INTRODUCTION
As pain is necessarily a subjective experience, research has been directed toward the development of more objective measures to correlate with self-reported pain severity scores. The representation of acute and chronic pain in forebrain neural networks has been mapped under many conditions using functional imaging techniques (Boly et al., Reference Boly, Faymonville, Schnakers, Peigneux, Lambermont and Phillips2008; Valeriani et al., Reference Valeriani, Betti, Le Pera, De Armas, Miliucci and Restuccia2008), but such tests have limited clinical applicability. Older tests utilizing autonomic and other physiologic parameters have not shown a stable variation with chronic pain intensity, possibly due to habituation and nonspecific factors such as the level of arousal (Gracely, Reference Gracely1999). A simple bedside test that would supplement the clinical examination and questionnaire scale items is lacking.
The most commonly used factor to validate the psychometric properties of pain questionnaires is assessment of pain-related interference with functions such as sleep, walking, and activities of daily living (Given et al., Reference Given, Given, Sikorskii, Jeon, McCorkle, Champion and Decker2008; Jeon, Given, Sikorskii, & Given, Reference Jeon, Given, Sikorskii and Given2009; Zelman, Dukes, Brandenburg, Bostrom, & Gore, Reference Zelman, Dukes, Brandenburg, Bostrom and Gore2005). Although the physical functioning construct may be more accessible to patients than items relating to pain itself, the scales used to tap this construct remain subject to the same recall and measurement biases of all self-reported symptom scales.
A more direct inference regarding pain-related physical impairment may be provided by mental motor imagery. A number of lines of evidence support the claim that mental motor imagery and action are subserved by the same processes. First, many investigators have demonstrated that there is a high correlation between the time required for normal subjects to produce a movement and the time required to mentally simulate that same movement (Sirigu et al., Reference Sirigu, Cohen, Duhamel, Pillon, Bubois, Agid and Pierrot-Deseilligny1995; Parsons, Reference Parsons1994; see Jeannerod, Reference Jeannerod1995). Second, mental motor imagery is associated with the same changes in somatic indices of behavior that are observed in action. For example, Decety and colleagues contrasted heart and respiratory rates during leg exercise at two levels of work (15 kg and 19 kg loads) and during mental simulation of the same exercise. Subjects reported a greater sensation of fatigue during imagined exercise with the larger load (Decety, Jeannerod, Durozard, & Baverel, Reference Decety, Jeannerod, Durozard and Baverel1993). Third, data from functional imaging studies support the claim that mental motor imagery and action rely to a considerable degree on the same neural apparatus (see Grafton, Arbib, Fadiga, & Rizzolatti, Reference Grafton, Arbib, Fadiga and Rizzolatti1996; Grezes & Decety, Reference Grezes and Decety2001 for a review).
Finally, performance on mental imagery tasks is influenced by the same factors that affect motor performance (Decety & Jeannerod, Reference Decety and Jeannerod1995; Fitts, Reference Fitts1954). Work by Parsons and colleagues is of particular relevance as it forms the basis for the present investigations. In an extended series of investigations (Parsons, Reference Parsons1987a; Reference Parsons1987b; Reference Parsons1994), normal subjects were asked to indicate whether a visually presented drawing depicted the right or left hand or foot. He found that reaction times (RTs) on this task were strongly correlated with the length of the trajectory through which the subject’s own hand, in its current position, would be rotated to match the depicted hand. In later investigations (1994), these investigators demonstrated a high correlation between RTs on the hand laterality judgment task and times required to actually move their hand to match the position of the pictured hand. Finally, Parsons repeatedly demonstrated that the trajectory through which subjects imagine rotating their hand or leg was constrained by biomechanical factors (Parsons, Reference Parsons1987a; Reference Parsons1987b; Reference Parsons1994).
If mental motor imagery is mediated by the same brain structures and procedures that underlie action, one would expect performance on mental motor imagery tasks to be influenced by the anticipated consequences of the action. Consistent with this expectation, previous studies of subjects with experimental (Hudson, McCormick, Zalucki, & Moseley, Reference Hudson, McCormick, Zalucki and Moseley2006) and chronic pain have demonstrated that motor imagery may be influenced by pain. We reported that subjects with Complex Regional Pain Syndrome (CRPS) involving one upper extremity were significantly impaired on a hand laterality task for the painful arm (Schwoebel, Friedman, Duda, & Coslett, Reference Schwoebel, Friedman, Duda and Coslett2001), and that this effect was eliminated by a treatment that reduced the pain (Schwoebel et al., Reference Schwoebel, Coronat and Coslett2002b; see also Moseley, Reference Moseley2004a; Moseley, Parsons, & Spence, Reference Moseley, Parsons and Spence2008).
We report data from an investigation exploring the utility of mental motor imagery as a measure of leg pain. In light of the considerations outlined earlier, we predicted that subjects with leg pain would be slower to respond to depictions of a painful leg. Thus, for subjects with unilateral leg pain, subjects would be expected to be slower than controls for the painful leg; subjects with bilateral leg pain, would be expected to be slower relative to controls for both legs. Importantly, as leg pain is typically exacerbated by movement, the slowing of response time would be expected to be most apparent for stimuli that require the greatest degree of mental rotation. In the context of the analyses described later, this would be manifested as a group by rotation interaction: subjects with leg pain would not only be slower than subjects without pain, but the slowing would be disproportionately manifested on those trials for which the mental rotation of the painful leg is greatest. Finally, as the predicted effects of pain are attributed to a slowing of mental rotation of a specific body part, the effects would not be expected for subjects with chronic pain in other parts of the body. Based on previous investigations (Fiorio, Tinazzi, & Aglioti, Reference Fiorio, Tinazzi and Aglioti2006; Moseley, Reference Moseley2004a; Reference Moseley2004b), we expected the effect on reaction time to be more substantial than on accuracy.
METHODS
Participants
Five groups of participant subjects were included. First, 40 subjects with pain involving one or both lower extremities of at least 3 months duration were recruited from the Pain Center at the University of Pennsylvania. Because subjects with bilateral leg pain are expected to differ from subjects with unilateral leg pain and subjects with unilateral right and left leg pain are expected to differ from each other, subjects with leg pain were divided into three groups: bilateral leg pain (BLP; n = 19; mean age 49.2 ± 8.8 [SD], 9 female), unilateral left leg pain (LLP; n = 11; mean age 53.2 ± 7.3, 7 female), and unilateral right leg pain (RLP; n = 10; mean age 49.5 ± 11.3, 6 female).
To control for the possible nonspecific effects of chronic pain, 42 subjects (22 female) with pain involving other parts of the body were also included. Pain control (PC) subjects ranged in age from 23 to 72, with a mean age of 48.1 (± 12.2). Pain control subjects were recruited from the Pain Center.
Subjects with pain were tested at the time of a regularly scheduled visit to the Pain Center while taking their usual medications. Two pain ratings were collected for each subject. First, subjects were asked to rate their current pain on a 0–10 scale using a visual analog scale. Second, they were asked to rate the severity of their pain “with movement” using the same scale. Mean rating of current pain was 6.1 ± 2.0 [SD] for the BLP subjects, 6.9 ± 2.5 for the LLP subjects, 6.3 ± 3.0 for the RLP subjects, and 5.9 ± 2.9 for the PC subjects; the four groups did not differ with respect to pain severity (all p values > .30). Mean ratings for pain with movement were 6.9 ± 3.4 for the BLP subjects, 7.45 ± 3.3 for the LLP subjects, 7.44 ± 3.2 for the RLP subjects, and 6.7 ± 3.1 for the PC subjects; the four groups did not differ with respect to pain with movement severity (all p values > .50).
Thirty-eight normal controls (16 female) without chronic pain were also included. Normal control (NC) subjects ranged in age from 22 to 81, with a mean age of 45.2 (± 18.5). The five groups did not differ with respect to age (all p values > .19). Subjects were paid for their participation. Consent was obtained according to the Declaration of Helsinki; the project was approved by the University of Pennsylvania Institutional Review Board (IRB).
Task
Subjects sat in a comfortable chair facing a computer screen. They were told that they would see a series of line drawings of a right or left foot in a number of different orientations and they were to indicate if the stimulus was a right or a left foot. Subjects maintained their hands in the palm down position with the index fingers of the left and right hands over the “z” and “m” keys, respectively; they depressed the “z” key in response to a left foot and the “m” key in response to a right foot. Subjects were instructed to respond quickly but accurately. Subjects were told not to move their feet during the testing. One normal control was excluded because she consistently moved her extremities. Although formal ratings of pain were not collected after the task, subjects were asked if their pain was altered by the testing; no subject reported noticing a change in pain during or after the test.
Stimuli included 12 depictions of each foot. Stimuli for the right and left feet were presented at the following angles of rotation: 0°, 30° internal, 60° internal, 90° internal, 120° internal, 150°internal, 180°, 150° external, 120° external, 90° external, 60° external, and 30° external (see Figure 1 for examples). In order to encourage subjects to adopt an egocentric perspective (that is, to view the stimulus from the same perspective that they typically saw their own foot), all drawings depicted the dorsum of the foot. Line drawings were presented in the center of the screen and were approximately 5° of visual angle.
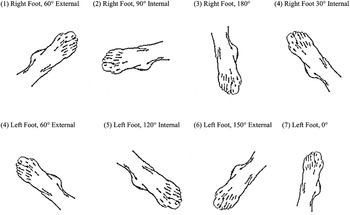
Fig. 1. Sample set of stimuli, 8 of the 24 presented images.
The task included eight repetitions of each of the 24 stimuli (12 right, 12 left) for a total of 192 trials. Stimuli were presented in a different random sequence on each administration of the task. Each trial began with the presentation of a fixation cross that persisted for one second before being replaced by a line drawing of the foot. The trial was terminated by depressing the “z” or “m” key. A new fixation cross was presented one second after the subject’s response. On average, the task lasted approximately 10–12 minutes.
RESULTS
Reaction Time (RT) and accuracy were recorded for each trial. For each subject, mean RT and accuracy were calculated for each of the 24 stimuli. For the RT analysis, trials on which the RT differed from the subject’s mean for that stimulus by more than 2.5 standard deviations were discarded. Trials for which the subject responded incorrectly were eliminated from the RT analysis. Finally, in order to exclude subjects who performed poorly because of factors such as a failure to engage in the task, an inability to understand the task, an inability to maintain set, or poor right/left discrimination, each subject’s performance on those trials that require minimal rotation of the foot (30° internal, 0°, 30° external, for each foot) was determined. Four PC subjects and one BLP subject performed at chance by this measure and were excluded from the analysis.
Control Performance
First, we analyzed the performance of the control group to confirm that performance was similar to that reported in previous investigations (Parsons, Reference Parsons1987a; Reference Parsons1987b; Fiorio et al., Reference Fiorio, Tinazzi, Ionta, Fiaschi, Moretto and Edwards2007). Within-subject analyses of variance (ANOVAs) in which rotation (12 conditions) and foot (R, L) served as factors were performed for the RT and accuracy data.
The RT analysis demonstrated significant main effects of rotation, F(11, 396) = 50.29, p < .001, and foot, F(1, 36) = 11.82, p < .001; subjects were significantly faster responding to right feet (1180.2 ± 77.5 vs. 1284.4 ± 90.4 ms [mean ± SE]). Similar findings were observed in the accuracy data. There was a main effect of rotation, F(11, 396) = 14.60, p < .001, and a trend for a main effect of foot, F(1, 36) = 3.69, p = .063. Subjects tended to be more accurate with the left foot (97.2% ± .6% vs. 95.7% ± .9%).
As in multiple previous reports, normal controls exhibited significant effects of rotation on both RT and accuracy. Second, like normal controls reported by Fiorio et al. (Reference Fiorio, Tinazzi, Ionta, Fiaschi, Moretto and Edwards2007), controls responded significantly more quickly to right as compared to left feet and tended to be more accurate for left as compared to right feet. These data strongly support the claim made by Parsons (Reference Parsons1987a; Reference Parsons1994) and reiterated by numerous additional investigators (Shenton, Schwoebel, & Coslett, Reference Shenton, Schwoebel and Coslett2004) that determining the laterality of a pictured body part entails the mental rotation of the subject’s own body part to match the position of the stimulus.
Overall Effects of Pain
To assess the effect of pain on performance, means for each of the five groups for each of the 24 conditions were calculated for RT and accuracy (see Figures 2 and 3). For the purposes of the statistical analyses reported later, the 12 rotation conditions were reduced to four categories by collapsing across stimuli that yielded similar performance in our subjects, as well as in previous reports from our lab (Shenton et al., Reference Shenton, Schwoebel and Coslett2004) and other investigators (Parsons et al., Reference Parsons1987a; Reference Parsons1987b). To this end, the 30° external, 0°, and 30° internal conditions were collapsed to generate the Minimal Rotation category and the 150° external, 180°, and 150° internal conditions were collapsed to generate the Maximal Rotation category. The 60° external, 90° external, and 120° external conditions were collapsed to generate the Moderate External Rotation category, whereas the 60° internal, 90° internal, and 120° internal conditions were collapsed to generate the Moderate Internal Rotation category.

Fig. 2. Mean reaction time for each rotation condition separated by (a) left foot and (b) right foot. “I” and “E” represent internal and external rotation of the foot, respectively.
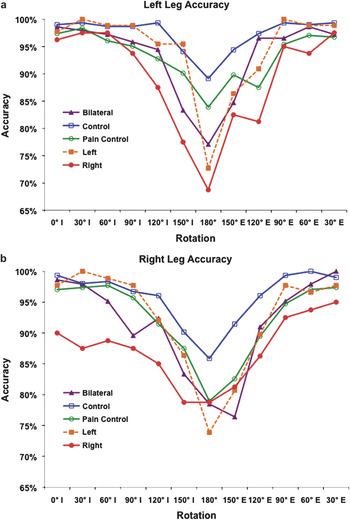
Fig. 3. Mean accuracy for each rotation condition separated by (a) left foot and (b) right foot. “I” and “E” represent internal and external rotation of the foot, respectively.
Mixed-design ANOVAs with foot (right, left) and rotation category (Minimal, Moderate Internal, Moderate External, and Maximal) as within-subject factors and group (RLP, LLP, BLP, PC, NC) as a between-subjects factor were performed for the RT and accuracy data. To address specific predictions regarding the performance of subjects with leg pain, planned comparisons were subsequently performed for both the RT and accuracy data in which each pain group was individually compared to the NC and PC groups. Analyses were performed with SPSS 16.
Analysis of the RT data from the omnibus ANOVA demonstrated a number of significant effects. First, there was a significant effect of foot, F(1, 110) = 9.54, p = .003; RTs for the right foot were significantly faster than for the left foot (1754.5 ± 73.7 vs. 1682.3 ± 68.0). There was also a robust effect of rotation, F(3, 330) = 167.5, p < .001; post-hoc tests demonstrated that the Minimal Rotation category (1282.5 ± 53.7 ms) was significantly shorter than all other categories (all p values < .001); furthermore, both the Moderate Internal (1591.4 ± 64.5) and Moderate External Rotation (1607.2 ± 66.2 ms) were shorter than the Maximal Rotation category (2392.4 ± 110.3 ms; all p values < .001), but did not differ from each other (p = .929). Finally, there was a main effect of group, F(1, 110) = 6.19, p < .001. Tukey’s post-hoc tests demonstrated that NC subjects (1237.9 ± 105.1 ms) were significantly faster than subjects with RLP (2271.6 ± 202.2 ms; p < .001) and subjects with LLP (1846.9 ±192.8 ms; p = .050), with a trend towards significance when compared to bilateral pain subjects (1690.2 ± 150.7, p = .107). There was also a significant difference comparing RLP subjects to pain control subjects (1545.3 ± 102.4, p = .015); all other comparisons were not significant.
There was also a significant rotation by group interaction, F(12, 330) = 3.54, p = .004, indicating that the groups differed in the extent to which they were slowed by rotation. All three groups of subjects with leg pain were slowed to a greater degree in the Maximal Rotation category than either control group. Finally, there was a significant three-way group by foot by difficulty interaction, F(12, 330) = 1.96, p = .032; this was explored further in the planned comparisons.
Effects of Pain on Rotation
As we predicted, the group by rotation interaction – that is, we hypothesized that leg pain would be associated with an impairment that was particularly prominent for stimuli requiring a larger degree of mental rotation – we further examined the group by rotation interactions with a series of planned comparisons. First, we compared the performance of normal controls to subjects with bilateral leg pain, unilateral left leg pain, and unilateral right leg pain. There was a rotation by group interaction for the comparison of the bilateral leg pain versus controls (p = .008) and for the unilateral right leg pain versus controls (p = .005); there was a trend for a rotation by group interaction for the comparison of unilateral left leg pain and controls (p = .084). In all instances, there was a greater RT cost for stimuli with increased rotation requirements for subjects with leg pain as compared to normal subjects. To determine if the group by rotation effects were simply a nonspecific effect of pain, each of the three groups of subjects with leg pain was contrasted with the pain control group. Once again, there were significant group by rotation interactions in the contrasts for the group with bilateral leg pain (p = .012) and unilateral right leg pain (p = .006). The rotation by group interaction was not significant for the comparison between the PC and LLP groups (p = .122). In all three instances, subjects with leg pain exhibited a greater effect of rotation than pain control subjects, suggesting that the deficit was not simply related to discomfort, medication, or other nonspecific effects. Finally, we compared normal controls to pain controls, to see if the group by rotation interaction can be simply attributable to having pain; we found no significant group by rotation interaction (p = .701).
As expected on the basis of previous reports (Fiorio et al., Reference Fiorio, Tinazzi and Aglioti2006; Moseley, Reference Moseley2004a; Reference Moseley2004b; Schwoebel et al., Reference Schwoebel, Friedman, Duda and Coslett2001; Reference Schwoebel, Coronat and Coslett2002b), effects were less substantial in the accuracy analysis. First, an omnibus ANOVA that included the five groups of subjects described earlier revealed significant main effects of rotation, F(3, 110) = 55.86, p < .001, and foot, F(1, 110) = 10.47. Although all groups of subjects with leg pain were less accurate than normal controls (RLP 88.07% ± 22.33, LLP 93.47% ± 16.74, BLP 92.25% ± 16.57, NC 96.59% ± 9.77), the group effect was only marginally significant. There was no group by rotation effect, F(12, 330) = 1.35, p = .238.
Correlations of Pain Ratings and Rotation Cost
Finally, a series of correlations for the 40 subjects with leg pain were calculated, with the variables being the subject’s current pain score, pain with movement score, and rotation cost, calculated as the difference between RTs in the minimal (330°, 0°, 30°) and maximal (150°, 180°, 210°) foot rotation conditions. There was a significant correlation between pain with movement scores and rotation cost (r = .362, p = .014); there was, however, no correlation between current pain score and rotation cost (r = –.032, p = .849).
As current pain and pain with movement scores (r = .333, p = .022) were significantly correlated, an additional analysis of the effect of pain with movement on rotation cost was assessed while controlling for current pain. Furthermore, as rotation cost could be correlated with baseline reaction time (e.g., RTs in the 0° rotation condition), we performed partial correlations to determine if there was a significant relationship between rotation cost and pain with movement score, while controlling for both current pain score and RT in the 0° rotation condition. We found a highly significant correlation between pain with movement and rotation cost, when controlling for current pain and RT in the 0° rotation condition (r = .438, p = .004). In contrast, the same analysis with pain controls found no significant relationship between rotation cost and pain with movement score (r = –.173, p = .313).
DISCUSSION
The data supported many of our hypotheses. First, we demonstrated that the foot lateralization judgment task employed here generates results similar to those reported by Parsons (Reference Parsons1987a) and subsequent investigators (Fiorio et al., Reference Fiorio, Tinazzi and Aglioti2006; Reference Fiorio, Tinazzi, Ionta, Fiaschi, Moretto and Edwards2007; Shenton et al., Reference Shenton, Schwoebel and Coslett2004) in normal subjects; NCs are significantly slower and less accurate when asked to identify feet that are maximally rotated relative to the minimal rotation conditions. As NCs were both faster and less accurate in responding to right feet, the foot effect observed for these subjects could be attributable, at least in part, to a speed-accuracy trade-off. Note, however, that for both feet in NC (and all other groups), subjects were slowest and least accurate with stimuli that were most discrepant from subjects’ feet at the time of testing; thus, the robust and systematic effects of rotation observed in both accuracy and RT cannot be attributed to a speed-accuracy trade-off.
Subsequent analyses in which subjects with bilateral, unilateral right, and unilateral left leg pain were compared to normal control subjects and pain control subjects largely confirmed the predictions outlined in the introduction. Subjects with leg pain on the right, on the left, or in both legs were slower and less accurate than NCs. Furthermore, as would be predicted if the effects of leg pain were exacerbated by a greater degree of mental rotation, there was a group by rotation interaction in the RT analysis for all three leg pain groups relative to NCs: Subjects with leg pain were slowed to a greater degree than controls in the Maximum Rotation category. Similar but less robust effects were observed in the accuracy analysis.
Although PC subjects were slower and less accurate than NCs, there was no rotation by group interaction when they were compared to NCs; thus, PCs were not disproportionately affected by stimuli that required greater degrees of leg rotation. Finally, we found rotation by group interactions for the comparisons between RLP and BLP groups and the PC group in the RT data. These data demonstrate that the impaired performance of subjects with leg pain is not simply a reflection of the nonspecific effects of chronic pain.
Finally, the fact that subjects with leg pain – but not the PC subjects – exhibited a highly significant correlation between pain with movement and RT difference between the baseline and maximal rotation conditions, even when baseline RT and baseline pain severity are controlled, suggests that the foot laterality task indexes pain that is associated with movement.
The demonstration that subjects with lower extremity pain are both slower and less accurate on this task than normal controls and, by at least some measures, subjects with chronic, non-leg pain, suggests that the foot laterality task may be a useful adjunct to the assessment of subjects with leg pain. Similar tasks to those described here have been profitably used to address putatively distinct representations of the human body brought on by other neurologic conditions such as stroke (Schwoebel & Coslett, Reference Schwoebel and Coslett2005), writer’s cramp (Fiorio et al., Reference Fiorio, Tinazzi and Aglioti2006), and cervical dystonia (Fiorio et al., Reference Fiorio, Tinazzi, Ionta, Fiaschi, Moretto and Edwards2007). The task possesses a number of attributes that may enhance its clinical utility. First, the task is brief and requires neither specialized equipment nor complex instructions. We have profitably employed mental motor imagery tasks in patients with substantial cognitive impairment (Coslett, Reference Coslett1998; Coslett, Saffran, & Schwoebel, Reference Coslett, Saffran and Schwoebel2002; Schwoebel et al., Reference Schwoebel, Coronat and Coslett2002a).
Second, the foot laterality task provides an assessment of pain without explicitly demanding a pain rating. The implicit nature of the task may have important implications for several reasons. First, subjective ratings of pain may be complicated by subject expectancy effects or other psychological factors; although formal data are lacking, one might speculate that the use of an indirect measure may minimize the role of attention, emotion, beliefs, and other cognitive factors in the evaluation of pain. In this context, it is noteworthy that no subject explicitly indicated that they believed the task to be a measure of pain severity; informal debriefing of subjects indicated that most were unaware of purpose of the task.
Although data from the task reported here are lacking, there is some evidence that mental motor imagery tasks such as the foot laterality task may be useful in the assessment of malingering or factitious pain disorders. Maruff and Velakoulis (Reference Maruff and Velakoulis2000) reported an investigation in which normal subjects were asked to feign weakness of one arm while imagining reaching to targets of varying sizes; normal subjects exhibited an inverse relationship between the size of a target to which they reach and the time required to touch the target, a speed-accuracy trade-off formalized in Fitts’ Law (Fitts, Reference Fitts1954). The authors found that while feigning weakness of one arm, the time required to perform imagined movements of the “weak” arm increased significantly. Critically, the speed-accuracy trade-off that characterizes normal performance on their motor imagery task was not observed in the behavior of the “weak” arm, a finding consistent with the claim that the speed-accuracy trade-off expressed in Fitts’ Law operates at an unconscious level. Furthermore, a subject diagnosed with “conversion disorder” did not exhibit the normal speed-accuracy trade-off on the imagined reaching task, whereas a subject with an arm injury did exhibit the anticipated effect. These data raise the possibility that the absence of the highly reliable and robust rotation effect on the foot laterality task could serve as a marker of poor effort or nonphysiologic performance.
Future evaluation of the mental motor imagery test will also need to include an assessment of known confounders of chronic pain such as depression, anxiety, and catastrophizing. Some predictions might be made based on the features of these mental states. For instance, depressed individuals with chronic pain may be excessively slow in their imagined movements relative to nondepressed chronic pain patients. Catastrophizers may exhibit a greater rotation cost in accuracy or RT (i.e., greater decrement in performance with maximal rotation stimuli) relative to noncatastrophizers who report a similar degree of pain.
Although the assessment was limited to asking subjects whether they regarded the task to be uncomfortable or painful, it is noteworthy that subjects in this and previous investigations did not report pain. Similarly, in our previous investigations (Schwoebel et al., Reference Schwoebel, Friedman, Duda and Coslett2001; Schwoebel et al., Reference Schwoebel, Coronat and Coslett2002b) involving laterality judgments for pictures of hands in subjects with CRPS involving the upper extremity, subjects did not report pain during or after the task. This observation contrasts with findings from a number of investigators demonstrating that motor imagery may either decrease (MacLachlan, McDonald, & Waloch, Reference MacLachlan, McDonald and Waloch2004; Moseley, Reference Moseley2004b; Ramachandran & Rogers-Ramachandran, Reference Ramachandran and Rogers-Ramachandran1996) or increase (Gustin et al., Reference Gustin, Wrigley, Ganevia, Middleton, Henderson and Siddal2008) pain. Perhaps of greatest relevance to the current study is the demonstration by Moseley et al. (Reference Moseley, Parsons and Spence2008) that mental motor imagery induced pain and swelling in subjects with CRPS involving the arm. One potential explanation for the discrepancy between Moseley et al.’s (Reference Moseley, Parsons and Spence2008) results and the data reported here appeals to the distinction between implicit and explicit movement. Moseley et al. (Reference Moseley, Parsons and Spence2008) presented images of a right or left hand and subjects were asked to imagine themselves producing the movement that would be required to make their hand match the position of the picture hand; indeed, subjects were instructed to imagine executing the movement twice before responding. Instructions emphasized that they were not to imagine watching themselves making the movement, but to imagine actually performing the movement. In contrast, in our study, subjects were not instructed to explicitly imagine moving their hands, but simply to indicate whether the depicted stimulus was a right or left hand. As we (Schwoebel & Coslett, Reference Schwoebel and Coslett2005) reported, substantial differences in performance on tasks involving explicit as compared to implicit movements in a study of 70 subjects with unilateral stroke, one possible reason for the discrepancy between our findings and those of Moseley et al. (Reference Moseley, Parsons and Spence2008) is that the implicit motor task engages a neural representation that is, in part, distinct from that underlying explicit motor imagery.
We (Schwoebel et al., Reference Schwoebel, Coronat and Coslett2002 a&b; Schwoebel & Coslett, Reference Schwoebel and Coslett2005) and others (Djikerman & de Haan, Reference Djikerman and de Haan2007; Sirigu, Grafman, Bressler, & Sunderland, Reference Sirigu, Grafman, Bressler and Sunderland1991) have argued for distinctions between representations of the body that differ with respect to the extent to which they are accessible to consciousness. The real-time, on-line depiction of the body that articulates with the motor system for action – a representation that corresponds to Head and Holmes’ classical view of the “body schema” – is typically unconscious. Alternatively, one’s set of beliefs, attitudes, and emotional response to one’s body – sometimes referred to as the “body image” (Schwoebel & Coslett, Reference Schwoebel and Coslett2005; Sirigu et al., Reference Sirigu, Grafman, Bressler and Sunderland1991) or “self-image” – is typically conscious and is intimately related to one’s sense of agency (Gallagher, Reference Gallagher, Bermudez, Marcel and Eilan1995). The fact that subjects with leg pain perform abnormally on a mental rotation task that doesn’t require an explicit judgment about pain suggests that the experience of chronic pain alters the often unconscious representation of the body that underlies movement planning. Furthermore, the fact that subjects do not report pain in this task, but do report pain and exhibit signs of inflammation when asked to explicitly simulate movements of a painful body part, suggests that the experience of pain may be intimately linked to the sense of agency that underlies action.
Previous work with a task in which subjects are asked to judge the laterality of a visually presented hand has raised the possibility that performance may be influenced by factors such as inattention to the painful hand (e.g., Moseley & Wiech, Reference Moseley and Wiech2009). Similarly, one might postulate that pain on one side serves to induce a response bias toward that side. We believe these to be unlikely explanations for our findings. First, it is not clear that inattention or a response bias would predict the major finding of this study: Subjects with pain in their legs were not only slower and less accurate, but, crucially, exhibited a greater decrement in performance as a function of degree of rotation than normal and pain control subjects. Second, the effects were evident in subjects with bilateral leg pain for whom such possible accounts are not applicable. We suggest that the systematic effect of stimulus rotation, as well as the fact that there is a significant correlation between slowing of mental rotation and ratings of pain with action, but not with ratings of current pain, support the claim that the leg lateralization task is mediated by the same representation of the body that underlies action. More specifically, we contend that imagined movements are mediated by a “forward model” that not only specifies the timing and force of muscle contractions, but also anticipates the sensory consequences of that action (Desmurget & Grafton, Reference Desmurget and Grafton2000). On this account, subjects are slower to respond to stimuli that would require large amplitude rotations, because those movements are likely to be associated with greater pain (Moseley, Reference Moseley2004a). Additionally, the fact that subjects exhibit a significant correlation between slowing for large amplitude movements and ratings of pain with movement, but not nonspecific pain, is consistent with the hypothesis that the anticipation of movement related pain underlies the effects reported here.
ACKNOWLEDGMENT
This work was supported by the National Institutes of Health (RO1 NS046049 to HBC).
Appendix 1: Reaction Time and Accuracy Data by Group







