Introduction
The aging brain is susceptible to several pathological changes that can adversely affect cognition and other aspects of brain function. Alzheimer’s disease (AD) is reported to be the most common cause of dementia among older adults (Cummings & Mega, Reference Cummings and Mega2003; Fratiglioni, De Ronchi, & Aguero-Torres, Reference Fratiglioni, De Ronchi and Aguero-Torres1999; Plassman et al., Reference Plassman, Langa, Fisher, Heeringa, Weir, Ofstedal and Wallace2007) but AD pathology can be present in older adults with only mild cognitive impairment (MCI) or normal cognition (see, for example, Bennett et al., Reference Bennett, Schneider, Arvanitakis, Kelly, Aggarwa, Shah and Wilson2006). Other types of pathologies often co-exist or exist in isolation without concomitant AD. In particular, evidence of Lewy body disease (LBD) and cerebrovascular disease (CVD) are also common. Teasing apart the influence of these different pathologies on cognitive function remains challenging and continues to be a source of debate in clinical practice and research.
Conceptually there is reason to believe these types of pathology may differentially affect specific domains of cognitive function. Episodic memory deficits are a hallmark of AD and are thought to reflect the early distribution of AD pathology in medial temporal lobe structures (Baudica et al., Reference Baudica, Dalla, Thibaudetc, Smagghec, Remyd and Traykov2006; Nestor, Scheltens, & Hodges, Reference Nestor, Scheltens and Hodges2004). Deficits in other cognitive domains such as language, visuospatial, and executive functions also occur as AD progresses to involve other areas of neocortex. While there is a substantial literature on the neuropsychological manifestations of AD, many of these studies do not have neuropathological verification or quantification of disease status. Furthermore, AD pathology is not a unitary concept. The hallmark pathological changes in AD include both amyloid beta peptide plaques and hyperphosphorylated paired helical filament tau protein-rich neurofibrillary tangles. There may be differential relationships between these two pathologies and cognition. Studies have suggested that cognitive measures are more highly correlated with tangle burden than plaque burden (Giannakopoulos et al., Reference Giannakopoulos, Herrmann, Bussiere, Bouras, Kovari, Perl and Hof2003; Guillozet, Weintraub, Mash, & Mesulam, Reference Guillozet, Weintraub, Mash and Mesulam2003). Pathology localization may also influence cognition. Tangle pathology occurs in medial temporal structures in early stages of AD progression and spreads to cortical areas as the disease progresses (Nelson et al., Reference Nelson, Abner, Schmitt, Kryscio, Jicha, Santacruz and Markesbery2009; Nestor et al., Reference Nestor, Scheltens and Hodges2004) while plaque distribution tends to be more widespread and cortically based (Arnold, Hyman, Flory, Damasio, & Van Hoesen, Reference Arnold, Hyman, Flory, Damasio and Van Hoesen1991).
LBD, while documented to be common in post-mortem studies, is relatively more difficulty to recognize clinically and has poorer diagnostic reliability when comparing clinical diagnosis to pathologically confirmed diagnosis (Papka, Rubio, & Schiffer, Reference Papka, Rubio and Schiffer1998). Several studies have suggested that LBD is associated with a somewhat different cognitive profile when compared to AD. For example, some but not all studies cite more impairment in visuospatial abilities in LBD (Preobrazhenskaya, Mkhitaryan, & Yakhno, Reference Preobrazhenskaya, Mkhitaryan and Yakhno2006; Simard, van Reekum, & Myran, Reference Simard, van Reekum and Myran2003), more executive/attentional problems (Aarslanda, Londosa, & Ballarda, Reference Aarslanda, Londosa and Ballarda2009; Molano et al., Reference Molano, Boeve, Ferman, Smith, Parisi, Dickson and Petersen2010), and less severe episodic memory deficit (Hamilton et al., Reference Hamilton, Salmon, Galasko, Delis, Hansen, Masliah and Thal2004).
The relationship between CVD and cognition is less well defined. Infarctions have been shown to increase the likelihood of cognitive impairment and dementia and to have additive effects when combined with AD (Schneider, Boyle, Arvanitakis, Bienias, & Bennett, Reference Schneider, Boyle, Arvanitakis, Bienias and Bennett2007; Schneider, Wilson, Bienias, Evans, & Bennett, Reference Schneider, Wilson, Bienias, Evans and Bennett2004). Several previous reports suggest that CVD is associated with greater impairments on tests that tap various aspects of executive functions (Cohen et al., Reference Cohen, Poppas, Forman, Hoth, Haley, Gunstad and Gerhard-Herman2009; Traykov et al., Reference Traykov, Baudic, Thibaudet, Rigaud, Smagghe and Boller2002). Subcortical CVD, in particular, can disrupt the integrity of corticostriatal circuits that course through frontal white matter tracts. The plausible cognitive manifestation of dysfunction in these circuits includes deficits in working memory, executive control functions, and other cognitive functions sub-served by prefrontal regions. Much of the literature on the relationship between CVD and cognition comes from studies of persons with clinically or radiologically diagnosed CVD. Because neuroimaging studies may not always differentiate small infarctions from other pathology, findings from these studies are difficult to interpret (Bowen, Barker, Loewenstein, Sheldon, & Duara, Reference Bowen, Barker, Loewenstein, Sheldon and Duara1990; Knopman, Reference Knopman2007). The use of postmortem measurements of CVD and cognitive function potentially offers a more direct comparison.
Although there is great interest in the relations between cognition and common neuropathologies, there are very few studies with sufficiently large samples to examine complex cognitive-pathological associations. A small number of studies have examined the association between specific neuropathological variables and cognitive function proximal to death (see, for example, Chui et al., Reference Chui, Zarow, Mack, Ellis, Zheng, Jagust and Vinters2006; Erten-Lyons et al., Reference Erten-Lyons, Woltjer, Dodge, Nixon, Vorobik, Calvert and Kaye2009; Sze et al., Reference Sze, Troncoso, Kawas, Mouton, Price and Martin1997) but are limited because of long intervals between autopsy and cognitive testing and examination of only a restricted number of both pathological and cognitive variables.
In the current study, we used a modeling approach that allows the simultaneous assessment of multiple correlated dimensions of the pathology-cognition process and applied it to a relatively large sample of older adults that had been well characterized in terms of cognitive function and had undergone postmortem brain autopsy. The primary goal was to examine the independent relationships between cognitive domains and indices of neuropathology commonly associated with aging and cognitive decline. To accomplish this, we used data from two longitudinal cohort studies of older adults—the Religious Orders Study (ROS) and the Memory and Aging Project (MAP) (Bennett et al., Reference Bennett, Schneider, Arvanitakis, Kelly, Aggarwa, Shah and Wilson2006; Bennett, Schneider, Buchman, et al., Reference Bennett, Schneider, Buchman, Mendes de Leon, Bienias and Wilson2005)—to separately identify the dimensionality of (a) the battery of neuropsychological tests used in these studies and (b) observed measures associated with neuropathological changes characteristic of AD. We then examined how these AD dimensions and measures of LBD and CVD were associated with the different cognitive domains. We also examined the association of brain atrophy, measured by brain weight, independent of AD, LBD, and CVD neuropathology.
Method
Participants
MAP and ROS are two independent community-based, prospective clinical cohort studies of risk factors for incident AD currently being conducted by the Rush University Medical Center and the Rush Alzheimer’s Disease Center. Recruitment, exclusion and inclusion criteria, and diagnostic procedures have been previously described in detail (Wilson, Barnes, & Bennett, Reference Wilson, Barnes and Bennett2003; Wilson, Beckett, et al., Reference Wilson, Beckett, Barnes, Schneider, Bach, Evans and Bennett2002; Wilson, Mendes, et al., Reference Wilson, Mendes, Barnes, Schneider, Bienias, Evans and Bennett2002). All protocols were approved by, and informed consent was obtained in accordance with the policies of the Institutional Review Board at Rush University Medical School. Briefly, both studies recruit older individuals without dementia who agree to receive clinical and psychological evaluation each year and to donate their brain for postmortem examination. The annual attrition rate in both cohorts is below 1% among survivors and the autopsy rate exceeds 80% in both cohorts. Besides sharing similar clinical and pathologic findings (Bennett et al., Reference Bennett, Schneider, Arvanitakis, Kelly, Aggarwa, Shah and Wilson2006), these studies also share a common 17-test neuropsychological battery and follow the same standard protocol and criteria for clinical diagnosis.
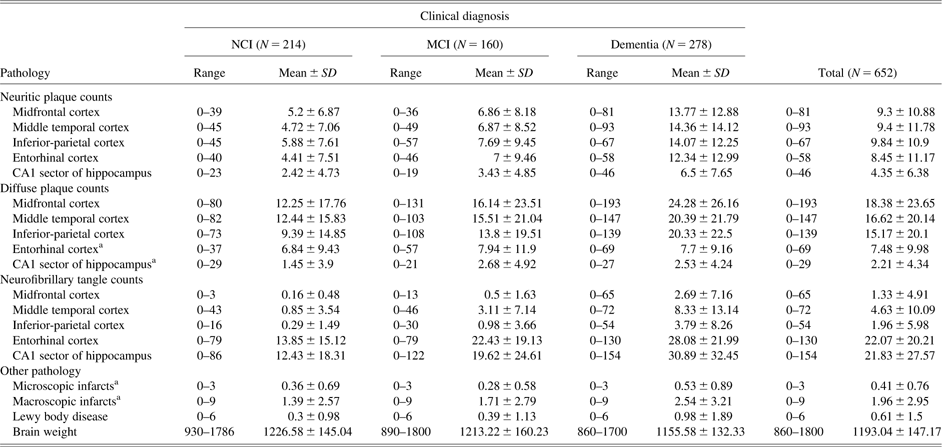
Table 1 presents a summary of the sample characteristics by clinical diagnosis before death. The sample consisted of 652 subjects with complete data on most of the neuropathological variables included in the analysis (sample sizes for individual variables ranged from 497 to 652). The sample was predominantly white, non-Hispanic (95%), with a mean age at death of 87 years (SD = 6.67; range, 66–104), an average education level of 16.76 years (SD = 3.69; range, 3–30), and gender composition of 60% female. The MMSE scores obtained at the last evaluation ranged from 0 to 30 (mean = 21.41; SD = 8.90). Approximately 29% of the participants were Apolipoprotein (APOE) ε4 carriers. In the last clinical evaluation, nearly a third of the sample was diagnosed as not cognitively impaired (NCI), 24% as MCI, and the remaining 43% as Alzheimer’s or other form of dementia.
Table 1 Demographic and clinical characteristics of the combined sample by last clinical diagnosis

Note. NCI = no cognitive impairment; MCI = mild cognitive impairment; MMSE = Mini-Mental State Examination.
aAnalyses of variance F-tests and χ 2 tests did not produce statistically significant differences by clinical diagnostic group for education (F = 0.11, p = 0.899), gender (χ2 = 1.03, p = 0.598), and racial composition (White, non-Hispanic) (χ 2 = 0.10, p = 0.951). Using a family-wise error rate of 0.002, all the remaining tests yielded a significant group effect.
bThe summary scores on cognitive tests are from the last valid clinic evaluation before death. Clustering of tests by domain is based on previous studies.
Neuropsychological Measures
The present analysis used scores on 17 widely used neuropsychological tests obtained at the last examination before death (see Table 1). The tests have been previously categorized into five cognitive domains (Wilson et al., Reference Wilson, Barnes and Bennett2003; Wilson, Beckett, et al., Reference Wilson, Beckett, Barnes, Schneider, Bach, Evans and Bennett2002): (1) episodic memory measured by Immediate story, Delayed story, Word list memory, Word list recall, East Boston immediate, and East Boston delay; (2) semantic memory assessed with Boston naming, the National Adult Reading test; and two semantic categories (Animals and Fruits) of verbal fluency from the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) test; (3) working memory measured by three tests: Digit Span Forward, Digit Span Backward, and Digit Ordering; (4) visuospatial ability measured with Judgment of Line Orientation and Standard Progressive Matrices; and (5) perceptual speed which involved two scales: Number Comparison and the oral version of the Symbol Digit Modalities test. (For a comprehensive exposition of the administration protocol of these cognitive scales, see Wilson, Mendes et al., 2002.)
Neuropathological Measures
Postmortem Indices
Postmortem indices were obtained from a standard neuropathology protocol described in Bennett, Schneider, Bienias, Evans, and Wilson (Reference Bennett, Schneider, Bienias, Evans and Wilson2005). The average interval from last psychological evaluation to brain autopsy was 6.8 months (SD = 4.24; range, 0.04–22 months). AD pathological variables of interest in this study included counts in a 1 mm2 area of greatest density of neuritic plaques (NP), diffuse plaques (DP), and neurofibrillary tangles (NFT) from five brain regions: hippocampal CA1 sector, entorhinal cortex, midfrontal, middle temporal, and inferior parietal cortices. In addition, we included a single summary measure of Lewy bodies obtained from six brain regions: substantia nigra, the entorhinal cortex, midfrontal gyrus, middle temporal cortex, inferior parietal cortex, and anterior cingulate gyrus. Given the highly skewed distributions produced by the AD neuropathology count measures we recoded the values into deciles. The potential loss of information due to the discretization of these measures into deciles was offset by gains in meeting distributional assumptions of latent variable models and improvements in overall model fit.
A summary measure representing the number of chronic microscopic infarctions (determined according to procedures described in Schneider et al., Reference Schneider, Wilson, Bienias, Evans and Bennett2004) and the total volume of macroscopic infarcts (henceforth, macro-infarcts) were also included in the analysis. Chronic microscopic infarctions (henceforth micro-infarcts) were defined as lesions only visible through examination of histological sections prepared from the following regions: midbrain, midfrontal, middle temporal, inferior parietal cortex, hippocampus, entorhinal cortex, and any other dissected blocks. See Table 2 for a summary of all neuropathology variables by clinical diagnostic group. Finally, brain weight was included in a model as a measure of nonspecific brain pathology not captured by the specific neuropathologies examined. All neuropathological measures were modeled as continuous exogeneous (independent) variables predicting continuous endogeneous (dependent) cognitive measures.
Table 2 Pathologic characteristics of the combined sample by clinical diagnosis (unscaled data)
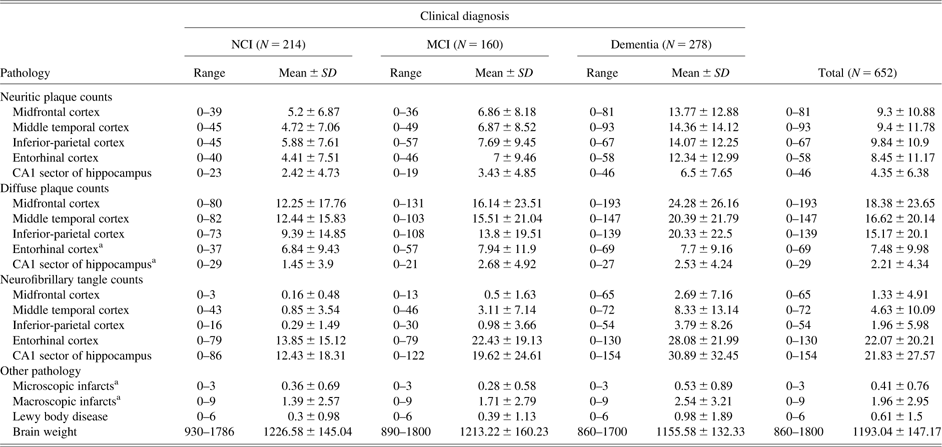
Note. NCI = no cognitive impairment; MCI = mild cognitive impairment.
aNo statistically significant differences between clinical groups were found for: diffuse plaque counts in the entorhinal cortex (F = 0.588, p = .556) and the hippocampal (F = 3.77, p = .024) regions; microscopic infarctions (F = 2.43, p = .089); and total volume of macro-infarcts (F = 3.35, p = .036).
All the remaining F-tests were significant for the group main effect (overall per test α = .003 with Sidak post-hoc adjustment for multiple comparisons). Post-hoc comparisons between NCI and Dementia groups as well as MCI and Dementia groups were statistically significant for all outcome measures with the exception of diffuse plaque counts for the middle temporal cortex and for the inferior parietal cortex. The last two measures only produced statistically significant post-hoc comparisons between NCI and dementia groups.
Data Analysis
Confirmatory Factor Analysis (CFA) models
The model building and analyses were performed using a multi-step procedure. The first step applied CFA based on structural equation methodology, to examine if the previously reported five-factor structure of the neuropsychological tests used in MAP & ROS studies provided an adequate representation of the data or if there was a better fitting factor structure.Footnote 1 Alternative factor structures were specified and evaluated in terms of overall fit and theoretical meaningfulness.
Given the fact that LBD and measures of CVD (e.g., macro-infarcts, micro-infarcts) were relatively infrequent in any brain region, making the estimation of CFA parameters unstable due to data sparseness issues; we chose to focus on the study of the dimensionality and structure of measures associated with neuropathological changes typical of AD. Consequently, summary measures of LBD and CVD were included in the model as independent observed explanatory variables instead of constructs derived from a factor analytic approach.
The assessment of the underlying factor structure of the 15 AD neuropathology variables, comprised of NPs, DPs, and NFTs from five brain regions, began with a model with each major AD pathology included as a unitary factor. A series of competing models varying the factor structure were subsequently tested. All models were fitted using MPLUS 5.2 (Muthén & Muthén, 1998–Reference Muthén and Muthén2008). The models were tested using sample variance-covariance matrices as input and parameters were estimated using robust maximum-likelihood (MLR) to handle non-normality and missing data. To increase the reliability of each model solution evaluation, we used multiple indices of fit: the Tucker Lewis fit indexes (TLI), the comparative fit index (CFI), the root-mean-squared error of approximation (RMSEA) and its 90% confidence interval, and the ratio χ 2/df (Jöreskog, Reference Jöreskog1969). (See online supplement material for additional detail.) Composite reliabilities were further reported as measures of the quality of the multidimensional factor structure (Raykov, Reference Raykov1998; Raykov & Shrout, Reference Raykov and Shrout2002).
Multiple-Indicator-Multiple-Cause (MIMIC) model
Once the final CFA models were determined, the next step was to use a MIMIC model (Jöreskog & Goldberger, Reference Jöreskog and Goldberger1975) to simultaneously assess the effect of both latent and observed neuropathology covariates (formative indicators) on the multiple latent cognitive domains. That is, the multiple cognitive latent factors were specified as outcomes predicted or influenced by neuropathology latent factors and three observed measures represented by single indicators: microscopic infarctions, volume of macro-infarcts, and brain weight (adjusted for sex and height). To study the overall contribution of brain weight as a formative indicator, we tested: a model including brain weight and a model without. The full analytical model is displayed in Figure 1.

Fig. 1 MIMIC model explaining the relationship between neuropathology and cognition. For simplicity, the model does show the correlations among the exogenous factors and observed variables and the correlations among the disturbances of the cognitive factors assumed in the estimation of the parameters.
The assessment of fit for the MIMIC modeling stage used the same criteria used to evaluate the CFA model fit. Additionally, we used Pratt’s (Reference Pratt1987) normalized measure of relative importance (Thomas, Hughes, & Zumbo, Reference Thomas, Hughes and Zumbo1998; Thomas, Zhu, & Decady, Reference Thomas, Zhu and Decady2007) to compare the relative contribution of independent variables in the model. All models were adjusted for education, age, and sex.
Results
Confirmatory Factor Analyses of the Cognitive Measurement Model
Table 3 summarizes the relative fit of the sequence of CFA models evaluated for validating or testing the previously published (Wilson et al., Reference Wilson, Barnes and Bennett2003; Wilson, Beckett, et al., Reference Wilson, Beckett, Barnes, Schneider, Bach, Evans and Bennett2002) five-factor cognitive structure. Most fit indices produced by the original five-factor model (Model 1) were within the established thresholds (RMSEA = 0.079; CFI = 0.942; and TLI = 0.926). However, the upper critical value for the RMSEA exceeded the cutoff of 0.08 for a reasonable error of approximation and the ratio χ 2/df was borderline (4.71). An inspection of modification indicesFootnote 2 (MI) for indicators of misfit revealed a large residual correlation between two semantic fluency categories: Animals and Fruits.
Table 3 Comparison of fit indices of competing models by latent construct
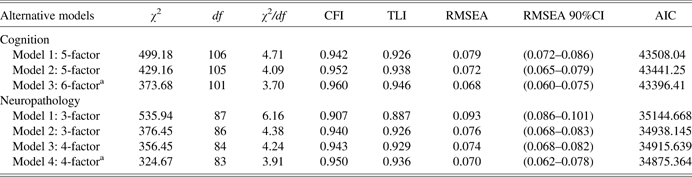
Note. CFI = comparative fit index; TLI = Tucker-Lewis Index; RMSEA = root mean square error of approximation; AIC = Akaike Information Criterion.
aSelected model.
By allowing the residual errors to correlate, the fit of the less constrained model (Model 2) relative to Model 1 was better (Δ χ 2 = 70.02; Δ df = 1; p = .001). As shown in Table 3, the corresponding fit indices for Model 2 also improved slightly. Instead of correlating residuals, which may indicate covariation due to a method effect, a competing non-nested model (Model 3) was tested allowing the subscales Animals and Fruits to load on a separate construct labeled as Verbal fluency. Of the three models tested, the six-factor Model 3 produced the best overall fit statistics (RMSEA = 0.068; CFI = 0.960; and TLI = 0.946), the lowest AIC value (AIC = 43396; the lower the better), and also a clinically meaningful and interpretable latent structure. Consequently, Model 3 was selected for further analyses.
Confirmatory Factor Analysis of the AD Neuropathology Measurement Model
We tested four alternative CFA models assessing the underlying structure of the 15 AD neuropathology indicators representing the counts of NPs, NFTs, and DPs in five different brain regions. The first model (Model 1) hypothesized a three-factor latent structure with single dimensions of NPs, DPs, and NFTs. As shown in Table 3, Model 1 failed to satisfy most of the established cutoff criteria for adequate fit (for example, RMSEA = 0.093, 90% CI (0.086, 0.101); χ 2/df = 6.16; and TLI = 0.887). The inspection of MIs for Model 1 revealed a sizable residual correlation between the neurofibrillary tangle counts for the entorhinal cortex and hippocampal sector. Allowing the estimation of this residual correlation improved the model fit to the data (RMSEA = 0.076; CFI = 0.940; and TLI = 0.926). The improvement of Model 2 over Model 1 was also significant (Δ χ 2 = 159.49; Δ df = 1; p = .001), however, the 90% CI for the RMSEA was slightly above the 0.08 threshold (0.068, 0.083).
Model 3 specified a four-factor structure, with two dimensions of NFTs, one involving medial temporal structures (entorhinal cortex and hippocampus) and the second defined by neocortical regions (mid frontal, mid temporal, inferior parietal). This parcellation corresponds to the well-known temporal progression of NFT pathology in AD. Model fit was improved in comparison to Model 1 (AIC = 34915.64 vs. AIC = 35144.67 for Model 1). Yet, the 90% CI for the RMSEA was also above the 0.08 cutoff criterion. Further examination of the MIs yielded by Model 3 revealed a large residual correlation between the NFT counts of the frontal and parietal brain regions relative to the other MIs in the model. Allowing this correlation to be estimated resulted in an improved fit and reasonably adequate measurement model for the neuropathology variables. Therefore, the four-factor model (Model 4) was adopted as the optimal measurement model for all the subsequent MIMIC analyses.
Relationship Between the Cognitive Factors and the Neuropathological Variables
Results of the full MIMIC model with and without brain weight as an exogenous predictor are summarized in Table 4. Both models produced fit indices well within reasonable thresholds. Using normalized Pratt’s measures (δj) as a reference to compare the relative importance of explanatory variables in the model, the NFT factor (defined by three measures taken from the frontal, temporal, and parietal brain regions) made the largest relative contribution to the prediction of five of the six cognitive outcomes (episodic memory, semantic memory, fluency, working memory, and visuospatial ability) and was significantly related to all six. The NP factor appeared to have a relatively strong effect on perceptual speed and visuospatial ability and a high relative importance for episodic memory and fluency. Lewy bodies were found to be a significant negative predictor of all six cognitive outcomes, making the largest relative contribution to visuospatial ability. The medial temporal NFT factor (measured by tangle counts in the entorhinal cortex and hippocampal regions) was a marginal predictor of working memory and was among the top most relevant predictors in visuospatial ability and episodic memory. Diffuse plaque counts were not significantly associated with any of the cognitive domains examined. This result was consistent across models whether brain weight was excluded or included.
Table 4 Results of the MIMIC model by cognitive endogenous factors

*p < .05; **p < .01.
aModel 1 fit indices (CF1 = 0.951; TLI = 0.940; RMSEA = 0.045, 90% CI (0.042, 0.049)).
bModel 2 fit indices (CF1 = 0.947; TLI = 0.936; RMSEA = 0.044, 90% CI (0.041, 0.047)).
cStandardized regression coefficient estimates using the variance of observed outcome, predictor, and latent measures.
dMarginal correlation between the response variable and the predictor.
eNormalized relative importance.
With respect to CVD measures, volume of macro-infarcts was negatively and significantly associated with all six cognitive factors, following Lewy bodies in relative importance. Additionally, micro-infarcts had a significant negative effect in models that excluded brain weight for two cognitive outcomes: fluency (t = −2.08; p = .038) and episodic memory (t = −1.98; p = .048). Micro-infarcts were also significantly related to semantic memory (Model 1: t = −2.35; p = .019; Model 2: t = −2.17; p = .031) and perceptual speed (Model 1: t = −2.26; p = .024; Model 2: t = −2.10; p = .036) regardless of the model evaluated.
Finally, brain weight was positively and significantly related to all six dimensions of cognitive performance. Adding brain weight to the MIMIC model as a predictor variable steadily increased the proportion of variance explained (R2) in the latent cognitive construct with values ranging from 1.10% (perceptual speed) to 3.40% (working memory). R2 values for the model including brain weight spanned from 34.3% (working memory) to 48.7% (episodic memory). The ordering of variables produced by Pratt’s measures also revealed that brain weight was among the four most important predictors of semantic memory, working memory, and fluency outcomes.
Discussion
To our knowledge this is one of the largest cognitive–neuropathological correlation studies to date. As such, it provides a means to better understand how different types of neuropathologies of aging (and possibly their localization within the brain) relate to specific cognitive impairments. The first two steps toward addressing this goal involved determining the underlying factor structure of both the neuropathological variables and the neuropsychological variables. Results of the CFA of AD indicators showed that the AD pathology was best represented by four separate factors (medial temporal tangles, cortical tangles, diffuse plaques and neuritic plaques). This finding agrees with the hypothesized sequence of development of AD pathology wherein NFT pathology first emerges within medial temporal lobe structures (Markesbery, Reference Markesbery2010) but ultimately progresses to include neocortex (Braak & Braak Reference Braak and Braak1991, Reference Braak and Braak1997). Both the neuritic and diffuse plaques appeared to be best represented as unidimensional factors, suggesting there are not significant regional differences in the distribution of these two pathology types. This is consistent with a large body of literature that shows amyloid plaques to be widely distributed throughout the cortex during the clinical stages of AD (Cupidi et al., Reference Cupidi, Capobianco, Goffredo, Marcon, Ghetti, Bugiani and Giaccone2010). With regard to the five-factor model of cognitive variables used in previous studies (Wilson, Beckett, et al., Reference Wilson, Beckett, Barnes, Schneider, Bach, Evans and Bennett2002; Wilson et al., Reference Wilson, Barnes and Bennett2003) fit reasonably well. However, a six-factor model with a separate verbal fluency factor provided a better fit likely attributable to the executive component of these tests (Lezak, Howleson, Loring, Hannay, & Fischer, Reference Lezak, Howleson, Loring, Hannay and Fischer2004; Marczinski & Kertesz, Reference Marczinski and Kertesz2005).
After identifying the factor structure of both the neuropathological and neuropsychological variables, we proceeded to examine how the AD pathology factors, as well as the other neuropathological variables (macro-infarcts micro-infarcts, and LBs), related to the six cognitive domains. In contrast, medial temporal tangles had more select associations with cognition, relating only to working memory and ranking among the four top predictors for visuospatial ability and episodic memory. The differential relationship between cognitive domain and pathology by regional distribution, again, corresponds to the hypothesized early distribution of NFTs within medial temporal structures and the early hallmark clinical feature of AD (memory impairment; van der Flier et al., Reference van der Flier, van den Heuvel, Weverling-Rijnsburger, Spilt, Bollen, Westendorp and van Buchem2002). Interestingly, the magnitude of the relationship between cortical NFTs and episodic memory was even stronger than the relationship between medial temporal NFTs and episodic memory. Using Pratt’s measure of relative importance, cortical NFTs were approximately 3 times more important than medial temporal NFTs for predicting episodic memory. Such findings correspond to a growing body of literature suggesting that various aspects of memory are dependent on a distributed set of brain regions including prefrontal regions (Dickerson et al., Reference Dickerson, Feczko, Augustinack, Pacheco, Morris, Fischl and Buckner2009; Kirchhoff, Wagner, Maril, & Stern, Reference Kirchhoff, Wagner, Maril and Stern2002) and posterior parietal regions (Kuczynski et al., Reference Kuczynski, Reed, Mungas, Weiner, Chui and Jagust2008; Staresina & Davachi, Reference Staresina and Davachi2006; Walhovd et al., Reference Walhovd, Fjell, Brewer, McEvoy, Fennema-Notestine, Hagler and Dale2010). Although there is some literature reporting a specific relationship between medial temporal tangle pathology and episodic memory (Mitchell et al., Reference Mitchell, Mufson, Schneider, Cochran, Nissanov and Arnold2002), we are unaware of any previous studies that have compared the association of NFT across different regions with specific cognitive functions.
Finally, we examined the association between cognition and two types of AD plaques—diffuse and neuritic plaques. Findings demonstrated that neuritic plaques were independently related to perceptual speed and visuospatial ability. Such findings, consistent with older studies, showed neuritic but not diffuse plaques to be related to global measures of cognition (Arriagada, Growdon, Hedley-Whyte, & Hyman, Reference Arriagada, Growdon, Hedley-Whyte and Hyman1992; Duyckaerts & Hauw, Reference Duyckaerts and Hauw1997; Nagy et al., Reference Nagy, Esiri, Jobst, Morris, King, McDonald and Smith1995). Other studies looking at total amyloid load have not found a strong association with cognition independent of NFT (Bennett, Schneider, Wilson, Bienias, & Arnold, Reference Bennett, Schneider, Wilson, Bienias and Arnold2004). However, total amyloid load as an index of pathology incorporates both diffuse and neuritic plaques. Taken together, neuritic involvement appears to be critical to cognitive disruption.
Although AD is thought to be the most common cause of dementia in the elderly (Alzheimer’s Association, 2009), the presence of some degree of concomitant CVD is extremely common (Plassman et al., Reference Plassman, Langa, Fisher, Heeringa, Weir, Ofstedal and Wallace2007). Despite its prevalence, the nature of the independent effects of CVD on cognition has been widely debated in the literature. We examined the association between cognition and two markers of CVD: macroscopic and microscopic infarcts. A higher volume of macro-infarcts was consistently associated with worse performance across all cognitive domains. These relationships were weak in comparison to the some of the AD pathology–cognitive relationships. Micro-infarcts had smaller and less consistent relationships with cognitive domains, relating only to fluency and episodic memory in models without brain weight as a predictor and to semantic memory and perceptual speed in models where brain weight was a predictor.
Finally, LBD was significantly related to all six cognitive domains. While no clear pattern emerged of differential relationships across the cognitive domains, examination of Pratt’s relative importance measures suggests that LBD pathology was strongly associated with the visuospatial domain. This is in keeping with a body of research linking LBD pathology to visuoperceptual disturbances.
While our primary objective was to examine the relationship between specific types of neuropathology and cognition, we also examined the independent contribution of brain weight to cognitive function. Some speculate that neuronal hypotrophy may be one of the earliest pathological changes in AD (Iacono et al., Reference Iacono, Markesbery, Gross, Pletnikova, Rudow, Zandi and Troncoso2009), and that ultimately neuronal and synaptic deficits are central to cognitive deterioration (Duyckaerts, Delatour, & Potier, Reference Duyckaerts, Delatour and Potier2009). Although we did not have direct measures of neuronal loss, we hypothesized that brain weight would, in part, be an indirect measure of neuronal loss and therefore might relate to cognition even after accounting for the other specific pathology types. Brain weight may also help to account for white matter loss not captured by the other specific pathologies examined in this study. In fact, we found that brain weight accounted for up to 3.4% of additional variance in the six cognitive domains, independent of the more specific pathologies. Whether this finding is due to limitations in measuring AD, CVD, and LBD neuropathology, not measuring and modeling effects of other forms of neuropathology, or preexisting, lifelong differences in brain weight remains an important question for further research.
The present study has several strengths. The latent variable modeling approach facilitated efficiently capturing the complexity of the interrelationships between the multiple cognitive variables and measures of brain pathologies. While the ROS study participants represent a rather homogeneous group in terms of life-style and other variables, the inclusion of data from a community-based study such as MAP greatly increased the sample variability. Both cohorts were extremely well characterized in terms of cognitive function and neuropathology. Finally, follow-up and autopsy rates are high for both cohorts, greatly increasing internal validity.
There are also some limitations to this study. The analytical sample is not population-based, and participants were predominantly white with high levels of education, limiting, to some extent, the generalizability of results. Diseases such as CVD may have been under-represented in our sample due to these demographic factors. While the battery of cognitive tests covered diverse domains, the assessment of some aspects of executive functioning was limited. Additionally, the investigation of micro-infarcts was performed in a limited number of sections of the brain.
It is possible that floor effects may affect between-subject variability in the pathology-cognition associations. We tested, however, for floor effects using cognitive domain score quartiles and did not find serious distributional asymmetries in the lowest quartile that should have been taken into account in the analyses. The estimated skewness and kurtosis for each cognitive domain scores in the lower quartile were respectively as follows: episodic memory (−0.350; −0.640), semantic memory (−0.739; 0.273), working memory (−0.357; −0.828), perceptual speed (−0.575; −0.123), visuospatial ability (−0.535; −0.490), and fluency (−0.430; −0.753). Using as a reference recommended thresholds of ±1.00 for both skewness and kurtosis (Meyers, Gamst, & Guarino, Reference Meyers, Gamst and Guarino2006; Morgan, Griego, & Gloeckner, Reference Morgan, Griego and Gloeckner2001), none of the reported values indicated serious asymmetry caused by floor effects affecting between-subject variability in the observed pathology-cognition associations.
The recruitment plans for both MAP and ROS studies targeted a broad spectrum of non-demented individuals at baseline, and many developed cognitive impairment over the follow-up period thus the sample included participants who had normal cognition, mild cognitive impairment, and dementia. We included the full sample in analyses, and did not evaluate relationships within groups defined by degree of cognitive impairment. Diagnoses of normal cognition, MCI, and dementia are labels that arbitrarily divide these dimensions to help with communication of complex clinical information. Separate analyses within diagnostic subgroups would be problematic for addressing the effects of neuropathology on cognition for several reasons. Methodologically, this strategy restricts variability and decreases sample size and, therefore, statistical power, both of which obscure important effects. But there is a more compelling substantive problem with subgroup analyses. The relationship of neuropsychological test results to brain structure across the full range is of clinical importance, and this cannot be effectively studied within individual subgroups that are based on arbitrary divisions of that range. This is exemplified by studies that show small correlations of hippocampal volume with memory in normals (Van Petten, Reference Van Petten2004) in contrast to striking correlations in clinically heterogeneous samples (Grundman et al., Reference Grundman, Jack, Petersen, Kim, Taylor, Datvian and Thal2003; Mungas, Reed, Ellis, & Jagust, Reference Mungas, Reed, Ellis and Jagust2001; Petersen et al., Reference Petersen, Stevens, Ganguli, Tangalos, Cummings and DeKosky2001). While subgroup analysis might have value for specific purposes (for example, determining how individuals diagnosed with MCI respond to a specific treatment) our goal was to understand how neuropathology affects cognition, and for this reason, we included the full range of variables in the total sample.
The present study demonstrates relationships between multiple neuropathological changes common to aging and cognitive functioning that are pervasive in scope and complex in detail. Every domain of cognitive function was significantly and negatively related to some set of neuropathological markers. Notably, neocortical tangles demonstrated the strongest relationship to most cognitive domains, supporting the idea that Alzheimer’s disease is a major determinant of cognitive impairment in the elderly. To some degree, the anatomical distribution of pathological changes influenced patterns of cognitive impairment (i.e., medial temporal NT affected primarily episodic memory), although many common pathologies have rather diffuse effects. Finally, all of the specific neuropathology types (i.e., those associated with AD, LBD, and CVD) made independent contributions to cognitive impairment, supporting the notion that cognitive impairment is multi-determined.
Despite the positive findings of this study, it must also be recognized that the total amount of variance explained by both the specific neuropathological variables examined and brain weight did not exceed 48%, and for most cognitive domains only approximately a third of the variance was accounted for by these brain variables. While the development of new and more precise measures of neuropathology and brain integrity (i.e., neuronal or synaptic count) will help to further close the gap between structural brain abnormalities and the degree and nature of cognitive dysfunction, it is also likely that a significant discrepancy will remain. In fact, it was precisely within this context of an apparent disconnect between extent of neuropathology and extent of cognitive impairment that the concept of brain reserve was born (Katzman et al., Reference Katzman, Terry, DeTeresa, Brown, Davies, Fuld and Peck1988; Stern, Reference Stern2002)—the idea being that there are other factors, some genetic or biological, others environmentally based, that make the brain more or less resilient to neuropathologies of aging. Examination of some of the factors that account for this unexplained variance between cognition and brain pathology is the focus of the follow-up paper in this series.
Acknowledgments
The data for this study were provided by studies supported by the National Institutes of Health, National Institute of Aging (NIA); the Religious Orders Study (P30AG10161, R01AG15819), and the Rush Memory and Aging Project (NIA Grants AG17917 and AG10161), and the Illinois Department of Public Health. Data were provided by the principal investigator (PI) of these projects, David Bennett, MD. The research was supported in part by an NIA conference grant Conference on Advanced Psychometric Methods in Cognitive Aging Research, (R13AG030995) Dan Mungas, PhD, PI. The authors thank the study participants and the staff of the Rush Alzheimer’s Disease Center.