INTRODUCTION
Deficits in emotion recognition after traumatic brain injury (TBI) have been reported in response to static visual stimuli (McDonald et al., 2004; McDonald & Saunders, 2005), facial emotion (Jessimer & Markham, 1997; Milders et al., 2003), speech and vocal prosody (Hornak et al., 1996), and naturalistic videotaped portrayals (McDonald & Flanagan, 2004). Deficits of emotional perception and expression are associated with alexithymia, a multifaceted construct comprising: (a) difficulty identifying feelings; (b) difficulty distinguishing between feelings and bodily sensations of emotional arousal; (c) difficulty describing feelings to other people; (d) constricted imaginal processes evidenced by paucity of fantasies; and (e) a stimulus-bound, externally-orientated thinking style (Taylor et al., 1997, p. 29). Alexithymia has been linked with a range of psychopathology (Taylor et al., 1997) but recently, Becerra et al. (2002) introduced the concept of “organic alexithymia” to distinguish the constitutional deficits associated with a developmental history of affective, somatoform, and personality disorders from an acquired disorder following traumatic brain injury. They argued that characteristics of organic alexithymia may present in ways that are similar to “non-organic” alexithymia, but are usually more circumscribed in character and do not become manifest until after brain injury, implying a causal role for head trauma.
Houtveen et al. (1997) argued that alexithymia results from a deficit in interhemispheric communication involving the corpus callosum. Some support for this view comes from studies by Hoppe & Bogen (1976) and TenHouten et al. (1985, 1987). However, other studies have found that deficits in emotional perception are more frequent in patients with right hemisphere damage (Mandal et al., 1999), suggesting that the right hemisphere is superior for the perception of emotional stimuli (Adolphs et al., 1996, 2000; Borod, 2000; Kucharska-Pietura et al., 2003; Smith & Bulman-Fleming, 2004). Decreased emotional expression has also been documented following frontal lobe damage (Kandel et al., 1991; Stuss et al., 1992). Therefore, if alexithymia can be associated with dysfunction in a number of cerebral structures, it should be prevalent after head trauma because of the diffuse nature of such injuries and predominant involvement of the frontal structures. Consistent with this line of thinking, Williams et al. (2001) found a high incidence of alexithymia in a cohort of TBI patients. They screened 135 patients attending a GP surgery, using the Traumatic Brain Injury Questionnaire (Solomon & Malloy, 1992) and the Toronto Alexithymia Scale-20 (TAS-20) (Bagby et al., 1994a, 1994b) and found that 49% reported a history of head injury. Of these, 18% recorded TAS-20 scores consistent with alexithymia, significantly higher than the general population prevalence rate of 7% to 10% percent (Pasini et al., 1992).
Relationships between alexithymia and neuropsychological functioning have been examined in non-organic and organic alexithymia. Lamberty & Holt (1995) administered tests of verbal and visuo-spatial ability, the Beck Depression Inventory (Beck et al., 1981), and the Toronto Alexithymia Scale (Taylor et al., 1985) to a neurologically intact sample of combat veterans attending a psychiatric clinic. They found that TAS scores negatively correlated with measures of verbal intelligence and Stroop Word indices, suggesting that “non-organic” alexithymia may partly be a consequence of poorly developed verbal ability. Additionally, Lamberty & Holt reported a significant correlation between TAS scores and the Beck Depression Inventory, a relationship that could be anticipated if, as theorized, alexithymia reflects a deficit in the regulation of emotions and a syndrome that limits the extent to which individuals can modulate emotions by fantasy and dreams (Mayes & Cohen, 1992). Henry et al. (2006) compared a small number of TBI patients (N = 28) with demographically matched healthy controls (N = 31) on different factors of the TAS-20, executive function, and quality of life ratings and found that difficulty identifying emotions was associated with both executive dysfunction and poorer quality of life. They argued that following TBI, more attention should be paid to deficits in emotional awareness and expression because of their potential impact on psychosocial and neuropsychological recovery.
The aim of the current study was to determine, in a large sample of head injured patients, the prevalence of alexithymia and its relationship to severity of injury. A further aim was to examine the relationship between alexithymia and a broad range of neuropsychological functions, clustered according to domains of cognitive ability (Wood & Rutterford, 2006a, 2006b; Wood & Liossi, in press). The study also aimed to examine relationships between alexithymia and affective disorder after TBI. A number of hypotheses were examined: (1) the prevalence of alexithymia after head trauma will exceed a) reported prevalence in the general population, and b) in a matched group of orthopedic patients, included to control for demographic, familial and social differences that might exist between TBI participants and the general population; (2) a relationship would be found between high scores on the TAS-20 measure of alexithymia and low scores on measures of verbal ability (based on the notion of “no words for feelings”), analogous to findings reported in a psychiatric sample (Lamberty & Holt, 1995); (3) consistent with the findings of Henry et al. (2006), there would be a relationship between high alexithymia scores and poor performance on executive tests, but there was no expectation of relationships between alexithymia and other cognitive domains; and (4) the presence of alexithymia would be associated with high scores on the Beck Depression Inventory (BDI) and Beck Anxiety Inventory (BAI).
METHOD
Participants
TBI group
This consisted of a consecutive series of head injured cases referred for routine neuropsychological assessment and advice on rehabilitation, to the University Head Injury Clinic between 2004 and 2005 (N = 91). Data is also included from a convenience sample, comprising members of two local head injury support groups (N = 30). There was no difference between the consecutive or convenience samples in respect of injury severity or any other parameters referred to below. Of the 121 participants in the study, 75 (62%) were male. Injury severity was determined by the length of Post Traumatic Amnesia (PTA) (mean: 15.74 days; SD = 32.17, range 1–210) and Glasgow Coma Scores (GCS) at the time of hospital admission (mean: 9.89; SD = 4.54, range 3–15). The mean time between injury and assessment was 4.25 years (SD = 4.44 years, range 0.42–29.69 years). Mean age at injury was 36.10 years (SD = 13.88, range 13–69) and at assessment, 40.32 years (SD = 13.85, range 17–71). The cohort had achieved an average of 11.62 years of education (SD = 1.91, range 7–17). Pre-morbid intelligence was estimated using the National Adult Reading Test (NART-2) (mean 97.41; SD = 13.05, range 70–121). Prior to injury, 93% of the cohort was in full time employment. At the time of assessment, 60% were either unemployed or working as volunteers. None of the cohort had a formal history of psychiatric illness or any kind of pre-injury personality disorder that could be interpreted as evidence of alexithymia.
Control group
This consisted of 52 orthopedic patients, hospitalized following serious trauma, excluding head trauma. All control cases had been discharged from hospital during the previous six months but were still attending a hospital outpatient clinic. Of the 52 patients, 48.1% were male and the mean age at assessment was 37.17 years (SD = 12.47 years, range 18–63). None of the cohort had a formal history of psychiatric illness or any kind of pre-injury personality disorder that could be interpreted as evidence of alexithymia. The control group was drawn from the same socio-economic catchment area as the TBI group. The two groups did not differ in gender, socio-economic status (determined by pre-accident occupational level-Freelance Interview), years of education, or age at time of assessment (Gender: t (171) = −1.703, p > .05; Socio-economic status: df = 4, χ2 = .606, p > .05; Years of education: t (147) = .736, p > .05; Age at Assessment: t (167) = 1.406, p > .05).
Procedure
All participants completed the TAS-20; 35 (28.9%) of the TBI cohort scored ≤51 (no alexithymia), 16 (13.2%) scored 51–61 (possible alexithymia), and 70 (57.9%) scored ≥61 (alexithymia). The TBI cohort was divided into these three groups for all future statistical analysis. The three alexithymia groups were compared on standard demographic information, including gender and age at assessment. The three alexithymia groups did not differ on gender (No Alexithymia & Possible Alexithymia: t (49) = −.279, p > .05; No Alexithymia & Alexithymia: t (103) = 1.436, p > .05; Possible Alexithymia & Alexithymia: t (84) = 1.406, p > .05). However, some group differences were noted for age at assessment (No Alexithymia & Possible Alexithymia: t (49) = −2.982, p < .05; No Alexithymia & Alexithymia: t (99) = −3.680, p < .05; Possible Alexithymia & Alexithymia: t (81) = .210, p > .05). Ninety-nine participants agreed to complete a routine clinical neuropsychological examination. Demographic details and information relating to head trauma was obtained from general practitioner records and hospital case notes.
Ethical approval was obtained from the Department of Psychology, University of Wales Swansea and the South West Wales NHS Research Ethics Committee.
Measures
Participants who attended the University Head Injury Clinic were administered neuropsychological tests as part of a routine clinical assessment. The tests varied slightly in number and combination based on clinical circumstances at the time of assessment.
- National Adult Reading Test–Revised (NART) (Nelson, 1982). Half levels of split-half reliability (0.93), inter-rater reliability (0.96–0.98), and test-retest reliability (0.98) have been reported (Schlosser & Ivison, 1989).
- Wechsler Adult Intelligence Scale–3rd Edition (WAIS-III) (Wechsler, 1997). The WAIS-III has good reliability and validity in clinical populations (Tulsky et al., 1997).
- Wechsler Memory Scale—3rd edition (WMS-III) (Wechsler, 1997). Reliability coefficients for the subtests range from 0.74–0.93. Concurrent validity has also been demonstrated (Wechsler, 1997).
- Trail Making Test Part A and B (Lezak, 1995). Reliability has been reported as 0.98 for Part A and 0.67 for Part B (Lezak, 1995). Adequate internal consistency has been reported ranging from .26–.93.
The previously mentioned tests will be familiar to most neuropsychologists. However, the assessment included three executive measures and one test of information processing speed, which is new and may need more explanation:-
- The Hayling Test (Burgess & Shallice, 1997): a measure of response completion and the ability to suppress inappropriate responses. Patients have to complete 30 sentences from which the last word is omitted. In the first half of the test (initiation condition) they complete sentences with a sensible word. In the second half (inhibition condition) they have to supply a word that makes no sense in the context of the sentence (e.g., “London is a very busy .. banana“). To achieve this it is necessary to inhibit the dominant (automatic) response before generating the new one. In the initiation section of the test the total response time for all 15 items is the performance measure (Hayling A). In the inhibition section, two measures are derived: the total time to respond to the items (Hayling B) and the total number of errors (Hayling C), where an error is a response that is related to the sentence. All three measures are expressed as scaled scores. Test-retest reliability of the overall score on the Hayling test has been reported as 0.76 (Burgess & Shallice, 1997).
- The Brixton Test (Burgess & Shallice, 1997): a measure of rule attainment and rule detection. The patient is shown a 56-page stimulus book, one page at a time. All pages contain 10 circles in the same basic array, one circle is colored blue, all others are white. The patient has to predict where the blue circle will be on the next page, based on the sequence of previous pages. The total number of errors made on the task is summed and converted to scaled scores. The test-retest reliability coefficient of the overall score on the Brixton test has been reported as 0.71 (Burgess & Shallice, 1997).
- The Zoo Map Test (Wilson et al., 1996): This subtest from the Behavioral Assessment of Dysexecutive Syndrome (BADS) battery was designed to simulate real life situations. Participants are required to show how they would visit a series of designated locations, while following certain rules. The map and the rules are constructed so that there are only four variations on a route that do not infringe the rules. The first trial represents a high demand version of the task in which the planning abilities of the patient are rigorously tested. In the second, low demand, trial, the patient is simply required to follow written instructions. On both trials, the patient is expected to minimize errors by modifying performance on the basis of feedback. Raw scores on each test were converted to profile scores for analysis, as per the BADS manual. Inter-rater reliability across the BADS tests range from .88–1.00 (Wilson et al., 1996).
- Speed of Comprehension Test (Baddeley et al., 1992): a measure of the efficiency of language comprehension in which simple statements about the world have to be judged true or false. The patient is presented with a maximum of 100 statements to judge in a two-minute interval, so processing speed, as well as efficiency of comprehension, is measured. Reliability of the SCOLP is good with a coefficient of 0.93 having been reported (Baddeley et al., 1992).
The presence of alexithymia was determined using the 20-item Toronto Alexithymia Scale (TAS-20) (Bagby et al., 1994a, 1994b). This is composed of 20 items that participants endorse on a five-point Likert-type scale, ranging from “Strongly Disagree” to “Strongly Agree.” The TAS-20 total score can range from 20–100. A score ≥61 confirms alexithymia; 51–61 indicates “possible” alexithymia; ≤51 indicates an absence of alexithymia (Bagby et al., 1994a, 1994b; Parker et al., 1992). The Beck Anxiety Inventory (Beck et al., 1988) and Beck Depression Inventory (Beck et al., 1981) were used to measure affect.
Statistical Analysis
For the purpose of the analysis, the cognitive tests administered were grouped into domains, based on clinical attempts to categorize general areas of impairment following TBI (Brooks, 1990; Johnstone et al., 1995; Prigatano, 1986; Tate et al., 1991) rather than on the basis of statistical procedures. These groupings have been employed in previous studies (Wood & Rutterford, 2006a, 2006b).

RESULTS
Prevalence of Alexithymia
Consecutive and convenience samples
A test of proportion examined differences in the prevalence rate of alexithymia between the consecutive and convenience samples. No significant difference was obtained (Z = 0.685, p > .05). Therefore, any notion of bias between samples is excluded, allowing both samples to be combined for further analysis.
TBI and general population
A test of proportion revealed a significant difference in the prevalence rate of alexithymia between the TBI cohort (57.9%) and the general population rate (7% to 10%) reported by Pasini et al. (1992) (Z = 12.87, p < .0001).
TBI and orthopedic controls
The TBI cohort (57.9%) recorded a significantly higher prevalence rate of alexithymia than orthopedic controls (15.4%). The proportion of 0.579 (70 out of 121) individuals with alexithymia in the TBI cohort is significantly different from the orthopedic cohort proportion of 0.154 (Z = 5.147, p < .0001). Table 1, showing the mean TAS-20 total and sub-scale scores, shows that significant differences were recorded between the TBI and orthopedic control groups for the TAS-20 total score (t (171) = 5.955, p < .0001) and on all three individual sub-scale scores of the TAS-20 (Sub-scale score 1—difficulty identifying feelings: t (171) = 6.350, p < .0001; Sub-scale score 2—difficulty describing feelings: t (171) = 6.243, p < .0001; Sub-scale score 3— externally orientated thinking: t (171) = 1.977, p < .05).
Mean and standard deviation of TAS-20 individual sub-scale scores and total TAS-20 alexithymia score

Severity of Injury
A Pearson product-moment correlation was performed to assess the relationship between measures of alexithymia and injury severity. No relationship was found between measures of alexithymia and severity of injury as determined by length of PTA (r = −.053, p > .05 or Glasgow Coma Scores (GCS) (r = −.156, p > .05), consequently, severity of injury was not entered as a covariate when relationships between alexithymia, neuropsychological abilities, and affective state were examined.
Neuropsychological Correlates
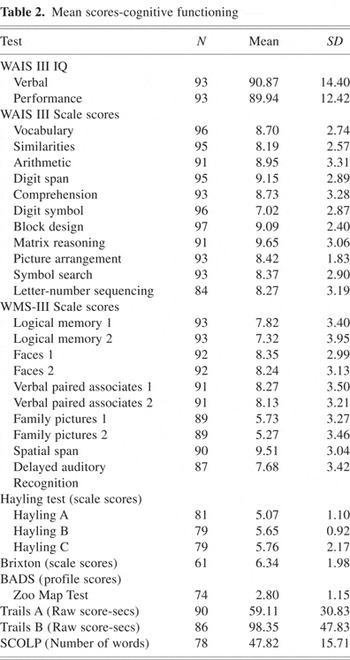
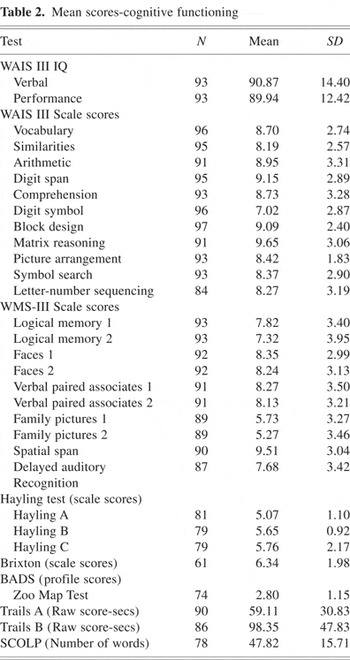
Mean scores for all neuropsychological tests can be found in Table 2.
Mean scores-cognitive functioning
A one-way between subjects multivariate analysis of variance (MANOVA), conducted within each cognitive domain (see Table 3) revealed a significant difference among the three alexithymia groups in two cognitive domains—verbal ability and sequencing. All the dependent variables in these two cognitive domains reached significance. There were no significant between group differences in the cognitive domains of “Visuospatial Reasoning” N = 90, F(3,85) = 1.27, p > .05, Wilks' λ = 0.916; “Executive Ability” N = 52, F(5,45) = 1.91, p > .05, Wilks' λ = 0.68; “Mental Speed” N = 71, F(5,75) = 1.11, p > .05, Wilks' λ = 0.891; “Verbal Memory” N = 87, F(5,80) = 1.33, p > .05, Wilks' λ = 0.852; “Non-Verbal Memory” N = 89, F(5,83) = 1.37, p > .05, Wilks' λ = 0.880.
Differences between alexithymia groups on neuropsychological domains and individual scale score measures
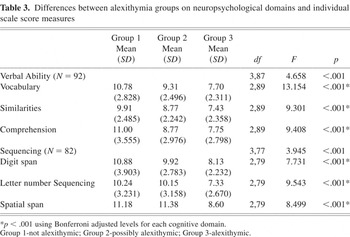
Post-Hoc comparisons using the Scheffe test revealed that for the cognitive domain Verbal Ability, the mean scores for group 1 (no alexithymia) differed significantly from group 3 (alexithymia), using a Bonferroni adjusted alpha level of .01, (Vocabulary, groups 1 & 3; p < .0001: Similarities, groups 1 & 3; p < .0001: Comprehension, groups 1 & 3: p < .0001). Group 2 (possible alexithymia) did not significantly differ from groups 1 or 3 on any of the three sub-tests within this cognitive domain (Vocabulary, groups 1 & 2; p > .05: groups 2 & 3; p > .05: Similarities, groups 1 & 2; p > .05; groups 2 & 3; p > .05: Comprehension, groups 1 & 2; p > .05; groups 2 & 3; p > .05).
A similar pattern of post-hoc between group differences was obtained for the cognitive domain–“Sequencing.” Groups 1 and 3 differed significantly on all three sub-tests using a Bonferroni adjusted alpha level of .01 (Digit Span, groups 1 & 3; p < .005; Letter Number Sequencing, groups 1 & 3; p < .005; spatial span, groups 1 & 3; p < .005). Additionally, Groups 2 and 3 differed significantly on two sub-tests within the “Sequencing” domain (Letter Number Sequencing, groups 2 & 3; p < .01: Spatial Span, groups 2 & 3; p < .05). Post-Hoc analysis did not reveal any other significant differences between alexithymia groups (Digit Span, groups 1 & 2; p > .05; groups 2 & 3; p > .05: Letter Number Sequencing, groups 1 & 2; p > .05: Spatial Span, groups 1 & 2, p > .05).
Affective Disorder
Group 1, 18.2%; Group 2, 14.3%; and Group 3, 25.5% reported mild-moderate problems of mood on the BDI (Depression: Group 1: M = 16.50, SD = 8.23; Group 2: M = 16.11, SD = 7.28; Group 3: M = 22.88, SD = 10.87). Furthermore, 27.3% of group 1, 14.3% of group 2 and 23.6% of group 3 reported anxiety problems on the BAI (Anxiety: Group 1: M = 16.28, SD = 10.824; Group 2: M = 16.14, SD = 10.45; Group 3: M = 22.36, SD = 13.12). However, one-way between-groups analysis of variance failed to identify significant between-group differences on either the Beck Depression Inventory (p > .05) or the Beck Anxiety Inventory (p > .05).
A Pearson Product-Moment Correlation was performed to assess the relationship between affective disorder and alexithymia. Significant correlations were obtained between scores on the Beck depression and anxiety inventories and alexithymia, measured by the TAS-20 (alexithymia and depression: r = 0.373, N = 60, p < .005; alexithymia and anxiety: r = 0.330, N = 60, p < .01), suggesting that alexithymia, depression, and anxiety may be related and overlapping constructs.
Multiple regression analysis techniques were employed to further examine the relationship between affective disturbance and alexithymia. The regression model, including BDI and BAI scores, explained 12% of the variance in TAS-20 total scores (adjusted R2 = .120, p < .01). Of the two model variables, neither BDI nor BAI scores made a statistically unique contribution to the prediction of TAS-20 total scores (BDI: β = .274, p > .05; BAI: β = .157, p > .05). Regression analyses examined how much variance could be explained within the individual sub-scale scores of the TAS-20 by BDI and BAI scores. The regression model accounted for 15.5% (p = .01) of the variance for sub-scale score 1 (Difficulty Identifying Feelings). BDI scores made a statistically unique contribution to sub-scale score 1 (β = .366, p < .05). BAI scores made no unique contribution (β = .096, p > .05). For sub-scale score 2 (Difficulty Describing Feelings), the regression model accounted for 8.5 per cent (p < .05) of variance in scores. BAI scores were found to make a significant contribution to sub-scale 2 scores (β = .344, p > .05), whereas BDI made no unique contribution (β = .004, p > .05). The regression model accounted for 8 per cent (p > .05) of the variance in sub-scale score 3 (Externally Orientated Thinking). Neither BDI nor BAI scores made a statistically significant contribution to predicting sub-scale 3 scores (BDI; β = .124, p > .05: BAI; β = .114, p > .05).
DISCUSSION
The results of this study confirm that the prevalence rate of alexithymia after TBI (57.9%) is much higher than both the reported general population rate of 7% to 10% (Pasini et al., 1992), and in a control group of patients who had recently suffered orthopedic injury (15.4%). There is no obvious explanation for why the TBI prevalence recorded in this study was higher than the 18% reported by Williams et al. (2001). The patients recruited to this study probably sustained more serious brain injuries than those in the Williams et al. study but this does not seem to be a viable explanation because we did not find a relationship between injury severity and presence or degree of alexithymia. At this stage of our research, all we can conclude is that TBI can result in an emotional deficit, reminiscent of alexithymia, in cases who, premorbidly, did not exhibit abnormalities of personality—a prima facia argument for organic alexithymia. We therefore agree with the recommendation of Henry et al. (2006), that the neuropsychological impact of TBI must be assessed in terms of both cognitive and emotional factors.
Significant differences were recorded between the TBI and orthopedic control groups on each of the TAS-20 sub-scale scores. Interestingly, the TBI patient cohort reported equal deficits across sub-scale 1 (Difficulty Identifying Feelings) and 3 (Externally Orientated Thinking) of the TAS-20 construct. The finding that the TBI cohort recorded difficulty identifying feelings is consistent with previous results (McDonald & Saunders, 2005; Milders et al., 2003) but also points out that such individuals fail to identify their own emotions as well as being unable to recognize emotions in others. The present cohort also tended to focus on the concrete details of external events (sub-scale score 3), demonstrating that TBI patients are less introspective, consistent with the findings of Henry et al. (2006).
Lamberty & Holt (1995) and Henry et al. (2006) have provided preliminary evidence suggesting a relationship between alexithymia, verbal ability and executive dysfunction. These findings were only partially confirmed in the present study, which employed a broader range of tests and a larger sample size than previous studies. A significant between group difference was only evident for the cognitive domains “Verbal Ability” (Vocabulary, Similarities, Comprehension), and “Sequencing” (Digit Span, Letter-Number Sequencing, Spatial Span). In both domains, higher scores on the TAS-20 were associated with poorer cognitive performance. A deficit in verbal cognition may underlie the “no words for feelings” concept of alexithymia. The poor performance on tests of sequencing abilities may reflect a lack of attentional control, giving some support to Henry et al's suggestion that deficits in cognitive control functions may have parallels in emotional functioning. However, the results of this study did not establish any direct link between executive dysfunction and alexithymia. Three ecologically valid tests of executive ability were used in this study compared to the one measure of executive function (verbal fluency) employed by Henry et al. It is possible that the verbal test of executive function is more directly related to alexithymia than the relatively new ecological tests used in this study. However, the multifactorial nature of executive function makes it difficult to draw any specific conclusions about relationships between alexithymia and any single (or specific group) of executive tests.
We found a significant correlation between alexithymia, depression, and anxiety, possibly reflecting restricted imaginal capabilities that limit the extent to which alexithymic individuals modulate anxiety and other emotions by fantasy and dreams (Mayes & Cohen, 1992). However, we failed to establish any significant differences between the three alexithymia groups and levels of depression or anxiety. Hendryx et al. (1991) suggested that measures of alexithymia may reflect aspects of depression and anxiety, rather than it being a unique personality construct that could increase the risk of secondary affective disturbance. However, data from the present study revealed that affective disturbance accounted for only 12% of the variance in TAS-20 total scores. Neither depression nor anxiety made a unique contribution to alexithymia scores, indicating that even though there is a relationship between affective disturbance and alexithymia, they should be considered as distinct constructs, consistent with the findings of Marchesi et al. (2000) and Parker et al. (1991) who conducted a factor analysis of the combined items of the TAS-20 and BDI, showing that alexithymia is a separate and distinct construct from depression Further support for this conclusion can be drawn from the finding that the orthopedic control group, who had suffered serious but (in most cases) transient disability, did not exhibit high rates of alexithymia, suggesting that high ratings by the TBI group were not simply an emotional reaction to injury.
This study has a number of limitations. The neuropsychological data is incomplete because the number and type of tests administered to patients was dependent on clinical circumstances at the time of assessment. Another limitation relates to the fact that some participants drawn from the head injury support groups declined to participate in a full neuropsychological assessment (N = 22). Therefore, whereas the study establishes links between alexithymia and both verbal and sequencing abilities, the conclusions are not based on the whole sample. The grouping of tests into domains for statistical analysis represents another potential limitation. The authors acknowledge that some tests could have been assigned to alternative domains, which could have influenced some parts of the analysis, thereby limiting the support given to the notion of “organic” alexithymia. Also, the relatively new executive tests used in this study may not relate to other, more traditional and widely used measures of executive function. However, Burgess and Alderman (2004) point out that patient's can show variable patterns of executive deficit and that one should not expect failure on one executive test to predict failure on another. It must also be acknowledged that, whereas a high prevalence of alexithymia is clearly present in a head trauma population, it is not possible to determine the role of brain injury in the acquisition of alexithymia. It is possible that the age differences separating the three alexithymia groups had an impact on emotional perception. We also accept that even though the patient cohort did not report a pre-accident history of psychiatric or personality problems reminiscent of alexithymia, sub-clinical levels may still have been present that were exacerbated by head trauma. Consequently, a pre-morbid aetiology cannot be ruled out. Criticism could also be made regarding use of the three sub-scale scores comprising the TAS-20. Some studies have failed to replicate this 3 sub-scale structure (Kooiman et al., 2002; Muller et al., 2003) and there has been no attempt to investigate the TAS-20 sub-scale structure in a head injured population. However, other studies (e.g., Loas et al., 2001) have confirmed the sub-scale structure in clinical and non-clinical samples and other research on head injured cases has employed the sub-scale structure (e.g., Henry et al., 2006). We therefore feel that an attempt to distinguish different components of alexithymia using the three sub-scale structure can be justified.
Whatever the relationship between head trauma and alexithymia, the results of this study emphasize the neuropsychological importance of assessing emotional as well as cognitive factors post-injury to see how they interact. It follows that there will be a need to develop instruments capable of measuring alexithymic characteristics in a brain injured population that may have predictive value for the quality of psychosocial outcome, or help distinguish organic alexithymia from emotional blunting associated with abnormal personality development.
ACKNOWLEDGMENTS
The information in this manuscript and the manuscript itself is new and original and is not currently under review by any other publication, and has never been published either electronically or in print. The authors have no financial relationships or conflict of interest to disclose.