Published online by Cambridge University Press: 12 February 2004
Deficits in visual-spatial ability can be associated with Parkinson's disease (PD), and there are several possible reasons for these deficits. Dysfunction in frontal–striatal and/or frontal–parietal systems, associated with dopamine deficiency, might disrupt cognitive processes either supporting (e.g., working memory) or subserving visual-spatial computations. The goal of this study was to assess visual–spatial orientation ability in individuals with PD using the Mental Rotations Test (MRT), along with other measures of cognitive function. Non-demented men with PD were significantly less accurate on this test than matched control men. In contrast, women with PD performed similarly to matched control women, but both groups of women did not perform much better than chance. Further, mental rotation accuracy in men correlated with their executive skills involving mental processing and psychomotor speed. In women with PD, however, mental rotation accuracy correlated negatively with verbal memory, indicating that higher mental rotation performance was associated with lower ability in verbal memory. These results indicate that PD is associated with visual–spatial orientation deficits in men. Women with PD and control women both performed poorly on the MRT, possibly reflecting a floor effect. Although men and women with PD appear to engage different cognitive processes in this task, the reason for the sex difference remains to be elucidated. (JINS, 2003, 9, 1078–1087.)
Parkinson's disease (PD) has been associated with deficits on visual–spatial tasks (Cummings & Huber, 1992; Growdon & Corkin, 1986; Stern & Mayeux, 1986). However, a recent meta-analysis of this literature (Waterfall & Crowe, 1995) suggested that visual–spatial deficits in PD are not universal because deficits are seen primarily on multifactorial visual–spatial tasks (e.g., Raven's Progressive Matrices) but not more unifactorial measures of visual–spatial ability (e.g., Judgment of Line Orientation, Embedded Figures Test). This conclusion was similar to those of Lazaruk (1994). Thus, the relationship between visual–spatial ability and PD is not clear.
One theory of cognitive dysfunction in PD suggests that these deficits are related to disturbance in the frontal–basal ganglia neural circuits important in executive functions such as attention and concentration, sequencing, working memory, and set-shifting (Brown & Marsden, 1990; Taylor & Saint-Cyr, 1995). This executive dysfunction theory was supported by a study that found a significant relationship between executive dysfunction and visual–spatial deficits in individuals with PD (Bondi et al., 1993). In a group of 19 individuals with mild to moderate PD, these investigators found that statistically controlling for the deficits in executive skills (e.g., Wisconsin Card Sorting Test, California Sorting Test) through an analysis of covariance eliminated the visual–spatial deficits (e.g., WAIS–R Picture Arrangement, Benton Facial Discrimination Test), but statistically controlling for the visual–spatial deficits did not alter the abnormal measures of executive function.
In contrast, Cronin-Golomb and Braun (1997) provide evidence for a visual–spatial deficit in PD that is independent of executive skills. In a group of 50 non-demented, non-depressed, individuals with mild to moderate PD, these investigators found deficiencies on Subtest A of Raven's Colored Progressive Matrices (RCPM) when compared to matched control subjects. Because Subtest A of the RCPM has a greater visual–spatial component than the remaining portions of the RCPM, and because the PD subjects' RCPM-A performance was related to other measures of visual–spatial ability (e.g., Luria's Mental Rotation Test, Standardized Road-Map Test of Direction Sense), but not measures of executive function (e.g., Stroop test, WAIS–R Picture Arrangement), they concluded that PD was associated with a visual–spatial problem-solving deficit.
Visual–spatial abilities as usually tested involve several distinct cognitive processes (Ekstrom et al., 1976), and the visual–spatial tasks used in the studies reviewed above are generally considered to be complex, involving multiple cognitive processes. Therefore, a significant problem with this literature is the visual–spatial tasks used and their dependence on other cognitive processes (Waterfall & Crowe, 1995). Mental rotation ability is correlated with other visual–spatial skills (Bryden, 1982; McGee, 1979; Stumpf & Eliot, 1999) and may offer a way of more directly examining this issue. However, mental rotation data in PD is also variable. Previous studies have found no significant differences in accuracy of mental rotation between individuals with Parkinson's disease and control subjects (Boller et al., 1984; Brown & Marsden, 1986; Goldenberg et al., 1986; Raskin et al., 1992; Smith et al., 1998; Taylor et al., 1986). One possible limitation of these negative studies, however, was the failure to use sensitive measures that limit verbal mediation and require both two- and three-dimensional rotations. For example, some studies (Boller et al., 1984; Brown & Marsden, 1986; Taylor et al., 1986) used tasks that are limited to two dimensions (e.g., letter rotation, map direction task) and could be verbally mediated. Other studies (Goldenberg et al., 1986; Raskin et al., 1992) used a manikin rotation task that can also be verbally mediated. However, a recent study using a tachistoscopic cube-figure presentation method similar to the mental rotation task of Shepard and Metzler (1971), which requires subjects to make same-different judgments when two objects are presented in different three-dimensional orientations, found that PD subjects were less accurate than control subjects in making “same” judgments (Lee et al., 1998). However, when compared to control subjects, these same PD subjects also had significantly faster response times when making “same” judgments, suggesting that the mental rotation deficit in these PD subjects might have been due to a speed–accuracy trade-off. Supporting this conclusion were the observations that slower response times for “different” judgments of three-dimensional stimuli, and “same” and “different” judgments of two-dimensional stimuli, were associated with normal accuracy. Thus, these findings do not differentiate whether these deficits in PD are due to problems in visual–spatial ability or deficiencies in psychomotor and mental processing speed which are common in PD. In addition, there was also no other testing to exclude the possible confound of an associated dementia.
Because it remains unclear if mental rotation deficits exist in non-demented individuals with Parkinson's disease, we studied a population of non-demented subjects with PD using the Mental Rotations Test (MRT) which requires the participant to recognize a target stimulus in different two- and three-dimensional spatial orientations (Linn & Petersen, 1985; Shepard, 1978; Shepard & Metzler, 1971). We also examined these PD subjects' performance on other tests of cognitive function, particularly executive skills, to ascertain the relationship between these other cognitive functions and mental rotation ability.
Twenty-eight men and 23 women with PD were recruited from the Neurology and the Neurosurgery clinics at the University of Florida Health Science Center as experimental subjects for this study. Most of these volunteers were being evaluated for surgical treatment of their PD, whereas the remaining few were seen in the clinic for their periodic neurological evaluation. Hospital volunteers, as well as family members and friends who accompanied the PD patients to the clinic were recruited as controls (28 men and 28 women). Exclusionary criteria for enrollment in this study (experimental and control subjects) included use of the left hand for writing, a history of learning disabilities, a history of other or concurrent neurological disorders, and a previous history of major psychiatric disorder prior to PD onset.
The presence of dementia was assessed in 105 of 107 subjects with the Mini Mental Status Exam (MMSE; Folstein et al., 1975), with a cut-off criterion of 27/30. A higher than usual cut-off criterion was used in this study to minimize the likelihood of a confounding neurodegenerative disease other than PD being present (Malapani et al., 1994; Reed et al., 1997). Two female control subjects did not receive the MMSE because they were gainfully employed at the time of testing and dementia screening was deemed unnecessary. The motor portion of the Unified Parkinson's Disease Rating Scale (UPDRS; Fahn & Elston, 1987) was administered to characterize the motor dysfunction of the PD subjects. The Parkinson's subjects were also screened for depression with the Geriatric Depression Scale (GDS; Yesavage et al., 1983). It should be noted that 9 PD subjects did not receive the GDS, and 2 PD subjects did not receive the UPDRS.
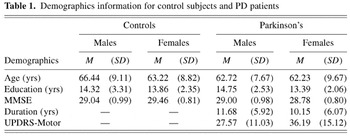
Demographic data for the respective groups are presented in Table 1. Please see Table 2 for the PD subjects' medications. There was no statistically significant difference in the numerical composition of the subject groups [χ2 = 0.612, (df = 1, N = 107) n.s.]. There were no significant differences between PD patients and control subjects in age or education, and no difference between male and female PD subjects in disease duration. However, the female PD patients had significantly more motor symptoms than male Parkinson's patients on the UPDRS Motor Scale (Mann-Whitney U = 189.50, p = .035). These UPDRS scores place the males with PD generally in the mild to moderate range of motor impairment (approximating a Hoehn & Yahr, 1967, Stage III to Stage IV), and the female PD patients in the moderate range of impairment (approximating a Hoehn & Yahr, 1967, Stage IV). Based on results from the UPDRS Motor Scale, 15 Parkinson's subjects exhibited predominantly right-sided symptoms, 11 exhibited predominantly left-sided symptoms, and 23 subjects exhibited bilateral symptoms. Results from the UPDRS Motor Scale also indicated that 27 Parkinson's subjects exhibited predominantly rigid–akinetic symptoms, 9 exhibited predominantly tremor, and the symptom presentation of 13 Parkinson's subjects could not be differentiated, exhibiting both rigid–akinesis and tremor to a similar degree. Based on responses to the GDS, 21 PD subjects reported no consistent symptoms of depression, 16 PD subjects reported symptoms of mild depression, and 5 subjects reported symptoms of moderate to severe depression (see Spreen & Strauss, 1998 for scoring criteria).
Demographics information for control subjects and PD patients
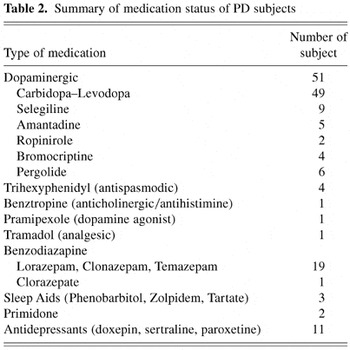
Summary of medication status of PD subjects
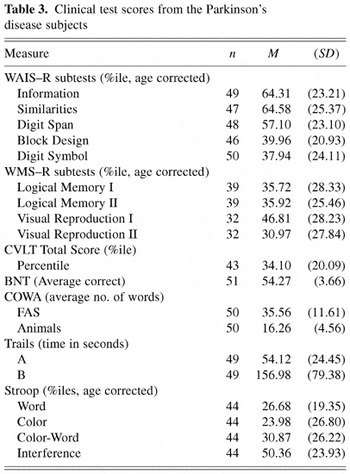
Parkinsonian patients also received neuropsychological testing as part of their clinical evaluation. These neuropsychological tests were selected to assess for current level of general intellectual skills, memory for verbal and visual–spatial information, language, visual–spatial ability, and executive functions involving speeded mental processing and set-shifting. Tests included the Information, Similarities, Digit Span, Digit Symbol, and Block Design subtests from the Wechsler Adult Intelligence Scale–Revised (Wechsler, 1981), Logical Memory I and II and Visual Reproduction I and II from the Wechsler Memory Scale–Revised (Wechsler, 1987), California Verbal Learning Test (CVLT; Delis et al., 1987), Boston Naming Test (BNT; Kaplan et al., 1983), Controlled Oral Word Associations (COWA; Spreen & Benton, 1977), Stroop Color Word Test (Golden, 1978), and the Trail Making Test (Trails; Reitan & Wolfson, 1985). These tests were administered and scored according to standardized instructions using normative data adjusted for age and education where available. Due to time constraints in the clinical evaluation, some of the PD subjects did not receive all of the tests (Table 3). Neuropsychological test results from the cognitive screening are also presented in Table 3. Review of Table 3 indicates that the level of cognitive functioning in this sample of Parkinsonian subjects is generally within normal limits (see Spreen & Strauss, 1998; Wechsler, 1981, 1987 for normative data), with no discrepancies between measures of crystallized knowledge (e.g., WAIS–R Information subtest) and verbal abstract reasoning (e.g., WAIS–R Similarities subtest). Performance on the WAIS–R Block Design, although variable, was within normal limits. These subjects also show no deficits in confrontation naming (e.g., Boston Naming Test), verbal fluency (e.g., COWA), or memory for either verbal or visual-spatial information (e.g., WMS–R, CVLT). Test results are noteworthy for some psychomotor slowing as seen on Trails B of the Trail Making Test. These test findings are generally consistent with the PD subjects' reported level of educational attainment. Taken together, these results do not suggest that these PD subjects have experienced any significant decline in general cognition. Further, it is noteworthy that the Parkinsonian's performances on tests requiring psychomotor output (e.g., Block Design, Digit Symbol, Trails A) were within the average range. Measures of executive function (e.g., Digit Symbol, Stroop, COWA, Trails A) were also within normal limits.
Clinical test scores from the Parkinson's disease subjects
Mental rotation ability was assessed with the Mental Rotations Test (e.g., Shepard, 1978; Shepard & Metzler, 1971) which can be administered either individually or in small groups of 2 to 4 volunteers (Vandenberg & Kuse, 1978). This paper-and-pencil test has 20 items with one target stimulus and four choices. Two of the four choices are correct, but are presented in different two- and three-dimensional planes, as if rotated to a different perspective. This test requires the participant to match the two correct choices to the target stimulus from four possible options. Because of the age range of these volunteers and possible difficulties of reduced vision, slight modifications were made to the test to facilitate performance. Test stimuli were enlarged by approximately 20% to enhance viewing (see Figure 1). Each example was presented on a separate page, and experimental test items were presented three per page. The participants responded on a separate answer sheet. Otherwise, test administration followed established procedures, including standardized instructions and a 10-min time limit (Vandenberg & Kuse, 1978). The dependent variable for this measure was the proportion of correct responses (number of correct items/total number of items attempted). Using a proportion correct score as the dependent variable was intended to correct for differences in psychomotor speed in test completion which may be a confound for individuals with Parkinson's disease.
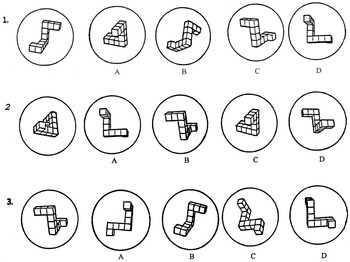
Mental Rotation Test example.
The total correct scores and proportion correct scores for the men and women of each experimental group are presented in Table 4. Review of Table 4 indicates that the total correct scores of the male and female control subjects are consistent with that of previous research using normal adults of a similar age (Wilson et al., 1975).
Mental Rotation Test scores

The proportion correct data from Table 4 was subjected to a 2 (group) × 2 (gender) ANCOVA, with age as the covariate. Gender was included as an independent variable because a large male advantage has previously been found on this task (Linn & Petersen, 1985). Age was included as the covariate to account for possible age effects on this task (Wilson et al., 1975). Consistent with this previous research (Wilson et al., 1975), age was a significant factor in this analysis [F(1,102) = 4.98, p = .028], and accounted for a small proportion of variance in this analysis, R2 = .01. This analysis also yielded a significant main effect for group [F(1,102) = 12.13, p ≤ .001], indicating that Parkinson's subjects (M = 55.94, SD = 12.30) were less accurate on the MRT than control subjects (M = 63.71, SD = 15.83). A significant main effect for gender was also found [F(1,102) = 18.55, p ≤ .001], indicating that men (M = 64.81, SD = 16.03) were more accurate on the MRT than women (M = 54.73, SD = 11.06).
The interaction between Group × Gender was also significant [F(1,102) = 5.21, p = .025]. Post-hoc analyses with Bonferroni correction (alpha = .008) on the age-corrected scores revealed that male control subjects were significantly more accurate than female control subjects [t(54) = 4.51, p ≤ .001], whereas there was no difference in accuracy between male and female PD subjects. Male control subjects were also significantly more accurate than male PD subjects [t(54) = 3.61, p = .001], although there was no difference in accuracy between female control and PD subjects.
A series of supplementary analyses were conducted to assess the relationship between subject characteristics of the Parkinsonian subjects (e.g., symptom presentation, symptom severity, illness duration, medication status, level of depression) and their spatial orientation performance. To determine if symptom laterality was related to mental rotation performance in these PD subjects, a two-factor ANCOVA was conducted on the proportion correct scores, with laterality (e.g., right, left, bilateral) and gender as a between-subjects variable. Although there was no significant gender difference in MRT accuracy within the PD group, gender was included as a between subjects variable to account for any variability in performance that may interact with symptom laterality. Age was included as a covariate to account for age effects, and maintain consistency with the analysis described above. No significant effect was found for laterality [right M = 56.10, SD = 13.90; left M = 58.90, SD = 14.29; bilateral M = 54.93, SD = 10.94; F(2,42) = .07, p = .93]. There was also no significant effect for age or gender, nor a significant interaction between Gender × Laterality.
To determine if the type of Parkinson's symptom (e.g., rigid–akinetic vs. tremulous vs. undifferentiated) exhibited by these subjects was associated with mental rotation ability, another ANCOVA was conducted on the MRT proportion correct scores, with predominant form of symptom and gender as the between subjects variables, and age as the covariate. This analysis revealed no significant difference in mental rotation accuracy associated with the PD symptom type [rigid–akinetic M = 57.45, SD = 14.36; tremulous M = 58.48, SD = 7.90; undifferentiated M = 51.96, SD = 10.45; F(2,42) = 1.09, p = .35]. Further, there was no main effect for age or gender, or an interaction between Gender × Predominant Symptom.
To assess the relationship between symptom severity and spatial orientation ability, a correlational analysis was conducted between the UPDRS score and the MRT accuracy score. To account for possible differences associated with sex, separate analyses were done for male and female PD subjects. Because the UPDRS score is based on a subjective ordinal scale, the proportion correct score was treated as rank order data for this analysis to allow comparison with the UPDRS score. The correlation between symptom severity and MRT accuracy for both men (rs = −.06) and women (rs = .26) was not significant.
To assess the relationship between the PD subjects' level of depression and spatial orientation performance, an ANCOVA was conducted on the proportion correct scores, with depression group (e.g., depressed, nondepressed) and gender as the between-subjects variables, and age as the covariate. Because of the small number of PD subjects reporting moderate to severe symptoms of depression, the data from these subjects was combined with the data of those reporting mild symptoms of depression to form one group. No significant difference in MRT accuracy were found associated with presence of symptoms of depression [non-depressed M = 58.25, SD = 9.76; depressed M = 53.11, SD = 12.81; F(1,37) = 2.17, p = .15]. There was also no significant effect for age or gender, or the interaction between Gender × Depression.
To evaluate the association between medication status and mental rotation accuracy, the PD subjects were divided into two groups. Group 1 consisted of those 27 individuals taking additional medications (e.g., analgesics, anxiolytics, sleep aids, antidepressants) that might interfere with cognitive function plus an additional two subjects taking Parkinsonian medications with significant cognitive side effects (e.g., trihexyphenidyl). The remaining 22 subjects taking only Parkinsonian medications with no significant cognitive side effects comprised Group 2. The MRT accuracy scores were then subjected to a 2 (medication status) × 2 (gender) ANCOVA, with age as the covariate. This analysis revealed no main effect for medication status [Group 1 M = 55.42, SD = 12.90; Group 2 M = 56.62, SD = 11.74; F(1,46) = 0.02, p = .89]. There was also no significant effect for age or gender, or the interaction between Gender × Medication Status.
Another correlational analysis was conducted between illness duration in years and MRT accuracy. Again, no significant relationship was found between disease duration and mental rotation accuracy for either men (r = −.08) or women (r = −.05).
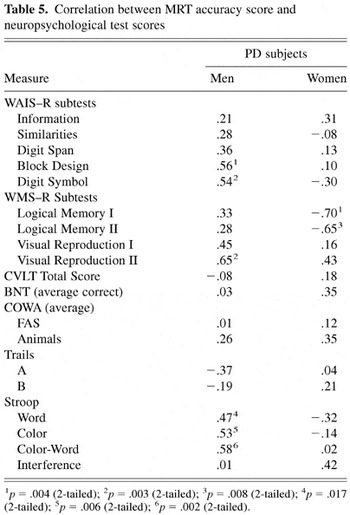
A final series of correlational analyses was conducted between the MRT proportion correct score and the neuropsychological test scores. Again, separate analyses were conducted for male and female PD subjects to account for possible differences associated with sex. These results are presented in Table 5. Review of these findings indicates that, for men, mental rotation accuracy was significantly correlated with the Wechsler Adult Intelligence Scale–Revised Block Design subtest, which requires visual–spatial organization and problem-solving, and the Digit Symbol subtest, which requires visual attention, scanning, and matching, as well as psychomotor speed and incidental learning. Mental rotation performance also correlated significantly with the speed of mental processing and response inhibition/cognitive set shifting on the Stroop Test, and delayed recall for visual–spatial information on the WMS–R. In contrast, women showed a significant inverse relationship between mental rotation accuracy and both immediate and delayed recall for verbal information on the WMS–R, indicating that higher mental rotation accuracy was associated with lower verbal memory. The correlations between the MRT proportion correct score and these cognitive test scores were further analyzed with Fisher r-to-Z transformation. Using Bonferroni correction (alpha = .006, one-tailed), the difference in correlations between PD men and women were significant for the Wechsler Adult Intelligence Scale–Revised Digit Symbol subtest (z = 3.00, p = .00135), Stroop Word Reading task (z = 2.81, p = .0026), the Wechsler Memory Scale–Revised Logical Memory tasks (Logical Memory I, z = 4.39, p = .0001; Logical Memory II, z = 3.43, p = .0003). The difference between correlations on the Stroop Color–Naming task was marginally significant (z = 2.44, p = .0073). The difference between correlations on the WAIS–R Block Design subtest (z = 1.70), Stroop Color-Word Reading task (z = 2.14), and the Wechsler Memory Scale–Revised Visual Reproduction II (z = 1.05) did not achieve significance.
Correlation between MRT accuracy score and neuropsychological test scores
The results of this study indicate that Parkinson's disease is associated with deficiencies in mental rotation in men. Specifically, men with PD demonstrated significantly lower scores on the Mental Rotations Test than men of similar age and education, whereas PD and control women performed at a similar low level. This floor effect indicates that the MRT is insensitive to visual–spatial deficits in PD females. Mental rotation performance in PD subjects was not affected by other factors, such as symptom laterality, symptom severity, symptom type, disease duration, medications, or mood. Because mental rotation is believed to be a basic component of visual–spatial ability (Bryden, 1982; Linn & Petersen, 1985; McGee, 1979; Stumpf & Eliot, 1999), these findings are consistent with previous research suggesting that PD is associated with diminished visual–spatial ability (Cummings & Huber, 1992; Growdon & Corkin, 1986; Stern & Mayeux, 1986). The reason men are impaired on the MRT is unknown, but there are several possible reasons.
The finding of a significant interaction between group and the subject's gender may provide some insight into the mechanisms that underlie mental rotation deficits in PD. Men typically perform better than women on tests of mental rotation (Linn & Petersen, 1985; see also Halpern, 1992; Maccoby & Jacklin, 1974), consistent with our findings. The mechanisms underlying this sex difference are not entirely known, but mental rotation ability is typically mediated primarily by the right hemisphere (Corballis, 1997; see also Benton & Tranel, 1993; Bryden, 1982; Lezak, 1995). There is also evidence that there are sex differences in the lateralization of visual-spatial processing (Harris, 1978; Hiscock et al., 1995; Levy, 1974; Levy & Reid, 1978), and this enhanced male asymmetry might explain the male advantage in visual–spatial tasks (see Levy & Heller, 1992, for review). It has been suggested that there are two different strategies used when performing the MRT task: one might be more verbal categorical (top–bottom, left–right), and the other more non-verbal and continuous (Shepard & Cooper, 1982). This latter strategy, in which the entire stimulus is mentally moved as if in three-dimensional space (Shepard & Cooper, 1982), might be a more efficient process, and based on the superior performance by control men, they are more likely to use this process.
The MRT performance of women with PD was not significantly different from that of control women. Notably, MRT accuracy of both control and PD women was not substantially above chance performance (50%, as defined by the overall probability of responding correctly to the test items; see Table 4), consistent with previous findings (see Wilson et al., 1975). That the men with PD did not perform statistically different from women in either group suggests that the men with PD lost the ability to use this mental rotation process.
The correlational analyses support the postulate that the men with PD might perform mental rotations differently than the women with PD. Men showed a significant relationship between mental rotation ability and executive functions involving mental processing and psychomotor speed (e.g., Stroop Word Reading, WAIS–R Digit Symbol) that was different from women, although this finding was less than consistent (see Stroop Color Naming, Color–Word Naming, Interference, Trail Making Test). Whereas the WAIS–R Digit Symbol subtest does not appear to localize well (see Lezak, 1995, for discussion), a recent functional imaging study revealed bilateral anterior cingulate activation, as well as right parietal and left frontal operculum activation, during performance on the Stroop task (Brown et al., 1999). In contrast, women showed a significant inverse relationship between mental rotation ability and verbal memory. Thus, consistent with the “cognitive trade-off” hypothesis (see Levy & Heller, 1992, for review), women who rely on verbal memory and other verbal mediation processes are more likely to perform more poorly on the MRT. These findings are consistent with the supposition that normally men and women use different hemispherically mediated cognitive processes in performing visual–spatial tasks (Halpern, 1992; Hampson & Kimura, 1992; Levy & Heller, 1992). Additional research on these tasks with normal subjects will help elucidate the nature of these relationships.
Slowed processing is not likely to explain the impaired MRT performance in the men with PD. Although there is some evidence of psychomotor slowing and difficulties with set-shifting in this sample of PD subjects (see Table 3, Trails B data), the majority of neuropsychological tests assessing psychomotor speed in the current study were within normal limits. Further, a proportion correct score was used as the dependent variable rather than the total correct score, thereby correcting for the total number of items each subject completed during the 10-min time limit of the test. This scoring approach was intended to control for the speed in which an individual subject completed the items on the test, and should have minimized the possible influence of psychomotor slowing. Consequently, mental rotation performance would be equated for each subject by taking into account the number of items completed.
While men with PD showed impaired mental rotation, deficient mental rotation performance can result from dysfunction in one of several possible components. In order for a person to compute a mental rotation, the individual must perceive the target stimulus and hold this perception in working memory as a mental image. The individual must then transform this mental image into a different perspective to learn if it matches each of the choices. With respect to visual perception, some visual disturbances have been reported in PD (Bodis-Wollner, 1990; Bodis-Wollner et al., 1987, 1991, 1993). However, these disturbances are thought to involve peripheral dysfunction in the retina rather than central cortical dysfunction, and these disturbances are responsive to dopaminergic treatment. Further, data from the neuropsychological assessment of our PD subjects do not indicate deficits in visual–spatial perception (Block Design) or working memory (WAIS–R Digit Span and Digit Symbol, WMS–R Logical Memory I and Visual Reproduction I) that could have accounted for the abnormal mental rotation performance of this sample of male PD subjects. Object imagery was not assessed in this sample, and a deficit in object imagery may account, in part, for the findings of this study. A deficit in imagery might be caused by the inability to create and maintain an internal representation of the object, which is consistent with the findings of overreliance on external environmental information in PD (Brown & Marsden, 1990). Other factors involved in mental rotation that could disrupt performance include the degree and/or dimension of the rotation (Corballis, 1997; Shepard, 1978; Shepard & Metzler, 1971), and these remain the topic of future research as well.
The anatomical localization of brain structures that subserve mental object rotation remains somewhat unclear. Although studies of individuals with lateralized brain damage generally indicate a right hemisphere advantage for mental rotation performance (Ditunno & Mann, 1990; Layman & Greene, 1988; Ratcliff, 1979; but see also Mehta et al., 1987), studies of mental rotation ability in normal subjects using tachistoscopic presentation procedures have been inconsistent in demonstrating a visual field advantage (Cohen & Polich, 1989; Corballis & McLaren, 1984; Corballis & Sergent, 1989; Fischer & Pelligrino, 1988; Jones & Anuza, 1982; Simion et al., 1980; Ueker & Obrzut, 1993; Van Strien & Bouma, 1990). Results from EEG studies are also inconsistent (Ornstein et al., 1980; Osaka, 1984). Functional imaging studies using magnetic resonance imaging have generally shown bilateral activation in both frontal and parietal regions (Cohen et al., 1996; Tagaris et al., 1997), although one study suggests that a hemispheric asymmetry exists that is dependent on whether the mental rotation is of an egocentric (left hemisphere) or extra-personal object-based (right hemisphere) transformation (Zacks et al., 1999). Studies of cerebral metabolism using positron emission tomography have generally found increased right hemisphere activation during performance of spatial orientation tasks (Deutsch et al., 1988; Gur et al., 1982; Harris et al., 2000). However, one study found no hemispheric asymmetry associated with mental rotation performance (Bulla-Hellwig et al., 1996), and another study found activity in left parietal region and basal ganglia during a mental rotation task involving alphanumeric stimuli (Alivisatos & Petrides, 1996).
Parkinson's disease is primarily a disorder of the basal ganglia, and these results indicate that the ability to perform mental rotations is a complex cognitive function that is dependent on intact function in the basal ganglia and their connections to the frontal lobes and parietal regions. Consequently, our results in the men are consistent with current theories of visual–spatial deficits in PD, which may be due to dysfunction in frontal–striatal circuits (Brown & Marsden, 1990; Taylor & Saint-Cyr, 1995), frontal–parietal systems (Cronin-Golomb & Braun, 1997), or parietal-striatal networks (Clower et al., 2002). Given these interconnections (see also Alexander et al., 1986, 1990; Middleton & Strick, 2000; Owen, 1997; Saint-Cyr, 2003, for reviews), dysfunction in one region can have a cascading effect that disrupts function in linked regions. However, it remains unclear if this deficit is primarily related to basal ganglia or cortical dysfunction. Most likely, however, this cognitive deficit is due to dysfunction within a distributed neural system that subserves visual–spatial perception, mental imagery, and mental manipulation of those images. Further research, however, is needed to elucidate the contribution of the different neuropsychological processes involved in spatial cognition, and to delineate the sex differences in the function of these respective processes.
Support for this research was provided, in part, by the National Parkinson's Foundation and the Medical Research Service of the Department of Veterans Affairs. We would like to thank Laura J. Grande, Dean Pettit, Jeff Anderson, Brenda Hanna-Pladdy, Robert Rhodes, Jeannine Mielke, Brian Shenal, and Sam Wu, for their technical assistance in completing this study. We would also like to thank David Mauger, for his assistance with the data analysis. These data were presented, in part, at the 29th Annual Meeting of the International Neuropsychological Society, Chicago, February 2001.

Demographics information for control subjects and PD patients

Summary of medication status of PD subjects

Clinical test scores from the Parkinson's disease subjects

Mental Rotation Test example.

Mental Rotation Test scores

Correlation between MRT accuracy score and neuropsychological test scores