the participants of the Pediatric Research Academic Initiative at SickKids Emergency (PRAISE) for identifying and participating in enrolment of study patients at the Toronto site. No conflicts exist. Members of the Biomarker and Quality of Life in Children with Traumatic Brain Injury group, by site: SickKids: Dr. Jamie Hutchison, Dr. Anne-Marie Guerguerian, Dr. Kathy Boutis, Dr. Martin Post, Sumaira Hussain, Judith Van Huyse, Amy Wilkinson, Helena Frndova. RCH: Dr. Vicki Anderson, Dr. Alison Crichton, Carmel Delzoppo, Mardee Greenham, Dr. Franz Babl, Dr. Warwick Butt. HSJ: Dr Miriam Beauchamp, Mariana Dumitrascu, Cindy Beaudoin, Dr. Catherine Farrell, Dr. Jocelyn Gravel, Charlotte Gagner.







