INTRODUCTION
Being born to a mother with epilepsy (0.3–0.4% of children, Olafsson et al., 1998) may affect the developmental outcome of the offspring in several ways. Prenatal exposure to antiepileptic drugs (AED) is associated with an increased incidence of major malformations (Tomson et al., 2004). Genetic factors may also contribute to developmental morbidity, possibly by modifying susceptibility to teratogenic agents (Duncan et al., 2001; Malm et al., 2002). Exposure to prolonged (Hiilesmaa, 1996) and multiple maternal seizures (Vinten et al., 2005) are additional risk factors. Maternal epilepsy, especially of the idiopathic variety, may imply a genetic predisposition to epilepsy in the offspring, although symptomatic epilepsies may also have a genetic basis (Winawer & Shinnar, 2005). Because epilepsy is multifactorial in origin, the risk contributed by genetics is generally modest, varying from the population rate of 1% to 2.8–8.7% in the offspring of a mother with epilepsy. Apart from raising risks for epilepsy, maternal epilepsy may also predispose offspring to impairment in brain maturation and ensuing neurocognitive dysfunction (Doose et al., 1996). These potential genetic influences on the offspring's development must be considered when evaluating the effects of maternal AED.
A recent Cochrane review (Adab et al., 2004) summarized the research on cognitive effects of maternal epilepsy from 1966 through 2003 and concluded that the available studies do not provide conclusive data to determine risks associated with AED. A retrospective study by Dean et al. (2002) found an increased risk or developmental delay associated with most AED, whereas prospective studies by Gaily et al. (1988) and Shapiro et al. (1976) did not find impaired IQ after prenatal exposure to phenytoin monotherapy. Vinten et al. (2005) found that prenatal valproate exposure was associated with impaired verbal IQ (VIQ) at 5–16 years of age in a retrospective study. A prospective study by our research group (Gaily et al., 2004) found also significantly reduced VIQ associated with polytherapy and valproate exposures, whereas there were no differences in performance IQ (PIQ) or full-scale IQ (FSIQ) between children exposed to carbamazepine in utero and controls.
In one of the few studies to examine specific neuropsychological skills, Gaily et al. (1990) observed significantly lower scores on tests of phonemic and visuospatial skills, though no overall IQ difference, in children of mothers with epilepsy compared with control children. These findings could not be explained by drug exposure but were associated with factors that suggested hereditary influences on test performance. Wide et al. (2002) reported lower locomotor function scores in phenytoin-exposed children compared with children exposed to carbamazepine, children without fetal exposure to AED, and controls at age 2–8 years. In Rovet et al.'s (1995) clinic-based study, impaired language skills were found in children aged 7–85 months whose mothers received phenytoin or carbamazepine during pregnancy. Impairment was more marked with phenytoin exposure. Limitations of the latter study include the lack of a control group exposed to maternal epilepsy without AED and the use of different tests for different age groups.
Potential age and gender differences in the effects of maternal epilepsy have not been examined in past studies. Variations between studies in participant ages might explain some of the lack of consistency in the findings, suggesting the need to take this factor into account. Justification for examining gender differences is provided by studies that reveal more pronounced effects of other types of neurological risks, such as very low birth weight and hypoxia, on males than on females (Kheirandish et al., 2005; Morse et al., 2006; Raz et al., 1995).
The primary objective of the present study was to investigate specific cognitive outcomes in children of mothers with epilepsy and to identify risks for cognitive impairment. Risk factors examined included fetal exposure to AED, maternal seizures, and type of maternal epilepsy. We compared the children from our previous study (Gaily et al., 2004) on a neuropsychological test battery to determine if maternal epilepsy had negative effects on outcomes other than VIQ and to explore epilepsy characteristics related to these negative effects. An additional aim was to determine if the effects of maternal epilepsy or of epilepsy characteristics varied with the child's age or gender.
METHODS
Participants
The basis for recruitment was the database of all 306 children born during the years 1989–1994 in the Department of Obstetrics and Gynecology of the Helsinki University Central Hospital (HUCS) to mothers with epilepsy. This database represented 71% to 95% of all infants born to mothers with epilepsy in the Uusimaa province during this period. Maternal drug intake and seizures during pregnancy were recorded prospectively. Control children were born in the same hospital during the same time period to mothers without epilepsy, who were matched pairwise with each mother with epilepsy on age, educational level, and parity. Because any siblings of the children in either group born during this period at HUCS were also included, the groups were not paired. Informed consent was obtained from one or both parents of all participating children. The ethics committees of the Hospital for Children and Adolescents and the Department of Neurology, HUCS, approved the study.
One hundred sixty-six of the 306 study children invited to participate were assessed, together with 136 of 276 controls. The eight children with VIQ and PIQ both below 75 were excluded, because the emphasis was on exploring the specific neuropsychological effects of maternal epilepsy on offspring. Furthermore, four children with either suspected of confirmed epilepsy, and six children with a history of more than one febrile convulsion were excluded. Thus 154 children (50% of the total sample) born to 120 mothers with epilepsy (study group) and 130 children (47% of the total sample) born to 110 mothers without epilepsy (control group) were included in the present study. There were no differences in the distribution of the maternal epilepsy medication between participants and non-participants (Gaily et al., 2004). Compared with the adult population of similar age in Uusimaa province (Tilastokeskus, 1999), more of our study and control families tended to belong to the middle educational categories, whereas parents who had completed only basic education were somewhat underrepresented in our sample. All mothers were ethnic Finns.
The children were assessed at an outpatients' clinic of HUCS in a single session lasting 1–2 hours. The neuropsychologist was not informed of the child's group assignment. The age at assessment was 5 to 11 years. Based on the distribution of ages in the total sample, age was classified as 5 years, 6–7 years, and 8–11 years. In addition to the measures described below, 6-subtest versions of the Finnish versions of Wechsler Preschool and Primary Scale of Intelligence, Revised (WPPSI-R, Wechsler, 1995) for 5-year-olds, or the Wechsler Intelligence Scale for Children, Revised (WISC-R, Wechsler, 1984) for older children, were administered as reported by Gaily et al. (2004).
A structured interview was conducted with the child's parent(s) by the study nurse while the child was being tested. Data was obtained on the child's postnatal developmental milestones, health (major illnesses yes/no), daycare (home/kindergarten), school program (special/mainstream class), and any learning problems. Additional data pertained to single parenthood, maternal and paternal health, drug treatment, and occupation and educational levels. Seven study and five control children had had one febrile convulsion. The groups did not differ significantly on any of these variables.
The distributions of maternal and paternal education, age, and gender in two groups are shown in Table 1. Although the groups did not differ in mean age or in the distribution of gender or maternal or paternal educational levels, proportionally more of the study group children were 5-year-old (52 study children versus 30 controls; χ(1,284)2 = 3.92; p = .048). Because of a tendency (p < .1) for proportionately more mothers of children who were exposed to valproate during pregnancy to be from the lower education categories (data not shown) maternal education was used as a covariate in the analyses.
Age, gender, and parental education in the study group and control group

Maternal epilepsies were classified according to the Proposal for revised Classification of Epilepsies and Epileptic syndromes (ILAE Commission, 1989) as described in Gaily et al. (2004). Three different epilepsy types were defined: idiopathic generalized epilepsy, cryptogenic/symptomatic partial epilepsy, and unclassified. In addition, there were two children of a mother with Baltic type progressive myoclonic epilepsy. Forty-six children had been exposed to maternal seizures (including auras) during pregnancy; out of these, 23 exposures were to one or more maternal generalized tonic-clonic seizures. The number of these seizures ranged from 1–11; only 4 children had been exposed to more than 5 seizures. Maternal epilepsy types, seizures, and AED during pregnancy are given in Table 2. Epilepsy type was not significantly associated with either maternal medications or seizures. Polytherapy was significantly associated with any seizures (χ(4,154)2 = 25.7; p < .001), and with generalized tonic-clonic seizures (χ(4,154)2 = 12.7; p = .01). Further information regarding epilepsy diagnoses and drug combinations is reported in Gaily et al. (2004).
Distribution of the study group children in subgroups based on exposure to maternal epilepsy types, medication and seizures during pregnancy

Neuropsychological Assessment
The assessment comprised 13 subtests of NEPSY: A Developmental Neuropsychological Assessment (Korkman et al., 1998) from the domains of attention, language skills, visuo-spatial skills, manual fine motor skills, and memory and learning. Standardized for Finnish children (Korkman et al., 1997), this test assesses cognitive skills in a number of ability domains, enabling analysis of cognitive strengths and weaknesses. The subtests used were chosen to provide a broad-based assessment. Previous studies have demonstrated the sensitivity of the selected NEPSY subtests to reading disorder and attention disorder (Korkman & Peltomaa, 1991; Korkman & Häkkinen-Rihu, 1994; Korkman et al., 1998). In addition, the Grooved Pegboard test (Knights & Moule, 1968) was used as a test of manual dexterity. Age-corrected standard scores for the NEPSY subtests (mean = 10, SD = 3) were used in the analyses. Because there is no Finnish standardization for the Grooved Pegboard test, z-scores (mean = 0, SD = 1) were defined for each age group based on the performance of the control children. Inspection of the scores for each age group suggested normal distributions. The measures are described in further detail below by domain.
Attention
Measures of attention included the NEPSY subtests Auditory Attention and Response Set, and Visual Attention. The former subtest consists of two continuous performance tasks that assess the ability to be vigilant and to sustain selective auditory attention (part A of the test) as well as to shift and maintain a new and complex set involving conflicting and matching responses (part B). The stimuli are presented on audiotape. Parts A and B were analyzed separately. The Visual Attention subtest is designed to assess the speed and accuracy with which a child is able to focus selectively on and maintain attention to visual targets within an array.
Language skills
The language domain was evaluated by administering the NEPSY subtests Phonological Processing, Comprehension of Instructions, Speeded Naming, Comprehension of Sentence Structures (not included in the American standardization of the NEPSY), and Repetition of Nonsense Words. These subtests were designed to assess, respectively, phonological awareness and segmentation; processing verbal instructions of increasing complexity; rapid accessing and production of names of recurring colors, sizes, and shapes; understanding complex meanings conveyed by sentence syntax; and phonological encoding and decoding as measured by the repetition of nonsense words presented on audiotape.
Manual fine motor skills
Tests of this skill domain included the NEPSY subtest Manual Motor Sequences, and the Grooved Pegboard Test. The former subtest is designed to assess the ability to imitate a series of rhythmic movement sequences using one or both hands. In the latter timed subtest, the subject is required to place grooved pegs in 10 holes on a pegboard with each hand separately.
Visuospatial skills
Tests of these included the NEPSY subtests Arrows and Design Copying. The former subtest assesses the ability to judge line orientation by requiring the subject to indicate the two arrows out of eight on each page that are going to hit the centre of a target. The latter subtest assesses the ability to copy designs of increasing complexity.
Memory and learning
Tests of this domain included the NEPSY subtests Memory for Names, Narrative Memory, and Sentence Repetition. The first subtest assesses the ability to learn the names of pictured children over three learning trials and to recall them in a delayed condition. The second subtest estimates the ability to retell a story under free and cued recall conditions, and the third the ability to repeat sentences of increasing complexity and length.
Statistical analyses
Using the Statistical Package for Social Sciences 10.0 program, each test domain was subjected to a three-way multivariate analysis of covariance (MANCOVA). Fixed factors were group, age (5 years, 6–7 years, and 8+ years), and gender. Maternal education was included as a covariate.
To identify risk factors for poorer child outcomes within the study group, the children were subgrouped on the basis of the maternal epilepsy type and whether or not mothers had any seizures during their pregnancy with the child, as well as on the basis of medication exposure. Epilepsy types were maternal idiopathic generalized epilepsy (n = 60) and cryptogenic/symptomatic partial epilepsy (n = 81). The children of mothers with unclassified epilepsy (n = 11), as well as the two children of a mother with progressive myoclonic epilepsy, were excluded from analyses concerning effects of type. Children were subgrouped according to maternal AED during pregnancy using two approaches. First, each child was classified based on the number of AED during pregnancy (none, n = 38; monotherapy, n = 92; polytherapy, n = 24), regardless of type of AED; and second, each child was classified with regard to valproate exposure, either in monotherapy or polytherapy (no AED, n = 38; non-valproate AED, n = 94; valproate, n = 22).
Subgroup comparisons were conducted using MANCOVA with maternal education and gender as covariates, because no significant gender interaction was observed in the main group analyses. Comparisons were also made between children who were exposed to maternal seizures in utero and those who were not. Age was excluded as a factor in these analyses because of small cell sizes. After conducting subgroup analyses, contrast terms were employed to compare each of the AED or epilepsy type subgroups with the control group.
Similar univariate analyses of covariance (ANCOVAs) were used to examine group and subgroup differences in performance in each test domain. Domain-wise alpha for the group and subgroup analyses were.05, with Bonferroni correction applied in determining significance for within-domain univariate comparisons (α = .05/number of tests in domain). Post hoc tests or pairwise comparisons with Student t-test were carried out where appropriate. Box test was used to test the homogeneity of the variances of the groups. Levene correction was applied when groups' variances appeared unequal. Given inclusion of siblings and potential violation of the assumption of independence, analyses were repeated using data from only one child per family (the eldest within the sample period in cases of multiple siblings). The results of these analyses were similar to those reported later.
RESULTS
Comparison of Study and Control Groups
The test score means and standard deviations of the study and control groups are presented in Table 3. The MANCOVAs showed significant group effects for Attention, Memory, and Motor domains. For the Attention domain there was also a significant group × age interaction (Wilks' λ = .95, F(6, 528) = 2.47, p = .023). The effect for age was significant for Attention (Wilks' λ = .86, F(6,528) = 6.90, p < .001), Memory (Wilks' λ = .93, F(6,520) = 3.1; p = .006), and Motor (Wilks' λ = .92, F(6,516) = 3.5, p = .002) domains, and the effect for gender significant for Attention (Wilks' λ = .97, F(3,264) = 3.07, p = .03), Visual (Wilks' λ = .77, F(2,263) = 38.6, p < .001), and Motor (Wilks' λ = 0.95, F(3,258) = 4.8, p = .003) domains. The group × gender interactions were not significant. The effect for maternal education was significant for Attention (Wilks' λ = .94, F(3,264) = 5.47, p = .01), Verbal (Wilks' λ = .94, F(5,253) = 3.14, p = .009), Visual (Wilks' λ = .97, F(2,263) = 4.57, p = .01), and Memory (Wilks' λ = .92, F(3, 260) = 7.55, p < .001) domains.
NEPSY subtest, Pegboard, and IQ means (M) and standard deviations (SD) for the study and control groups with F(degrees of freedom) and p values from domainwise MANCOVAs

Univariate comparisons revealed that the study group scored lower than controls in Auditory Attention and Response Set A and B, Visual Attention, Memory for Names, Manual Motor Sequences, and Grooved Pegboard, both hands (Table 3). Analysis also revealed a group × age interaction for Auditory Attention A (F(2,269) = 6.82, p = .01; Fig. 1). According to follow-up tests, the group difference was significant for 5-year-olds (t(74.6) = −3.4, p = .001), approached significance for children 6–7 years (t(96) = −2.4, p = .019), but was not significant for children 8+ years. Gender effects were found on Auditory Attention B (F(1,268) = 8.2; p = .005), Design Copying (F(1,269) = 10.0; p = .002), Manual Motor Sequences (F(1,260) = 13.9; p < .001), and Arrows (F(1,265) = 40.7; p < .001), with girls performing better on the former three subtests and boys doing better on the latter subtest.

Means with 95% confidence intervals of scores on Auditory Attention A as a function of age in study and control groups. The number of children (n) in each group is presented by the corresponding bars.
The results changed little when FSIQ was added as a covariate in the above analyses. FSIQ was a significant correlate of performance. However, with this factor in analysis, the effect of maternal education was no longer significant. Group differences in the Memory domain were reduced to nonsignifance with FSIQ as a covariate.
Associations with AED Exposure
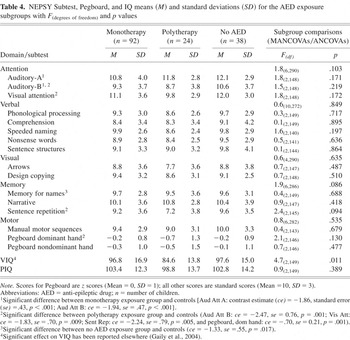
MANCOVAs conducted within the study group to determine the effect of number of AED (no AED, monotherapy, or polytherapy) failed to reveal significant group effects for any domain (Table 4). However, when medication exposure subgroups were compared with the control group using univariate contrasts, the subgroup not exposed to medication obtained lower scores than controls on Memory for Names, the monotherapy subgroup performed less well than controls on Auditory Attention A and B, and the polytherapy subgroup scored less well than controls on Auditory Attention B, Visual Attention, Sentence Repetition, and Pegboard, Dominant hand. Because the monotherapy subgroup consisted largely of carbamazepine exposures (76/92), of whom 35 were in the youngest age group, an additional analysis was undertaken to contrast the carbamazepine exposed subgroup to controls with age as a second fixed factor. The results of this analysis were similar to those reported earlier.
NEPSY Subtest, Pegboard, and IQ means (M) and standard deviations (SD) for the AED exposure subgroups with F(degrees of freedom) and p values
The comparison of the effects of valproate (alone or in combination with other AED) to other AED or no AED revealed a significant group effect for the Attention domain and the Memory domain (Table 5). When valproate exposure group was compared to each of the other subgroups separately, the group effect remained significant (valproate exposure vs. no AED exposure F(3,53) = 3.5, p = .018 for Attention domain, F(3,53) = 3.6, p = .020 for Memory domain; valproate exposure versus other AED exposure F(3,107) = 4.1, p = .009 for Attention domain, F(3,107) = 4.1, p = .009 for Memory domain). Corresponding univariate comparisons indicated group differences in Auditory Attention B and Sentence Repetition. According to post-hoc comparisons, scores on Auditory Attention B and Sentence Repetition were significantly lower for the valproate-exposed subgroup than for the other-AED-exposure or no-exposure subgroups. Comparisons of each subgroup with the control group revealed that the valproate-exposed subgroup scores on Auditory Attention B and Sentence Repetition were also significantly lower than those of the controls. The subgroup exposed to other AED scored also lower than the controls on Auditory Attention B, but there were no other significant differences between other subgroups and controls on the two subtests. The means with 95% confidence intervals for these two subtests for these medication subgroups and the control group are presented in Figure 2.
NEPSY subtest, Pegboard, and IQ means (M) and standard deviations (SD) for the VPA exposure subgroups with F(degrees of freedom) and p values


Means with 95% confidence intervals for scores on Auditory Attention B and Sentence Repetition in the medication exposure subgroups and the control group. The number of children (n) in each group is presented below the corresponding bars.
Association to VIQ (Gaily et al., 2004) was explored by running correlations between VIQ and the NEPSY subtests for the total sample (study and control groups combined) and for the valproate exposure subgroup. Correlations for the total sample were significant (p < .01) for all NEPSY subtests, ranging from 0.18 (Auditory Attention A) to 0.55 (Comprehension of Sentence Structures). The subtests that correlated significantly with VIQ in the valproate exposure subgroup were Sentence Repetition (r = .82, p < .001) as well as Phonological Processing (r = .57, p = .006) and Memory for Names (r = .56, p = .007). Scores on Sentence Repetition were significantly related to maternal serum valproate level during pregnancy (r = −.43; p = .047).
Associations With Type of Maternal Epilepsy and Seizures During Pregnancy
MANCOVAs conducted within the study group to examine the effects of type of epilepsy on neuropsychological performance revealed significant group effects for the verbal, visual, motor, and memory domains (Table 6).
NEPSY subtest, Pegboard, and IQ means (M) and standard deviations (SD) for the subgroups based on the type of maternal epilepsy with F(degrees of freedom) and p values

Results from univariate analyses indicated that the children of mothers with idiopathic generalized epilepsy scored significantly less well than those of mothers with partial epilepsy on Design Copying and Pegboard Dominant hand, whereas children of mothers with idiopathic generalized epilepsy performed significantly better than partial epilepsy subgroup on Narrative Memory. The effect found for the Verbal domain failed to be significant for any subtest taken singly, although the idiopathic epilepsy offspring tended to score slightly better. In univariate comparisons with controls, both epilepsy type subgroups differed from controls on the Auditory Attention A and Auditory Attention B, though the epilepsy type subgroups did not differ significantly from one another on these measures. Furthermore, the performance on Pegboard, Dominant hand, and Pegboard, Nondominant hand, was significantly poorer in the offspring of mothers with idiopathic epilepsy than in the controls.
Children whose mothers had had seizures during pregnancy did not differ from children not exposed to seizures on any of the measures nor were differences found when children exposed to maternal generalized tonic-clonic seizures during pregnancy were compared with the rest of study group or with the controls.
DISCUSSION
Despite similar IQ scores, children of mothers with epilepsy in this study performed less well than controls on neuropsychological tests related to attention, memory, and manual dexterity. This result is consistent with findings reported previously by Gaily et al. (1988, 1990). Whereas impairments in attention were mainly seen in the younger children in the study group, impairments related to memory and hand fine motor skills showed no age interaction. There was no interaction between gender and maternal epilepsy and thus nothing to suggest specific male vulnerability to effects of maternal epilepsy.
When subgroups exposed to maternal monotherapy (mostly carbamazepine), to polytherapy, or to no AED were compared to the controls, deficits in attention were seen in both AED exposure subgroups. The polytherapy exposure subgroup also had lower memory scores than the controls; however, so did study group children without AED exposure. The polytherapy exposure subgroup also had lower manual motor scores.
Our previous findings (Gaily et al., 2004) suggested that valproate exposure might have lowered the verbal IQ. In the present study, valproate exposure alone or in polytherapy was associated with lower scores on Auditory Attention B and Sentence Repetition, compared with the controls but also when compared with other AED exposure subgroups.
Both epilepsy type subgroups (maternal idiopathic generalized epilepsy and maternal partial symptomatic epilepsy) had lower scores on attention than the controls. In addition, maternal idiopathic epilepsy offspring had lower manual motor scores than the controls. Short generalized tonic-clonic seizures or absences during pregnancy were not a risk for cognitive deficits in this study, which also agrees with earlier observations (Gaily et al., 1988).
Possible Mechanisms Explaining Findings
Difficulties with attention, memory, and psychomotor speed are among those considered typical for learning disorders associated with epilepsy (Aldenkamp et al., 1990). They are also among the most common cognitive side effects for AED (Loring & Meador, 2001). Our findings are compatible with genetic and AED-related contributions to the cognitive deficits found in offspring of mothers with epilepsy. The genetic predisposition manifested in the mother as epilepsy may be expressed in the offspring as a susceptibility to cognitive dysfunction, or a tendency to seizure disorder, or both (Doose et al., 1996; Winawer & Shinnar, 2005). A seizure disorder may occur as undetected EEG disturbance without overt seizures. Such a disturbance may have insidious cognitive effects, either in the form of so-called transitory cognitive impairment (Binnie, 1993) or through an accumulating process over time (Aldenkamp & Arends, 2004). The immature brain is believed to be especially vulnerable to the untoward effects of epileptiform EEG discharges (Holmes, 2001). Children in the study subgroup who were not prenatally exposed to maternal AED scored less well on Memory for Names than the controls, supporting the possibility of either genetically induced brain dysfunction or proneness to subtle discharge in this subgroup. The genetic risk is more evident in idiopathic than in symptomatic epilepsies (Winawer & Shinnar, 2005). The slower fine motor performance of the idiopathic epilepsy offspring is thus also compatible with genetic explanation.
Deficiencies in simple auditory attention in study children were found only in younger participants, and appeared now to be associated with AED exposure. To our knowledge, this finding has not been reported previously. This result suggests an age-related sensitivity of this test domain to the effects of maternal AED, or the possibility of a more extended period of neural recovery for the auditory-linguistic system compared with other cognitive domains (Aylward, 1997). On the other hand, hereditary inclination to EEG-sharp-wave activity has been seen more frequently in children less than seven years of age and often disappears in the course of maturation (Doose et al., 1996). Because children in Finland start school at the age of seven, it is also possible that this acts as a “leveler,” evening out differences caused by varied early environment.
Auditory Attention B was also low in the total study group relative to controls but selectively more impaired in the valproate-exposed subgroup. This task requires rather complex mental programming and working memory. Another subtest on which valproate exposure subgroup scored low was Sentence Repetition, a measure of primary working memory span. We have previously reported significantly lower VIQ in association with valproate exposure in these same children (Gaily et al., 2004). Sentence Repetition correlated significantly with VIQ in the valproate exposure group, the correlation being higher than in the total group (.82 vs. 52). This might indicate that VIQ is more dependent on working memory in this subgroup. There was also a significant correlation to maternal serum valproate level. A recent study (Vinten et al., 2005) found that valproate-exposed children scored lower on measures of “freedom from distractibility” and memory. Our findings suggest that impaired verbal working memory may be one factor leading to low VIQ in children with prenatal VPA exposure.
Representativeness of the Sample and Limitations of the Study
Approximately 40% to 50% of the population of children born to mothers with epilepsy in the Uusimaa region participated in our study. The mothers were comparable with Uusimaa population on the whole as regards education. The present sample was biased towards normality, because all children with low overall level were excluded. Study and control children had similar developmental and health histories. The slight excess of siblings in the study group did not explain our findings.
Although maternal education explained a significant portion of the variance in the test performance, this effect appeared in the study and the control group alike. Maternal education was used as a covariate in all of the analyses to control for any unbalance in the medication subgroups. Because we did not measure maternal IQ, which was found to explain much of valproate-exposed children's cognitive outcome by Eriksson et al. (2005), the possibility that social or genetic disadvantage contributed to our findings cannot be excluded.
Because the number of children exposed to valproate monotherapy was small, significant confounding by polytherapy cannot be excluded. Because epilepsy type was not included in the analysis of AED effects caused by small subgroups, this factor also remains a potential confounder. However, there was no association between epilepsy type and AED as such. Thus, this confounding effect does not seem likely.
CONCLUSION
The children of mothers with epilepsy scored significantly lower than the controls on measures of attention, memory, and fine-motor function. Our findings thus agree with previous research in that maternal epilepsy has negative effects on neurocognitive development of the offspring, but are among the first to indicate the nature of cognitive deficits or to show age dependent effects. Group differences on auditory attention appeared AED-related and were found only in younger children. Memory impairments were more marked in but not limited to the subset of the study group exposed to maternal medication in utero. Slower manual motor scores were associated with both polytherapy exposure and idiopathic maternal epilepsy, thus supporting the view that both genetic factors and medication effects contribute to deficits in offspring. Valproate-exposed children obtained lower scores than other children in the study group or controls on tasks demanding complex information processing and auditory working memory. This finding was strongly associated with their lower Verbal IQ. Although the neuropsychological test performance of the study group fell below that of the controls on several subtests, mean scores generally fell in the average range, indicating a lack of clinically significant impairment for the majority of the study children. The observation that some of the deficits were found only in the younger children might also indicate a favorable outcome in the long run. The effects of valproate appeared more pronounced; however, this finding should be confirmed by further studies with larger groups of children exposed to valproate monotherapy. The associations to maternal epilepsy type and age emphasize the need to consider these factors in future studies on effects of maternal AED.
ACKNOWLEDGMENTS
The authors thank Ville Hiilesmaa, MD, Riitta Matila, MD, and Erja Kaaja, PhD, for providing the obstetric database and prenatal exposure data; Kristiina Tuomainen, RN, for interviewing the parents and taking care of the practical arrangements of this study; Mervi Kotila, MD, Tuula Nylund, MD, Ali Bardy, MD, and Marja-Liisa Granström, MD, for re-evaluating maternal epilepsy diagnoses; and Juhani Vilkki, PhD, and Lauri Tarkkonen, PhD, for valuable guidance and statistical advice. Supported by a research grant from Helsinki University Hospital.
Declaration of competing interests: None of the authors have any competing interests.










