INTRODUCTION
The older population in Australia continues to grow at a rapid rate. In 2016, 3.7 million people were aged ≥65 years, representing 15% of the population (Australian Institute of Health and Welfare, 2017). By 2056, the number of older Australians is projected to reach 8.7 million, representing approximately 22% of the population (Australian Bureau of Statistics, 2013). Importantly, as the older population increases, so too will the proportion of people living with cognitive decline.
Very mild cognitive changes (e.g., mildly slowed information processing or occasional forgetfulness) are known to be part of the normal ageing process; however, more significant cognitive decline is often caused by neurodegenerative diseases such as Alzheimer’s disease (AD), the most common type of dementia (accounting for up to 53.7% of dementia cases) (Lobo et al., Reference Lobo, Launer, Fratiglioni, Andersen, Di Carlo, Breteler, Copeland, Dartigues, Jagger, Martinez-Lage, Soininen and Hofman2000). Cognitive decline may also be caused by other factors (e.g., cardiovascular disease or depression), and even where this is nonprogressive or more gradually progressive, it may still have a negative impact on psychological well-being and day-to-day functioning (Deary et al., Reference Deary, Corley, Gow, Harris, Houlihan, Marioni, Penke, Rafnsson and Starr2009; Samuels, Reference Samuels2014).
Importantly, some older people may experience cognitive decline that is more than expected as part of the normal ageing process, but is not severe enough to warrant a diagnosis of dementia. This ‘intermediate’ state is known as mild cognitive impairment (MCI). MCI is diagnosed when an individual subjectively experiences cognitive changes and also demonstrates impairment on objective neuropsychological testing; however, this does not interfere with daily functioning or has only minimal impact (Winblad et al., Reference Winblad, Palmer, Kivipelto, Jelic, Fratiglioni, Wahlund, Nordberg, Bäckman, Albert, Almkvist, Arai, Basun, Blennow, De Leon, DeCarli, Erkinjuntti, Giacobini, Graff, Hardy, Jack, Jorm, Ritchie, Van Duijn, Visser and Petersen2004). Nonetheless, and perhaps critically, MCI is viewed as a risk factor and possibly a prodromal marker of progressing to dementia, as 10–15% of older adults with MCI will convert to dementia per year (Gauthier et al., Reference Gauthier, Reisberg, Zaudig, Petersen, Ritchie, Broich, Belleville, Brodaty, Bennett, Chertkow, Cummings, de Leon, Feldman, Ganguli, Hampel, Scheltens, Tierney, Whitehouse and Winblad2006) – a much higher rate than that seen in healthy older adults. However, 40–50% of people with MCI may remain stable over time or may even return to normal (i.e., age-appropriate) cognitive functioning (Mitchell & Shiri-Feshki, Reference Mitchell and Shiri-Feshki2009). Given this variability in outcome, efforts to delineate the underlying pathology and likely prognosis of MCI have suggested the utility of classifying it further into subtypes, including amnestic or nonamnestic, and single domain or multiple domain (Petersen, Reference Petersen2004). Older people who present with predominant impairment in episodic memory are labelled amnestic MCI (aMCI), and this subtype is considered to be at higher risk of progressing to AD, while those with impairment in other cognitive domains (e.g., language, executive function, processing speed, and visuospatial skills) are labelled nonamnestic MCI (naMCI). This subtype is considered more likely to progress to a non-AD dementia such as vascular dementia or Lewy body dementia (Busse, Hensel, Guhne, Angermeyer, & Riedel-Heller, Reference Busse, Hensel, Guhne, Angermeyer and Riedel-Heller2006; Petersen, Reference Petersen2004; Petersen et al., Reference Petersen, Doody, Kurz, Mohs, Morris, Rabins, Ritchie, Rossor, Thal and Winblad2001). Thus, irrespective of the suspected aetiology, older people with MCI represent an important target group for strategies and interventions to compensate or improve their cognitive difficulties, in order to maintain their capacity for independent functioning.
Mitigating Cognitive Change: The Use of Compensation
Older adults have been shown to compensate for memory difficulties using memory strategies; moreover, they tend to use such strategies more frequently than young adults, which is thought to be due to their increased awareness of cognitive decline (de Frias, Dixon, & Backman, Reference de Frias, Dixon and Backman2003).
In general, memory strategies are categorized as internal aids (e.g., chunking or mental imagery), external aids (e.g., using shopping lists or calendars), and reliance aids (e.g., asking for reminders from others). Across the literature, self-report instruments have typically been used to ascertain the frequency and type of memory strategies used by older adults. One example is the Memory Compensation Questionnaire (MCQ) (de Frias & Dixon, Reference de Frias and Dixon2005; Dixon, de Frias, & Backman, Reference Dixon, de Frias and Backman2001). Previous research indicates that strategy use in general, as well as preference for a particular type of strategy, may relate to factors such as cognitive functioning and insight. While strategy use appears to be higher in those with MCI compared to healthy older adults (Schmitter-Edgecombe, Parsey, & Lamb, Reference Schmitter-Edgecombe, Parsey and Lamb2014), a recent study showed that older individuals with normal cognitive function as well as those with MCI tend to use memory strategies more frequently than individuals with AD (Tomaszewski et al., Reference Tomaszewski, Schmitter-Edgecombe, Weakley, Harvey, Denny, Barba, Gravano, Giovannetti and Willis2018). Additionally, memory strategy use may rely more heavily on particular cognitive domains. For instance, more frequent use of compensation strategies has been associated with both stronger episodic memory and better executive functioning (Tomaszewski et al., Reference Tomaszewski, Schmitter-Edgecombe, Weakley, Harvey, Denny, Barba, Gravano, Giovannetti and Willis2018). Furthermore, executive function has been considered more important in the use of internal aids compared to external aids (Bouazzaoui et al., Reference Bouazzaoui, Isingrini, Fay, Angel, Vanneste, Clarys and Taconnat2010). Participants with greater executive function tend to use internal aids most frequently, perhaps because internal aids require more cognitive effort (e.g., relating to strategic encoding and retrieval processes).
Rationale for the Current Study
Due to the current lack of effective pharmacological treatment for cognitive decline, interventions focusing on compensatory strategies offer a promising approach to mitigate the effects of cognitive decline and thereby promote independent and effective functioning. In this regard, the use of compensatory strategies as part of therapeutic cognitive interventions has been widely investigated in older people with MCI and shows great promise as a preventive technique in this group (Mowszowski, Batchelor, & Naismith, Reference Mowszowski, Batchelor and Naismith2010; Reijnders, van Heugten, & van Boxtel, Reference Reijnders, van Heugten and van Boxtel2013). For example, Kinsella and colleagues (Reference Kinsella, Ames, Storey, Ong, Pike, Saling, Clare, Mullaly and Rand2016) studied the effect of memory compensation strategy training in healthy older adults and those with aMCI, and found that compensation strategy training was associated with improved independence and social engagement. In another randomized controlled trial, where 81% of participants were diagnosed with MCI, participants who received a 7-week combined cognitive training and psychoeducation intervention incorporating instruction and practice in the use of compensatory strategies showed improvement in memory, self-reported depressive symptoms, and subjective sleep quality compared to controls (Diamond et al., Reference Diamond, Mowszowski, Cockayne, Norrie, Paradise, Hermens, Lewis, Hickie and Naismith2015).
While such interventions are therefore worthwhile, to date, there is insufficient understanding of how strategies are routinely used by people with MCI. To our knowledge, no studies have yet investigated whether there are differences in memory strategy use between those with aMCI and naMCI. Similarly, few studies have investigated the relationship between strategy type and cognitive function among people with MCI, and to our knowledge, no studies have explored broader concepts relating to strategy use in MCI, such as personal investment in using strategies, motivation to do so, or awareness of the need for compensatory strategies. This information would be invaluable to clinicians, in order to make more specific or tailored recommendations, so that therapeutic interventions can be more targeted, meaningful, and effective for individuals. Therefore, the aims of this study are to: (1) comprehensively investigate memory strategy use in people with MCI, including a comparison of strategy use between those with aMCI and naMCI; (2) explore the relationship between cognitive functioning and the types of memory strategies, personal investment in memory strategies, and awareness and motivation to use cognitive strategies in MCI; and (3) explore whether memory strategy use is related to other markers of functioning in people with MCI, such as aspects of health, day-to-day functioning, and psychological well-being.
METHODS
Participants
Participants included in this cross-sectional study were part of a research cohort from the Healthy Brain Ageing (HBA) Clinic at the Brain and Mind Centre, the University of Sydney. The HBA Clinic is a specialized research clinic for older adults ≥50 years old, with reported new onset cognitive difficulties or mood disturbance within the last 5 years. Exclusion criteria for this clinic are (1) Mini-Mental State Examination (MMSE) score <20 (Folstein, Folstein, & McHugh, Reference Folstein, Folstein and McHugh1975), (2) history of schizophrenia or other nonaffective psychiatric disorder, (3) intellectual disability or insufficient English for standardized neuropsychological assessment, (4) history of stroke, head injury with loss of consciousness >30 min, or other neurological disease (e.g., epilepsy), and (5) substance dependence. Participants attending this clinic undergo comprehensive neuropsychological, neurological, and psychological assessments to inform clinical diagnoses and management.
For the current study, we selected a subsample of participants who, following this comprehensive assessment process, were diagnosed with MCI on clinician consensus (neurologist/geriatrician and neuropsychologists) according to established criteria (Winblad et al., Reference Winblad, Palmer, Kivipelto, Jelic, Fratiglioni, Wahlund, Nordberg, Bäckman, Albert, Almkvist, Arai, Basun, Blennow, De Leon, DeCarli, Erkinjuntti, Giacobini, Graff, Hardy, Jack, Jorm, Ritchie, Van Duijn, Visser and Petersen2004) and who had completed the MCQ. We further excluded participants with MMSE score ≤24. A written consent was obtained from all participants, and this research was approved by the Human Research Ethics Committee of the University of Sydney.
Assessments
Use of memory strategies
The MCQ is a widely used 45-item, self-report questionnaire which assesses the variety and extent of memory strategy use on a daily basis (de Frias & Dixon, Reference de Frias and Dixon2005). Participants respond using a five-point Likert scale ranging from ‘never’ (rated 1) to ‘always’ (rated 5). Higher scores therefore represent more frequent strategy use. The 45 MCQ items are classified into seven domains. Three domains relate to strategy type: ‘internal’ (e.g., chunking or mental imagery; 10 items), ‘external’ (e.g., use of shopping lists; 8 items), and ‘reliance’ (e.g., asking for reminders from other people; 5 items). Two domains relate to personal investment in strategy use: ‘time’ (e.g., intentionally devoting more time to tasks to facilitate memory; 5 items) and ‘effort’ (e.g., putting in more effort to remember an important conversation; 6 items). The final two domains relate to awareness and motivation regarding the use of memory strategies: ‘change’ (e.g., changes in the need for memory compensation compared to 5–10 years before; 6 items) and ‘success’ (e.g., commitment to remembering an important conversation perfectly; 5 items). The psychometric properties of the MCQ (e.g., test–retest reliability, internal consistency, and convergent and discriminant validity) have been confirmed in several previous studies (de Frias & Dixon, Reference de Frias and Dixon2005; Dixon & Hultsch, Reference Dixon and Hultsch1983; Dixon et al., Reference Dixon, de Frias and Backman2001).
Cognitive functioning
Standardized neuropsychological tests and semistructured interviews were conducted by clinical neuropsychologists to assess cognitive functioning. For descriptive purposes, the MMSE was used to measure global cognition (Folstein et al., Reference Folstein, Folstein and McHugh1975), and the Wechsler Test of Adult Reading was used to estimate premorbid intellectual ability (Wechsler, 2001). Learning and memory were assessed using the Rey Auditory Verbal Learning Test (Lezak, Reference Lezak1995) and the Logical Memory subtest from the Wechsler Memory Scale – 3rd edition (Wechsler, 1997b), respectively. The Trail Making Test Part A was used to measure processing speed, and Trail Making Test Part B was used to measure cognitive flexibility (Reitan, Reference Reitan1979). Working memory was assessed using the Digit Span subtest from the Wechsler Adult Intelligence Scale – 3rd edition (Wechsler, 1997a). Finally, the DKEFS Colour-Word Interference Test was used to measure aspects of executive functioning (specifically, response inhibition and set shifting) (Delis, Kaplan, & Kramer, Reference Delis, Kaplan and Kramer2001). Age- and (where possible) education-adjusted standardized scores (e.g., Z scores or age-scaled scores) were calculated for all tests.
Additionally, a close informant of each participant completed questionnaires relating to changes in participants’ cognition and behaviour. These informants identified themselves as either partner/spouse of the participants (50.7%), other relatives (e.g., a child; 22.3%), or close friend (9.5%). Measurements included the Cambridge Behavioural Inventory-Revised, a 45-item questionnaire relating to cognitive and behavioural changes, whereby higher scores indicate higher frequency of symptoms (Wear et al., Reference Wear, Wedderburn, Mioshi, Williams-Gray, Mason, Barker and Hodges2008), and the Revised Memory and Behaviour Checklist, a 24-item questionnaire assessing observable cognitive and behavioural problems as well as their impact on the informant, whereby higher scores indicate increased frequency and impact, respectively (Teri et al., Reference Teri, Truax, Logsdon, Uomoto, Zarit and Vitaliano1992).
Medical burden
Participants were assessed by a neurologist or geriatrician to obtain a detailed medical history. Current medical burden was recorded using the Cumulative Illness Rating Scale – Geriatric version (Miller & Towers, Reference Miller and Towers1991), which provides a comprehensive overview of medical conditions across different body systems (e.g., vascular, respiratory, etc.). Total score and total endorsed were calculated, with higher scores representing greater medical burden.
Psychosocial functioning
Participants completed a series of questionnaires relating to various aspects of psychosocial functioning. General health-related disability was assessed using the World Health Organisation Disability Assessment Schedule, with a 36-item version applied to participants who are working and a 32-item version used for those not in work. A standardised total score (0–100) was calculated to reflect overall disability, with higher scores representing higher subjective disability or loss of function (World Health Organization, 2000). Quality of life (QoL) was measured with the abbreviated World Health Organization Quality of Life scale (BREF, 26 items). A standardised total score was calculated to reflect overall QoL, with higher scores representing better quality (World Health Organization, 1996). Subjective memory functioning (irrespective of memory strategy use) was assessed using the Everyday Memory Questionnaire (EMQ). A total score was calculated, with higher scores representing greater memory problems (Royle & Lincoln, Reference Royle and Lincoln2008). Finally, subjective sleep quality was measured using the Pittsburgh Sleep Quality Index. A global score was calculated, with higher scores representing poorer sleep quality (Buysse, Reynolds, Monk, Berman, & Kupfer, Reference Buysse, Reynolds, Monk, Berman and Kupfer1989).
Mood assessment
Both subjective and clinician-rated measures were used to assess current mood. Participants completed the 14-item self-report Hospital Anxiety and Depression Scale (HADS) (Zigmond & Snaith, Reference Zigmond and Snaith1983) where scores ≥8 indicate clinically meaningful symptoms. Via interview, clinicians completed the 17-item Hamilton Depression Rating Scale (HAM-D) (Hamilton, Reference Hamilton1960), where scores ≥8 are suggestive of depression.
Data Analyses
All statistical analyses were conducted using the Statistical Package for the Social Sciences for Windows (IBM Corporation). First, all data were visually inspected using histograms and boxplots to detect outliers. Those outliers thought to be skewing the sample were curtailed to the next highest value plus one. Subsequently, differences in demographic and clinical variables as well as memory strategy use between the aMCI and naMCI groups were investigated using independent t tests for normally distributed variables, Mann–Whitney U tests for non-normally distributed variables, or the chi-squared test for categorical variables. The relationship between memory strategy use and neuropsychological, medical, psychosocial, and mood variables was then investigated using Pearson correlations for normally distributed data or nonparametric Spearman correlations for non-normally distributed data. All the correlation analyses were two-tailed with a manually adjusted p-value in order to account for family-wise multiple comparisons (see Results section for detailed breakdown of adjusted p-values for each family of outcomes). The variables that were significantly correlated with memory strategy use were then used as predictors in linear regression analyses, where p was set at .05.
RESULTS
Sample Characteristics
As shown in Table 1, 148 participants with MCI were included in the current study. This included 49 participants with aMCI and 99 participants with naMCI. On average, participants were 67.9 years old (range: 50–85 years, SD = 8.9) and 57.4% (i.e., 85/148) of the participants were female. Our sample was fairly highly educated, with an average of 13.8 years of education (SD = 3.3), and within the higher end of the average range in terms of estimated premorbid cognitive functioning (mean = 107.1, SD = 9.5). As expected, the average MMSE score (mean = 28.7, SD = 1.4) was within normal limits. In terms of mood, clinician ratings indicated normal levels of depressive symptoms (HAM-D mean = 5.14, SD = 4.6) and participants’ self-report scores also indicated normal levels of depressive symptoms (HADS depression mean = 4.91, SD = 3.72) and anxiety symptoms (HADS anxiety mean = 6.22, SD = 4.06).
Table 1. Mean and standard deviations for demographic and clinical data

Note: MMSE = Mini-Mental State Examination; WTAR-FSIQ = Wechsler Test of Adult Reading- Full scale IQ; HAM-D = Hamilton Depression Rating Scale; HADS = Hospital Anxiety and Depression Scale; EMQ = Everyday Memory Questionnaire.
a MMSE data were missing for one participant in the naMCI group.
b WTAR FSIQ data were missing for three participants.
c HAM-D data were missing for one participant.
d HADS data were missing for six participants.
e EMQ data were missing for one participant.
Between Group Differences: aMCI versus naMCI
As shown in Table 1, there were no differences between the aMCI and naMCI groups in age, education, premorbid IQ, depression symptoms, and anxiety symptoms; however, the groups differed in global cognition, sex ratio, as well as subjectively reported everyday memory problems. In terms of memory strategy use, Table 2 illustrates that the two groups did not significantly differ in their use of memory strategies, in relation to strategy type, personal investment in strategy use, or awareness and motivation in relation to using memory strategies (p > .007 for all comparisons). Therefore, all further analyses investigating relationships between memory strategy use and cognitive, medical, psychosocial, and mood data were analysed for the MCI group as a whole (n = 148).
Table 2. Differences in memory strategy use between aMCI and naMCI groups

a p-value was adjusted to .007 to control for multiple comparisons.
b For parametric tests (i.e., t tests), mean values were used. For nonparametric tests (i.e., Mann–Whitney U), median values were used.
Correlations Between Memory Strategy Use and Cognitive Functioning
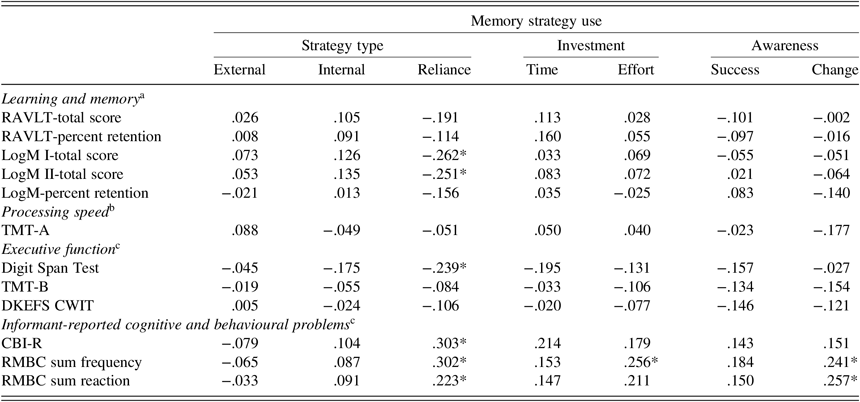
As shown in Table 3, in terms of strategy type, there were no significant correlations between external strategy use or internal strategy use and cognitive functioning in either the neuropsychological tests or informant ratings. However, several relationships were apparent in terms of reliance strategies. That is, increased use of reliance strategies was significantly correlated with poorer cognitive functioning, including poorer structured verbal learning and memory, as well as poorer working memory. Additionally, increased reliance strategy use was also significantly correlated with higher frequency and impact of cognitive/behavioural problems as rated by informants.
Table 3. Correlation between memory strategy use and cognitive functioning

Notes: RAVLT = Rey Auditory Verbal Learning Test; LogM I = Logical Memory Test I; LogM II = Logical Memory Test II; TMT-A = Trail Making Test, Part A; TMT-B = Trail Making Test, Part B; DKEFS CWIT = The Delis–Kaplan Executive Function System Colour-Word Interference Test (inhibition); CBI-R = Cambridge Behavioural Inventory-Revised; RMBC = Revised Memory and Behaviour Checklist.
Significant values are indicated by *.
a p-value was adjusted to .01 to control for multiple comparisons.
b p-value was adjusted to .05 to control for multiple comparisons.
c p-value was adjusted to .017 to control for multiple comparisons.
In terms of personal investment, there was no significant correlation between time invested into memory tasks and cognitive functioning, but increased effort invested into memory tasks was significantly related to higher informant-rated frequency of behaviour problems. For motivation and awareness, there was no significant correlation between motivation and cognitive functioning, but increased awareness of the need for memory compensation was significantly associated with higher informant-rated frequency and impact of cognitive/behavioural changes.
Correlation Between Memory Strategy Use and Medical, Psychosocial, and Mood Functioning
As shown in Table 4, strategy type was not significantly correlated with medical burden, psychosocial functioning, or mood; however, all three strategy types were positively correlated with the EMQ, indicating that increased strategy use was related to higher subjective memory complaints.
Table 4. Correlation between memory strategy use and medical, psychosocial, and mood functioning

Notes: CIRS-G = Cumulative Illness Rating Scale–Geriatric version; WHO-DAS = World Health Organization Disability Assessment Schedule; EMQ = Everyday Memory Questionnaire; PSQI = Pittsburgh Sleep Quality Index; WQOL = WHO Quality of Life Questionnaire (abbreviated); HADS = Hospital Anxiety and Depression Scale; HAM-D = Hamilton Depression Rating Scale.
Significant values are indicated by *.
a p-value was adjusted to .025 to control for multiple comparisons.
b p-value was adjusted to .017 to control for multiple comparisons.
c p-value was adjusted to .013 to control for multiple comparisons.
d p-value was adjusted to .017 to control for multiple comparisons.
With respect to personal investment, increased investment of time into memory tasks was significantly associated with increased subjective memory complaints, higher subjective disability, and poorer QoL relating to three domains (i.e., physical health, psychological functioning, and environmental factors). Similar patterns were evident in relation to investment of effort in the use of memory strategies. That is, increased effort was significantly correlated with greater subjective disability and increased memory complaints.
Finally, greater motivation to use memory strategies was significantly correlated with greater subjective disability, increased memory complaints, higher levels of depression and anxiety, and poorer QoL relating to physical health. Similarly, increased awareness of the need for memory compensation was significantly related to greater subjective disability, increased memory complaints, and increased depressive symptoms.
Predictors of Memory Strategies Use
After ensuring that all assumptions had been met, several multiple regressions were run to examine the relative contribution to memory strategy use from relevant cognitive, medical, psychosocial, and mood-related factors (see Table 5, below).
Table 5. Multiple regression analysis

Note: *p < .05; B = unstandardized regression coefficient; SE B = Standard error of the unstandardized coefficient; β = standardized coefficient; LogM I = Logical Memory Test I; LogM II = Logical Memory Test II; CBI-R = Cambridge Behavioural Inventory-Revised; RMBC = Revised Memory and Behaviour Checklist; WHO-DAS = World Health Organization Disability Assessment Schedule; WQOL = WHO Quality of Life Questionnaire (abbreviated); HADS = Hospital Anxiety and Depression Scale; HAM-D = Hamilton Depression Rating Scale.
A multiple regression model significantly predicted reliance strategy use from structured verbal learning and memory, working memory, subjective memory complaints, and frequency and impact of cognitive/behavioural problems as rated by informants (p < .001) with an R 2 of .342. Subjective memory complaints and working memory explained, respectively, 10.6% and 5.3% of the unique variance in reliance strategy use. The remaining variables together accounted for only 2.1% of the unique variance in reliance strategy use, and the remainder (11.9%) was shared variance.
In terms of time investment, a multiple regression model showed significant contributions to significant time investment from subjective memory complaints, general health-related disability, depression, and QoL relating to three domains (i.e., physical health, psychological functioning, and environmental factors), p < .001, adj. R 2 = .253. Subjective memory complaints explained 10.8% of the unique variance in time investment. The rest of the variables together accounted for only 5.0% of the unique variance in time investment, and the remainder (5.5%) was shared variance.
A significant regression equation was also found in relation to the effort invested in memory tasks (p < .005, adj. R 2 = .107), showing important contributions from informant-reported cognitive and behavioural problems, general health-related disability, and subjective memory complaints. In this model, subjective memory complaints (4.5%) and informant-reported cognitive problems (2.0%) uniquely explained 6.5% of the variance in effort, and the remainder (2.0%) was shared variance.
A significant regression equation was also found in relation to awareness of the need for memory compensation (p < .001, adj. R 2 = .239), where variables included subjective memory complaints, general health-related disability, informant-reported cognitive and behavioural problems, and depression. Here, subjective memory complaints uniquely accounted for 19.3% of the variance, whereas other variables accounted for only 2.6% of the unique variance. The remainder (2.0%) was shared variance. Despite this, as shown in Table 5, the regression model examining factors contributing to motivation to use memory strategies was nonsignificant (p >.05).
DISCUSSION
To our knowledge, this is the first study to investigate memory strategy use across MCI subtypes; moreover, it considerably expands on existing knowledge regarding relationships between memory strategy use and cognitive, medical, psychosocial, and psychological functioning in people with MCI. In particular, our focus, not only on strategy type but also on broader aspects of strategy use (i.e., personal investment in memory strategies, and awareness and motivation to use cognitive strategies), represents a novel area of exploration in people with MCI.
The first important and unique finding of the present study is that there is no significant difference in patterns of memory strategy use between individuals with aMCI and naMCI, even though we might expect the aMCI group to report more frequent memory strategy use, since by definition, their primary cognitive impairment lies in memory. Moreover, subjective everyday memory performance was reported to be significantly poorer in the aMCI group than the naMCI group, again suggesting that the former group might be more likely to benefit from using compensatory strategies to improve their everyday memory functioning, particularly since previous research has shown that increased memory strategy use is related to increased awareness of memory impairment (de Frias et al., Reference de Frias, Dixon and Backman2003). Indeed, this point was confirmed in the current study, where subjective memory complaints were shown to make the greatest contribution to various aspects of memory strategy use across all of the significant regression models. These results therefore suggest that individuals with aMCI, perhaps more so than naMCI, need to be educated regarding the potential utility of memory strategies and how best to use them for maximum effect.
In terms of the relationship between cognitive functioning and memory strategy use in people with MCI, it seems that the most prominent pattern indicates a general trend for those with poorer cognition (i.e., learning, memory, and working memory) to more frequently depend on strategies which rely on or involve other people. Moreover, our finding indicating there is a significant relationship between increased participants’ use of reliance strategies and more severe reaction ratings of informants to participants’ cognitive problems, suggests that this may also be causing greater burden on those family and friends, perhaps because they are the ones who are frequently called upon for assistance. With an abundance of research indicating that increased carer burden may cause poor physical and mental health in caregivers (e.g., Fekete, Tough, Siegrist, & Brinkhof, Reference Fekete, Tough, Siegrist and Brinkhof2017), it seems there may be additional positive implications to educating older people with MCI regarding optimal strategy use, as this may reduce the impact on those close to them and prevent them taking up a role as inadvertent ‘caregivers’.
The present study also found that participants with better working memory tend to use strategies less frequently. These results differ from previous research, which has shown that higher levels of executive function are associated with increased (internal) strategy use. One reason for this inconsistency may relate to the fact that executive functions reflect a broad array of subskills including planning, organization, problem-solving, multitasking, and so on. In our study, we employed measures of working memory, response inhibition, and cognitive flexibility, while other studies (e.g., Bouazzaoui et al., Reference Bouazzaoui, Isingrini, Fay, Angel, Vanneste, Clarys and Taconnat2010) have measured other aspects of executive function (i.e., abstract reasoning, problem-solving, set shifting, and phonemic verbal fluency). Interestingly, our study did not show a significant relationship between memory strategy use and cognitive flexibility or response inhibition, suggesting that working memory may play a more important role in day-to-day memory functioning compared to other aspects of executive functioning. Previous studies have demonstrated that working memory seems to be a prerequisite for goal-directed and purposeful cognitive functioning (Kennedy et al., Reference Kennedy, Coelho, Turkstra, Ylvisaker, Moore Sohlberg, Yorkston, Chiou and Kan2008) and that it is critical for planning and performing daily activities (Baddeley, Reference Baddeley1998; Klingberg et al., Reference Klingberg, Fernell, Olesen, Johnson, Gustafsson, Dahlstrom, Gillberg, Forssberg and Westerberg2005). It stands to reason, therefore, that if people with MCI have better working memory functioning, they may not feel the need to use reliance strategies as frequently, in order to accomplish daily tasks.
The other inconsistent finding compared to previous studies is the relationship between memory performance and strategy use. One previous study showed that increased external strategy use is associated with better memory performance in individuals with MCI (Aronov et al., Reference Aronov, Rabin, Fogel, Chi, Kann, Abdelhak and Zimmerman2015); however, in that study, external strategy use was the only type of strategy considered and frequency of use was rated by test administrators during a lab-based task of prospective memory. In our study, participants subjectively reported strategy use in everyday life, which may account for our results – that is, that better retrospective memory performance relates only to reliance strategies in individuals with MCI, and in fact, this reflects less frequent usage of this less dependable strategy type.
Our exploration of the relationship between memory strategy use and noncognitive factors produced both expected and unexpected findings. Increased QoL was associated with less investment in strategies, most likely because these individuals were satisfied with their current level of functioning. However, in terms of psychological functioning and self-reported disability, only factors relating to personal investment (especially the time invested in memory tasks) and awareness/commitment to strategy use showed significant relationships with anxiety, depression, and disability, and somewhat counterintuitively, this was in a positive direction – that is, those with greater depression/anxiety symptoms and disability, who might otherwise be expected to have poorer motivation/initiation in general, still reported spending more time and effort, and had greater motivation and commitment in relation to memory strategies. However, this could reflect a unique feature of our ‘help-seeking’ population, whereby participants had voluntarily presented to a specialist memory clinic with some insight into their cognitive and psychological symptoms, for the specific purpose of accessing optimal management as well as recommendations for strategies or interventions to prevent future cognitive decline. Thus, our sample may be generally more motivated or insightful than other adults with MCI in the larger community.
Overall, these results demonstrate that older adults with MCI routinely use memory strategies in daily life and that both frequency and type of strategy use relate to important aspects of cognitive and psychosocial functioning. These findings therefore present a valuable opportunity to consider how strategies may be facilitated by neuropsychologists and utilized for maximal benefit in clinical practice. For example, psychoeducation and cognitive training interventions, incorporating instruction and practice in the use of various compensatory and/or restorative strategies, are worthwhile and relevant for people with MCI (Coe, Martin, & Stapleton, Reference Coe, Martin and Stapleton2019; Dewar, Kapur, & Kopelman, Reference Dewar, Kapur and Kopelman2018; Mowszowski et al., Reference Mowszowski, Batchelor and Naismith2010) – however, these results also indicate that such interventions should ideally be tailored to the individual’s cognitive and psychosocial profile, paying particular attention to subjective memory complaints, objective deficits in working memory, attention, learning and memory, as well as depression, perceived disability, and QoL. These results also emphasize the value in clinicians seeking input from close family or friends regarding cognitive and/or behavioural changes in those with MCI as this may also influence the intervention plan in older people with MCI, psychoeducation should not only focus on reducing reliance strategy use but also on improving the consistency and effectiveness of external/internal memory strategy use in daily life, both to maximize benefit and reduce potential impact on family/friends.
This study has several strengths, including the use of a comprehensive assessment of cognitive functioning, medical burden, psychosocial function, and mood as well as informant-reported frequency and impact of cognitive/behavioural problems for the MCI participants, for the purposes of arriving at a thorough understanding of how these factors interact with memory strategy use. Furthermore, memory strategy use was divided into three subclasses – strategy type, personal investment, and self-awareness. This classification could not only help clinicians have a more detailed understanding about what type of memory strategy an individual with MCI might apply for the greatest benefit on a day-to-day basis but also give clinicians an indication of an individual’s commitment to and awareness of memory strategies. However, some limitations should also be noted. Firstly, participants in this sample were on average relatively highly educated, which (a) may not fully represent the population of older Australians and (b) may reflect some bias in strategy use, since a previous study has suggested that education may be a factor for strategy use (Saczynski, Willis, & Warner Schaie, Reference Saczynski, Willis and Warner Schaie2002). Secondly, some may consider the emphasis on a self-report measure of strategy use to be limiting in a population with cognitive impairment, as this may have reduced reliability. Indeed, individuals with MCI might lack awareness of subtle functional deficits and might overestimate their functional capacity (Okonkwo et al., Reference Okonkwo, Wadley, Griffith, Belue, Lanza, Zamrini, Harrell, Brockington, Clark, Raman and Marson2008). Nonetheless, we also note that by definition, those with MCI have at least some awareness of cognitive change and that poor insight would typically be indicative of more progressed cognitive impairment beyond the stage of MCI. A further limitation is that we did not examine strategies that take advantage of technology (e.g., set a reminder and alarm on mobile phone). Future studies may benefit from investigating strategy use related to technology and recruiting participants across a variety of settings in order to ensure a more representative sample of the older adult population in Australia.
The current study substantially expands on existing knowledge with respect to how individuals with MCI utilize memory compensation strategies in daily life. Due to lack of effective pharmacological treatment for cognitive decline, nonpharmacological approaches such as psychoeducation and cognitive interventions focusing on compensatory strategies are likely to be extremely valuable for maintaining day-to-day functioning and self-efficacy in daily life for people with MCI, particularly when these interventions are tailored to cognitive and psychosocial factors affecting uptake.
ACKNOWLEDGMENTS
LM is supported by a National Health and Medical Research Council (NHMRC) – Australian Research Council (ARC) Dementia Research Development Fellowship (grant number 1109618), and SLN is supported by a NHMRC Dementia Leadership Fellowship (grant number 1135639). There are no financial relationships with commercial interests and no conflicts of interest to report. We would like to acknowledge the assistance of all neurologists/geriatricians, neuropsychologists, and research psychologists at the Healthy Brain Ageing (HBA) Clinic in diagnosing patients as well as conducting neuropsychological and mood assessments.