Published online by Cambridge University Press: 01 September 2004
This study investigated two mechanism-based treatments for expressive aprosodia, a disturbance in emotional prosody thought to be governed by the right hemisphere. The 3 participants all suffered right CVA's resulting in expressive aprosodia. Presence of expressive aprosodia was determined by performance on two batteries of emotional communication. A single subject ABAC design was employed in which two treatments, one imitative and one cognitive linguistic, were assigned in random order. All participants in this study were randomly assigned to begin with the cognitive linguistic treatment. Probes of treated and untreated emotions were completed during baseline and therapy phases. Probe items were judged by a reliable, trained rater blind to time of testing. Visual and statistical analyses were completed. These analyses confirmed that both treatments were active. For example, effect size calculations confirmed modest to substantial treatment effects for both treatments in all 3 patients. Replication to increase confidence about treatment effect and enhance understanding of the neuromechanisms underlying aprosodia is underway. (JINS, 2004, 10, 786–793.)
Prosody is the collective name given to lawful changes in loudness, pitch, speech rate, rhythm, and melody critical to conveying both linguistic and emotional messages. Traditionally, the left hemisphere has been assigned the major role in controlling linguistic prosody and the right hemisphere in controlling emotional prosody. A disruption of the comprehension or expression of emotional prosody has been called aprosodia (Monrad-Krohn, 1947; Ross, 1981; Myers, 1999). In the most severe cases of receptive aprosodia, a listener may be completely ignorant of a speaker's emotional intent, especially when the propositional verbal message differs from the prosodic emotional message (Bowers et al., 1987; Heilman et al., 1984). In the most severe expressive cases, even strongly emotional content may be conveyed with little variation in intonation, rate, or stress (Tucker et al., 1997). The listener is left to guess about the speaker's emotional investment in the speech content. Expressive aprosodia can result from anterior cortical, posterior cortical, or subcortical lesions (Ross, 1981). Types of aprosodia, mirroring types of aphasia, have been posited (Ross, 1981). The emphasis of the present study is on expressive aprosodia whether or not it is accompanied by receptive deficits.
Despite expressive aprosodia's negative effects on human performance, only limited behavioral treatment data are available. Anderson and colleagues (Anderson et al., 1999) employed a single subject design with a 62-year-old man who exhibited aprosodia secondary to a right-hemisphere stroke. Three treatment phases were utilized: a prosody repetition strategy, a cognitive linguistic self-cueing strategy, and a facial expression cross-cueing strategy. Two weeks of treatment were alternated with 2 weeks of no treatment. Preliminary findings suggested the most powerful treatment effect resulted from the prosody repetition treatment. Stringer (1996) also treated a single case of aprosodia. His patient was a 36-year-old female whose aprosodia resulted from traumatic brain injury. He combined two treatments that he called pitch biofeedback and expression modeling. The pitch biofeedback component of the treatment provided acoustic feedback via the Visipitch. Expression modeling required the patient to imitate the clinician's tone of voice and facial expression. Treatment duration was two months. Prosody imitation, prosody production, gesture imitation, and gesture production all improved. Results from these two studies support cautious optimism about the influence of behavioral treatments on aprosodia. However, if behavioral treatments are to be made more robust and applied more confidently to patients with aprosodia, the need, in addition to more data, is for hypotheses about the underlying pathophysiology of aprosodia.
Two hypotheses about the control of expressive, affective prosody motivated the design of the two treatments employed in this study. The first hypothesis is that expressive aprosodia results from a programming deficit (van der Merwe, 1997). Support for this explanation is converging from a variety of sources, especially for persons having a purely expressive aprosodia. Models of motor control are one source. Van der Merwe (1997) describes multiple levels of motor control including planning, programming, and execution. For her, programming is the conversion of a strategy (planning) into programs or what she calls “tactics.” A breakdown in tactics is appealing heuristically as an explanation for the expressive deficit of aprosodic speakers who can and do describe themselves in the clinic as angry, happy, or sad but do so in an emotionless tone of voice and who do not have a traditional dysarthria.
Indirect support for a motor deficit explanation is also provided by the polyvagal theory of Porges (1995). He observes that glossopharyngeal, vagus, and accessory nerves originate in what he calls the same source nucleus, which is nucleus ambiguous (NA). He concludes that as a result “the NA efferent projections are involved … with processes associated with movement, emotion and communication.” It is branches of the vagus that innervate the larynx and are the primary contributor to the larynx's control of fundamental frequency (FO). It is FO that is the most critical component of emotional prosody. This chain of speculations about emotion and the larynx is not damaged by the observation (Pell & Baum,1997) that both left and right hemispheres contribute to emotional prosodic processing. The hemispheres appear to make different contributions with the right having greater control over graded changes than the left. Graded changes in FO across several units of speech (often called intonation) are a primary contributor to affective communication.
If expressive aprosodia does indeed result from a breakdown in the hierarchically organized stages of cortical and subcortical preparations for a motor response, then a treatment program aimed at improving motor performance should be successful. Thus, the imitative–motor treatment was designed to improve motor performance.
The other hypothesized explanation for expressive aprosodia is that expressive aprosodia results from degradation of a modality specific nonverbal affect lexicon (Bowers et al., 1993). The notion is that the prosodic output lexicon comprises “species-typical … prosody” and that it represents a “knowledge base that appears to be right-hemisphere dependent in humans.” This nonverbal affect lexicon was compared to the verbal lexicon of the left hemisphere.
In the left hemisphere, the verbal lexicon is likely instantiated in a pattern associator network linking distributed concept representations in association cortices to articulatory motor representations in Broca's area and adjacent opercular areas 4 and 6. By analogy, the affective lexicon may be instantiated in a pattern associator network linking predominantly nondominant hemisphere association cortices and limbic structures with right prefrontal and premotor cortex. When connectionist networks are damaged, they exhibit graceful degradation. That is, they do not produce completely novel responses reflecting new operational principles. Instead, the probability of producing the correct response is reduced and incorrect responses tend to be near misses. Even networks damaged to the point that they are incapable of generating any response at all may still contain a great deal of knowledge residing in the remaining undamaged connections (Plaut, 1996). Our second treatment for affective aprosodia, the cognitive linguistic treatment, is based upon the hypothesis that the affective lexicon pattern associator has been damaged to the point that it cannot produce any appropriate responses. However, because of graceful degradation, there is the possibility of potentiating the expression of the residual knowledge by both enhancing limbic input and by training participants in an explicit intonation strategy, which presumably enhances prefrontal input to the affective lexicon pattern associator.
The purpose of this study, then, is to determine the outcome of two mechanism-based treatment approaches, an imitative motor treatment and a cognitive linguistic treatment in three participants with expressive aprosodia using a single subject design.
The 3 participants, 2 men and 1 woman, were right-handed, native English speakers who suffered a right hemisphere CVA with resulting expressive aprosodia (see Table 1 for a listing of relevant participant demographic information). Presence and severity of aprosodia was independently determined by four trained raters who judged each participant's performance on two emotional communication batteries, the Florida Affect Battery (Bowers et al., 1998), and an unpublished emotional expressive battery being developed by the Cognitive Neuroscience Laboratory at the University of Florida. The Florida Affect Battery (Bowers et al., 1998) assesses the ability to identify spoken prosody as well as facial expression of emotional affects. The emotional expressive battery has participants perform a series of subtests, the first three test ability to imitate syntactic and emotional prosody, and the other three to produce syntactic and emotional prosody to command. Participants were also given the Mini Mental Status Examination (MMSE) (Folstein et al., 1975), and were assessed for visual spatial disorders using the Rey-Osterieth Complex Figure Test (Rey-O; see Lezak, 1983) and Judgment of Line Orientation (JOLO; Benton et al., 1983). Mood assessment scales, including the Geriatric Depression Scale (Yesavage et al., 1983) and Beck Hopelessness Scale (Beck & Steer, 1988), were also administered to rule out depression and other neuropsychological explanations for aprosodia. Table 2 lists pre-treatment scores on these tests for all participants.
Subject demographic information

Pre-treatment cognitive testing and mood assessment scores
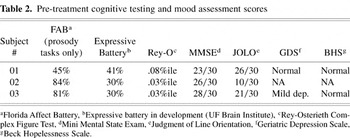
Participant 01 demonstrated reduced ability to change rate, pitch, or loudness in speech. He was able to vary pause time. His aprosodia was moderately severe. He did not demonstrate any signs of dysarthria or lingual, palatal, or facial weakness. He told his therapist that others had sometimes complained of trouble understanding him when he spoke, but that he did not believe it to be a significant problem. His judgment of angular orientation of lines as demonstrated by performance on the JOLO was within normal limits. His performance on the Rey-O showed visual spatial skill deficits involving accurate inclusion and integration of details.
Participant 02 demonstrated almost no changes in rate, pause time, pitch, or loudness in speech. Her aprosodia was severe. She did not demonstrate any signs of dysarthria or oromotor or facial weakness. She did not complain about her speech. Her judgment of angular orientation of lines as demonstrated by performance on the JOLO was significantly impaired. Her performance on the Rey-O showed difficulties including and accurately integrating visual information and details.
Participant 03 demonstrated limited changes in rate, pause time, pitch, and loudness in speech. His aprosodia was moderately severe. In addition, he presented with a mild dysarthria with consonant imprecision. He also presented with a left facial droop. He did not complain about his speech. His judgment of angular orientation of lines as demonstrated by performance on the JOLO was moderately impaired. His performance on the Rey-O was impaired. He omitted several visual details and had some difficulty organizing the remaining details.
This study investigated two mechanism-based treatments for expressive aprosodia, an imitative treatment and a cognitive linguistic treatment. Both treatments followed a six-step cuing continuum. Maximum cueing was provided in the first step and was systematically decreased as the patient progressed to the final step. For example, in the first step of the imitative treatment, the clinician provided a model sentence using the correct emotional prosody and the participant attempted to imitate using the same emotional tone of voice. In the ensuing steps the participant moved from immediate imitation of the clinician's model to independent production of the sentence using the target emotional tone of voice. In the first step of the cognitive linguistic therapy, the participant was provided with the name of the target emotion, the vocal characteristics of that emotional tone of voice, and a picture showing the appropriate facial expression for the target emotion. These cues were systematically removed as the participant successfully moved through each step. The steps for both experimental treatments and the corresponding stimuli are outlined in Table 3.
Experimental treatment steps
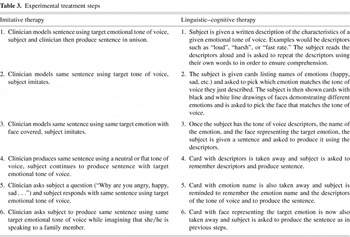
Prior to each treatment session, the participant was administered a series of probes to be described further under outcomes measures. Each treatment session consisted of training on nine sentences (three each of happy, sad and angry) presented in random order. Treatment for each sentence began with step one and was continued through step six, unless the participant failed to produce three consecutive correct responses. The participant was given a maximum of fifteen attempts at each step. If a participant failed to move through a given step, the next randomly ordered sentence was introduced and the process was repeated.
The co-authors and colleagues generated the treatment stimuli by compiling a list of sentences invoking an affective response. Of these sentences, the 20 sentences eliciting the strongest affective response for each emotion amongst the co-authors and colleagues were selected as stimuli. All sentences were semantically congruent with their accompanying emotional tone of voice. These sentences were then divided into three main lists, one to be treated during imitative treatment, one to be treated during cognitive linguistic treatment, and one that was never treated but was used as a control.
A single subject ABAC design with replication across 3 participants was employed. During the initial no-treatment phase (A), stable baselines for verbal production of five emotional tones of voice were established and verified via the C-statistic (Tryon, 1982). Both treatment phases (B and C) were approximately 1 month in duration and consisted of 20 treatment sessions. The average treatment session length was 1 hr. The two treatments were assigned in random order and were separated by a second no treatment phase that was also 1 month in duration. All three participants were randomly assigned to receive cognitive linguistic treatment during the first treatment phase, followed by imitative treatment.
Probes of four treated emotions (happy, sad, angry, and neutral) and probes of one control, or non-treated emotion (fear), were completed eight times during both pre-treatment baseline phases (A phases), daily prior to the start of therapy (both B and C phases) and during the post testing that directly followed both treatments.
There were a total of 50 probe sentences completed during each baseline session and 45 sentences preceding each treatment session. The probes were administered by presenting a sentence written on a card to the participant, who was then asked to say the sentence aloud using a particular tone of voice (e.g., Patient was shown “Our house is on fire” and asked, “Please say this sentence using a fearful tone of voice”).
The corpus of probe sentences included sentences that were considered control probes (fearful tone of voice was never treated), some that were considered generalization probes (sentences which were never treated but were produced using trained tones of voice—angry, sad, happy), and sentences that were actively treated. The entire corpus was randomized to create daily probe lists.
All probes were audiotaped. Audiotaping was done on a Marantz portable cassette recorder (model pmd430) for the first two participants in this study. All subsequent audiotapes were made on Tascam digital audio tape recorders (model DA-P1). Each probe item was scored as “plus” if correctly conveying, or “minus” if incorrectly conveying the requested emotional tone of voice. Scoring was done online during the session by the therapist, and was also later judged by a trained rater blind to the time of testing. The judgments of both the therapist and trained rater were based solely on verbal expression, facial expression was not a factor.
The trained rater was a speech–language pathologist with two years of experience in evaluating the prosody of emotionally intoned sentences. Training for this rater included familiarization with the descriptions of features for each emotion including respective changes in pitch, loudness, and rate. The rater also took part in research group sessions during which tapes of aprosodic speakers were discussed and individual features rated. Acoustic analysis of selected pre- and post-treatment data subsequently supported the trained rater's judgments. The trained rater's judgments were the data used in all analyses. Both intra- and inter-judge reliability were calculated using 20% of each participant's productions. Intra-judge reliability for the trained rater was acceptable (Kendall's Tau of 0.75, p < .001). Inter-judge reliability based on judgments by the trained rater and another experienced clinician (B.H.) was also acceptable (Kendall's Tau of 0.79, p < .001).
Data were analyzed visually and statistically. Visual inspection of probe data was completed by three judges, all speech-language pathologists, all with at least three years experience judging data via visual inspection. Figure 1 displays the graphs the judges used for the visual analysis. They were asked to judge the stability of both baseline phases for each participant and then to consider the relative slope and height of the data displays during the two treatment phases. They received similar directions for judging displays for the untreated emotion.

Percent correct on treated and untreated probe stimuli.
For the statistical analyses, effect sizes (Robey et al., 1999) were calculated for each participant for each therapy. The effect size for treatment one was calculated by subtracting the mean of the correct responses on the eight baseline probes from the mean of the correct responses on the twenty therapy probes, divided by the standard deviation of the baseline probe data. The effect sizes for each participant for the second therapy were calculated in an identical manner using data from the second therapy. The formula used to calculate the effects sizes (ES) is as follows:

Three judges unanimously agreed that visual displays of probe data (see Figure 1) showed evidence of treatment effects from both treatments for all three participants. No evidence of generalization to the untreated emotion was noted for any participant. Obvious differences in response to the treatments for the 3 participants exist.
Participant 01 was the most variable, particularly during the cognitive linguistic treatment, suggesting that the cognitive linguistic treatment initially disrupted pretreatment performance without providing a stable alternative. Despite the variability, best performance during cognitive linguistic treatment was clearly superior to pre-treatment performance. However, it is also true that this participant's worst performance during cognitive linguistic was worse than baseline performance. It may be that treatment temporarily destabilized the participant's verbal expression of prosody by discouraging his abnormal pattern, before a stable, new response was predictably available. Variability continues during the baseline testing prior to the imitative treatment and relative stability does not occur until the final six or seven imitative treatment sessions. Relative stability was maintained at post-treatment testing. Participant 01 was the longest post-stroke and therefore would have spent the most time post-stroke using altered prosodic contours.
Participant 02 was 6 months post stroke and demonstrated stable baseline performance. A treatment effect does not emerge until the final sessions of the cognitive linguistic treatment. Following discontinuation of treatment, performance seems to retreat to pre-treatment levels. Considerable variability occurs after introduction of the imitative treatment but the overall level of treatment effect is also higher than during the cognitive linguistic treatment. Again, a treatment effect appears to be maintained at post-testing.
After a stable baseline period, Participant 03, who was 4 months post stroke, shows a dramatic treatment effect for the cognitive linguistic treatment. The imitation treatment was limited by a ceiling effect. When this participant reached 100% accuracy on probes of treated emotions, treatment was discontinued. Treatment effects appear to have been maintained at post-testing.
Examination of effect sizes (see Table 4) confirmed modest to substantial treatment effects for both treatments in all three participants. For Participant 01, the effect size was 1.224 for cognitive linguistic treatment and 1.183 for imitative. Participant 02 showed an effect size of .660 for cognitive linguistic treatment and 2.542 for imitative. Effect sizes for Participant 03 were 11.518 for the cognitive linguistic treatment and 2.015 for imitative. These differences are moderate to large by traditional standards (Cohen, 1988) and are within the range of effect sizes in the aphasiology literature for a variety of treatments and aphasia diagnoses (Robey, 1994).
Z scores for change associated with first (cognitive–linguistic) and second (imitative) therapies

This was a phase I study of mechanism-based treatments for expressive aprosodia. The data suggest that both treatments improve production of affective prosody. Clearly, however, more individuals with aprosodia must be treated before confident conclusions can be reached. In addition, because all three participants began with the cognitive linguistic treatment, it is impossible to sort out the effects of treatment order. The most one can say is that a cognitive linguistic treatment for aprosodia is active and that a subsequent imitative treatment adds to the activity. Sorting through the order effects, as part of a systematic effort to increase the number of persons treated, is the focus of ongoing research.
Within the study's limitations, the data on generalization are also informative. First, the treatment did not generalize to the untreated emotion, fear. The finding that the treatment does not generalize to an untreated emotional prosody suggests that the representation that contains the information needed to produce this prosody is independent of the representations needed to produce other emotional prosodies. It is also true that fear was the most impaired emotion as was evidenced by the extremely low baseline performance. This was not anticipated and is not easily explained. In a subsequent study we will treat fear to ascertain whether it is amenable to treatment.
However, performance on an unpublished expressive aprosodia battery currently undergoing standardization does suggest generalization. Specifically, all 3 participants improved by at least 20 percentage points. Participant 03, who showed the greatest effects sizes for therapy probes, more than doubled his expressive aprosodia score from 30% to 65%. Until this tool is standardized and a larger sample of patients is treated, these data will not be analyzed statistically. It can be posited, however, that changes on this measure will parallel those on the probes that were the main outcome of this study. Investigation of generalization to extra-clinical settings is also underway.
Receptive aprosodia was measured using a standardized battery (Florida Affect Battery; Bowers, et al., 1998). Participant 01 presented pre-therapy with a moderate receptive prosody impairment, participants 02 and 03 had only mild receptive prosody deficits. Changes from pre- to post-therapy in the receptive aprosodia scores of the 3 participants were positive but relatively inconsequential; all were at or under 5 percentage points. All three subjects responded to the treatments for their accompanying expressive aprosodia but it does not appear from the receptive aprosodia data that receptive deficit predicts response to treatment. This is only a hypothesis however, because these data will not be analyzed statistically until more subjects have been treated.
No conclusions about the relationship of site of lesion to most active treatment are possible. Participant 01, who had a medial frontal lesion and might have been expected to be most responsive to the imitative treatment, responded similarly to both treatments. Participant 02, who had involvement of the centrum semi-ovale, internal capsule, and portions of the striatum, responded most positively to the imitative treatment, consistent with the prediction. Participant 03, for whom we had no imaging data but who presented with a dense left hemiplegia suggesting motor system involvement, responded most robustly to the cognitive linguistic treatment. More data will be necessary if hypotheses about the pathophysiology of expressive aprosodia are to be supported. For example, the case for an executive deficit will be strengthened by evidence of robust effects to the imitative treatment in participants with expressive aprosodia that is unaccompanied by receptive aprosodia.
Despite the limitations of this phase I study, the evidence it provides of treatment effects for aprosodia is important. First, aprosodia has functional consequences, because normal relationships are handicapped by an inability to signal emotion. Therefore, active treatments raise the possibility of functional impact. Furthermore, there is a relative dearth of treatment data about any of the myriad deficits of right hemisphere lesions. Foremost among these is anosagnosia or denial of signs and symptoms. These three participants had differing degrees of anosagnosia clinically, however all 3 were faithful in their attendance at treatment sessions. And all three improved. This suggests that aprosodia and other right hemisphere deficits may be amenable to behavioral treatments.
Additional participants with expressive aprosodia are currently being treated. With more data the answers to several questions may become clearer. Specifically, the relative activity of both the cognitive linguistic and imitative treatments may be established. In addition, their effects on extra-clinic functioning may emerge. With this clarification may come additional understanding of the underlying pathophysiology of aprosodia. Clinicians and theoreticians may both be rewarded.
Supported in part by VA Office of Research and Development, Rehabilitation R&D Service, Brain Rehabilitation Research Center and by NIDCD/NIH Grant P50 DCO3888. We would like to thank S. Freshwater, Ph.D., for technical assistance in this study.

Subject demographic information

Pre-treatment cognitive testing and mood assessment scores

Experimental treatment steps

Percent correct on treated and untreated probe stimuli.

Z scores for change associated with first (cognitive–linguistic) and second (imitative) therapies