INTRODUCTION
There is growing interest in accurately quantifying motor activity patterns during the acute as well as chronic phases of stroke. This interest is motivated by the increasing appreciation that clinical decisions and rehabilitation outcomes following stroke can be significantly enhanced if motor deficits are adequately quantified (Calautti et al., Reference Calautti, Jones, Persaud, Guincestre, Naccarato, Warburton and Baron2006; Hingtgen, McGuire, Wang, & Harris, Reference Hingtgen, McGuire, Wang and Harris2006; Lindberg et al., Reference Lindberg, Roche, Robertson, Roby-Brami, Bussel and Maier2012; Lum, Burgar, Kenney, & Van Machiel Der Loos, Reference Lum, Burgar, Kenney and Van Machiel Der Loos1999; Tamburini, Mazzoli, & Stagni, Reference Tamburini, Mazzoli and Stagni2018; Thrane, Emaus, Askim, & Anke, Reference Thrane, Emaus, Askim and Anke2011). Quantitative solutions such as wrist-based accelerometers, video-based assessments, and robotic technologies are, therefore, gaining popularity because they provide a more reliable and precise measurement of limb use after stroke. Such solutions allow repeated, stable and accurate measurements, decrease subjectivity or bias associated with self-reported measures, and can be carried over to settings outside the clinical environment.
Quantification of movement patterns has also enabled a better understanding of how various factors influence the evolution of particular motor patterns after stroke. For example, Thrane et al. (Reference Thrane, Emaus, Askim and Anke2011) demonstrated a strong influence of the level of motor impairment on the pattern of arm use post-stroke. This study examined how “arm movement ratio” (calculated as the ratio of the duration of ipsilesional to contralesional arm use measured using wrist-based accelerometers) varied with the degree of motor impairment estimated using the Fugl-Meyer Assessment (FM) score. They showed an inverse relationship between arm movement ratio (ipsilesional/contralesional) and degree of impairment: as contralesional FM score increased/improved, arm movement ratio decreased. In other words, subjects tended to use their contralesional arm more frequently at lower impairment levels.
Another study (Lang, Wagner, Edwards, & Dromerick, Reference Lang, Wagner, Edwards and Dromerick2007) had also previously demonstrated a similar relationship between impairment level and the duration of affected arm use over a 24-hr period in acute stroke patients. This study reported minimal use of the affected arm in the inpatient setting, and moreover, showed that 7 of 10 impairment measures were related to affected arm use. Similar results have been reported across other studies (Gebruers et al., Reference Gebruers, Truijen, Engelborghs, Nagels, Brouns and De Deyn2008; Lang et al., Reference Lang, Wagner, Edwards and Dromerick2007; Reiterer, Sauter, Klosch, Lalouschek, & Zeitlhofer, Reference Reiterer, Sauter, Klosch, Lalouschek and Zeitlhofer2008; Siekierka-Kleiser, Kleiser, Wohlschlager, Freund, & Seitz, Reference Siekierka-Kleiser, Kleiser, Wohlschläger, Freund and Seitz2006; Wang et al., Reference Wang, Lin, Wu, Chung, Pei and Teng2011) that have examined the relationship between various clinical measures and arm use patterns, in both acute and chronic stroke.
Unfortunately, almost all these prior studies have ignored the impact of the hemisphere of brain damage on the relationship between clinical impairment measures and arm use patterns. This is despite growing evidence that damage to the left or right hemisphere produces distinct behavioral deficits (Mani et al., Reference Mani, Mutha, Przybyla, Haaland, Good and Sainburg2013; Schaefer, Haaland, & Sainburg, Reference Schaefer, Haaland and Sainburg2009; Schaefer, Mutha, Haaland, & Sainburg, Reference Schaefer, Mutha, Haaland and Sainburg2012; Winstein & Pohl, Reference Winstein and Pohl1995), and that these differential deficits hold significant implications for rehabilitation (Sainburg, & Duff, Reference Sainburg and Duff2006; Sainburg, Maenza, Winstein, & Good, Reference Sainburg, Maenza, Winstein and Good2016). For example, a large body of work has shown that motor control (Haaland, Prestopnik, Knight & Lee, Reference Haaland, Prestopnik, Knight and Lee2004; Mani et al., Reference Mani, Mutha, Przybyla, Haaland, Good and Sainburg2013; Schaefer et al., Reference Schaefer, Haaland and Sainburg2009; Winstein & Pohl, Reference Winstein and Pohl1995), error correction (Schaefer et al., Reference Schaefer, Mutha, Haaland and Sainburg2012) and learning (Garry, Kamel & Nordstrom, Reference Garry, Kamen and Nordstrom2004; Mutha, Sainburg, & Haaland, Reference Mutha, Sainburg and Haaland2011a,Reference Mutha, Sainburg and Haalandb) are all differentially affected in patients with left versus right hemisphere damage when they are tested on point-to-point reaching movements.
Furthermore, differential impact of left versus right hemisphere damage has also been shown in everyday tasks (Bernspång & Fisher, Reference Bernspång and Fisher1995; Haaland et al., Reference Haaland, Mutha, Rinehart, Daniels, Cushnyr and Adair2012; Poole, Sadek & Haaland, Reference Poole, Sadek and Haaland2009). For instance, patterns of arm use, assessed during instrumented as well as regular activities of daily living using bilaterally worn wrist accelerometers, are strongly influenced by the hemisphere of damage (Haaland et al., Reference Haaland, Mutha, Rinehart, Daniels, Cushnyr and Adair2012; Rinehart, Singleton, Adair, Sadek, & Haaland, Reference Rinehart, Singleton, Adair, Sadek and Haaland2009). Specifically, it has been noted that right hemisphere damaged (RHD) patients use their contralesional arm less than left hemisphere damaged (LHD) patients, while ipsilesional arm use is not different between the two groups. Thus, given that LHD and RHD give rise to differential patterns of arm use, and that patterns of arm use are related to impairment levels, we reasoned that the side of brain damage post-stroke would modulate the relationship between arm use and impairment.
The aim of this study was, therefore, to examine potential differences in the relationship between impairment and arm use in individuals with LHD or RHD. We used the FM score as the measure of impairment while arm use patterns were derived from accelerometer data as subjects performed the Arm Motor Ability Test (AMAT) (Kopp et al., Reference Kopp, Kunkel, Flor, Platz, Rose, Mauritz and Taub1997) and Functional Impact Assessment (FIA) (Heaton et al., Reference Heaton, Marcotte, Rivera Mindt, Sadek, Moore, Bentley and Wolfson2004). Given arm preference effects (in our case, strong pre-morbid right arm preference), we predicted that use of the contralesional, affected arm would be different in LHD and RHD subjects for low impairment levels (high FM scores). However, for low FM scores (higher impairment), we considered the possibility that the substantial impairment might override arm preference effects, making the influence of hemisphere of damage on the relationship between arm use and impairment less certain.
METHODS
Participants
This study was approved by the Institutional Review Board of the New Mexico Veterans Affairs Healthcare System and study procedures conformed to the Declaration of Helsinki. Fifty-eight participants with unilateral stroke (28 LHD, 30 RHD) participated in this study after providing written informed consent. All the participants were right handed before their stroke; pre-morbid right-handedness was confirmed via the Edinburgh Handedness Inventory (Oldfield, Reference Oldfield1971). Inclusion criteria were as follows: stroke patients must be in the chronic phase (i.e., more than 6 months post-stroke), and they should have had no prior history of severe psychiatric, motor, or other neurological disorder other than stroke. Subjects were excluded if they had a history of substance abuse or peripheral movement restrictions from neuropathy or orthopedic injuries. Besides demographic variables, we also recorded their grip strength in the contralesional and ipsilesional arms using hand-held dynamometers and auditory comprehension abilities using the sequential commands subtest of the Western Aphasia Battery (Kertesz, Reference Kertesz1982).
Lesion measurement
Magnetic resonance imaging (48 patients) and computed tomography (10 patients) scans were obtained from the stroke patients. These images were normalized to the MNI-152 template using SPM8 and custom Matlab code. Lesions were then traced on individual anatomical image slices and the traced lesions were converted back into volumes. Volumes from all patients within each stroke group were overlaid in MRICron to create overlap images showing areas of damage common to all patients within a group (Figure 1). Broadly, all subjects showed widespread damage to cortical and subcortical regions. Most patients had damage to the basal ganglia, internal capsule, and insular cortex. Most subjects also had damage in sensorimotor cortex (Brodmann areas 4, 6, 3, 1, and 2).
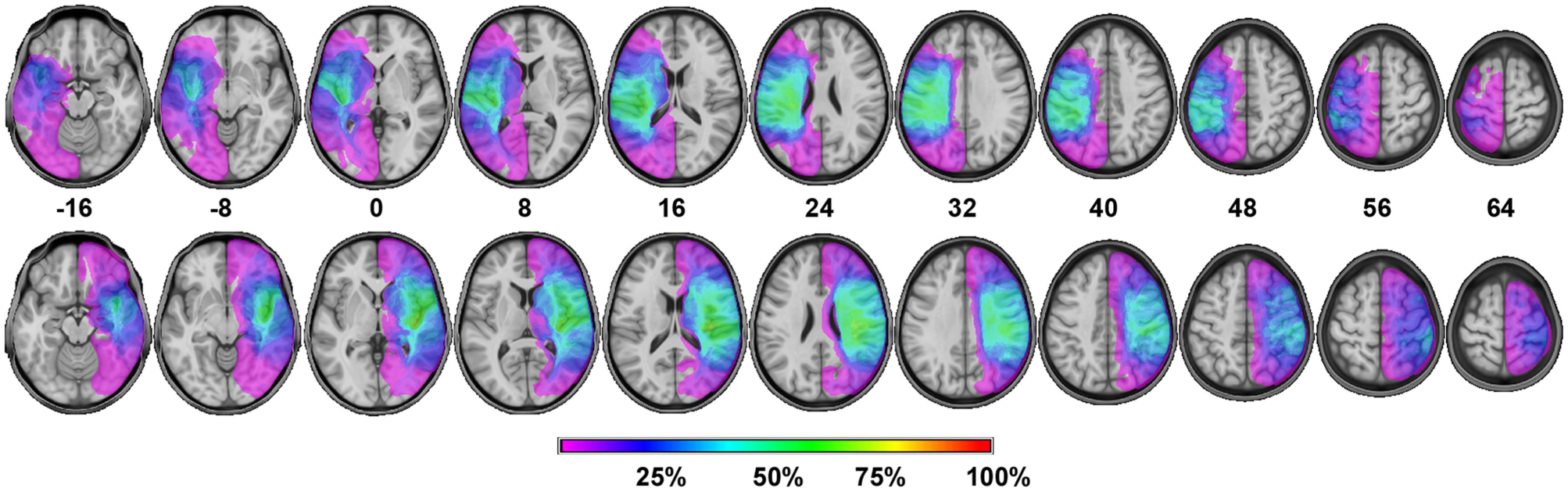
Fig. 1 Lesion overlap images for the LHD (top) and RHD (bottom) patient groups. Slice numbers depict MNI coordinates in the horizontal plane. The different colors represent the percentage of patients with damage to that region of the brain.
Arm impairment assessment
We used the upper extremity component of the FM scale to quantify the level of contralesional impairment post-stroke (Fugl-Meyer, Jääskö, Leyman, Olsson, & Steglind, Reference Fugl-Meyer, Jääskö, Leyman, Olsson and Steglind1975; Gladstone, Danells, & Black, Reference Gladstone, Danells and Black2002). This scale primarily assesses loss in control and joint individuation (e.g., wrist dorsiflexion or palmar flexion); some sub-tasks also provide information about loss of strength. The maximum score on the upper extremity component of the FM scale is 66, and this score is commonly used to define the degree of hemiparesis in patients (for instance, patients with scores below 25 are generally considered to be “severely” hemiparetic). The FM has an extremely good inter-rater as well as intra-rater reliability and validity; it is also able to detect small changes in impairment levels (Gladstone et al., Reference Gladstone, Danells and Black2002; Page, Levine, & Hade, Reference Page, Levine and Hade2012; Platz et al., Reference Platz, Pinkowski, van Wijck, Kim, di Bella and Johnson2005; See et al., Reference See, Dodakian, Chou, Chan, McKenzie, Reinkensmeyer and Cramer2013).
AMAT
The AMAT is well-validated tool for assessment of activities of daily living (ADLs) (Kopp et al., Reference Kopp, Kunkel, Flor, Platz, Rose, Mauritz and Taub1997). We used a modified AMAT that consisted of 12 items; one item (prop on extended arm) of the standard AMAT was excluded because of difficulty level. Tasks on the AMAT involve activities such as drinking, combing hair, and opening a jar. In this study, while each task was modeled by the experimenter, no explicit instructions were given regarding which arm to use or what speed to maintain; instead participants were told to perform the task as they would in their day-to-day life. Scoring in the AMAT is based on the quality of the movement, but this measure itself was not critical in the current study. Rather, we were interested in the pattern of arm usage during the AMAT, which were derived using accelerometers worn on the wrists during AMAT performance.
FIA
Performance on instrumented ADLs (IADLs) was assessed using the FIA (Heaton et al., Reference Heaton, Marcotte, Rivera Mindt, Sadek, Moore, Bentley and Wolfson2004). The FIA assesses accuracy of performance on simulated tasks related to shopping, money management, meal preparation, medication management, and communication. The score on the FIA ranges from 0 to 115, and the scoring emphasis is on whether the performance is functional or not. Additionally, the FIA is not timed. The FIA has good construct validity and is highly correlated with self-report measures of functional independence after stroke (Sadek, Stricker, Adair, & Haaland, Reference Sadek, Stricker, Adair and Haaland2011). Again, our interest here was on the pattern of arm use during the FIA rather than the actual score obtained on this test.
Arm use patterns
Arm use patterns were obtained via accelerometers (GT1M monitors) worn on both wrists during the AMAT and FIA. The monitors contain piezoelectric crystals that are sensitive to motion and, thus, measure changes in acceleration during movement. The signals provided by these accelerometers are sampled, digitized, and represented by a series of numbers called “raw counts” (Uswatte et al., Reference Uswatte, Miltner, Foo, Varma, Moran and Taub2000). These numeric values obtained for each arm reflect the intensity of movement for an epoch (2 s in our case). As our primary measure of interest was duration of movement and not the amplitude, the raw counts were transformed using a validated threshold filter technique which yields a valid and reliable measure of movement duration (Uswatte et al., Reference Uswatte, Giuliani, Winstein, Zeringue, Hobbs and Wolf2006).
Each epoch with a raw count ≥1 was assigned a value of 1 for the right arm and 4 for the left arm, and each epoch with a raw count of <1 was assigned a value of 0 for the right arm and 2 for the left arm. Values obtained from this transformation were summed to determine arm use during each epoch. A value of 2 indicates that neither arm was in use, 3 meant that only the right arm was used, 4 meant that only the left arm was used, and 5 suggested that both the arms were used. The duration of arm use (right only, left only, and bilateral) was then expressed as a percentage of the total AMAT or FIA duration.
Statistical Analyses
The JMP statistical package was used for statistical analysis. Differences in pattern of arm use across groups (LHD, RHD), arm (ipsilesional, contralesional, bilateral) and task (AMAT, FIA) were first determined using three-way analysis of variance (ANOVA). The relationship between arm use and impairment as a function of hemisphere of damage was examined using linear regression. Significance levels were set at 0.05 for all comparisons.
RESULTS
Group Characteristics
Table 1 provides descriptive characteristics for patients in the two stroke groups. The RHD and LHD groups were not statistically different in terms of age (p=.1046) or education (p=.629), but there was a marginally greater number of females in the RHD compared to the LHD group (p=.0560). The degree of arm preference before stroke was not statistically different across groups (p=.1346), with almost all subjects demonstrating strong pre-morbid right-handedness. There was also no significant difference between the two groups in terms of lesion volume (p=.2808), time post-stroke (p=.7587), and degree of upper extremity motor impairments assessed using the FM exam (p=.2493).
Table 1 Demographic and clinical data

Note. Numbers represent the mean for the various parameters, with SDs in parentheses (except for sex).
a Laterality Quotient ranges from +100 (strongly right handed) to – l00 (strongly left handed).
b Western Aphasia Battery, Sequential Commands, maximum score is 80 (Kertesz, Reference Kertesz1982).
c Grip strength from dynamometer are expressed as standardized t scores; mean=50, SD=10.
d Maximum score on the uper-extremity component of the Fugl-Meyer test is 66.
However, the two groups differed significantly on their language comprehension abilities (F1,56=8.41; p=.0053) with LHD patients exhibiting poorer comprehension, as expected (Bonilha et al., Reference Bonilha, Hillis, Hickok, Den Ouden, Rorden and Fridriksson2017; Cloutman et al., Reference Cloutman, Gottesman, Chaudhry, Davis, Kleinman, Pawlak and Hillis2009; Damasio, Reference Damasio1992; Dronkers, Wilkins, Van Valin, Redfern, & Jaeger, Reference Dronkers, Wilkins, Van Valin, Redfern and Jaeger2004; Eggert, Reference Eggert1977). We ensured that these comprehension difficulties did not influence the FM examination by repeating instructions whenever necessary.
Patterns of Arm Use in the Two Stroke Groups
Figure 2 shows the pattern of arm use for the AMAT (Figure 2A) and the FIA (Figure 2B) for the two stroke groups. Our statistical analysis (group, arm, task ANOVA) revealed a significant group×arm interaction (F2,280=21.5069; p<.0001; partial eta squared=0.125). However, the three-way interaction was not significant (F2,280=0.9765; p=.3777; partial eta squared=0.0064), indicating that the relationship between arm used and side of damage (i.e., the group×arm interaction) was not different for the two tasks. We, therefore, used the pooled FIA and AMAT data for further investigation of group differences in the pattern of arm use. A separate group×arm ANOVA on the pooled data showed a significant interaction effect (F2,286=19.8109; p<.0001; partial eta squared=0.115).
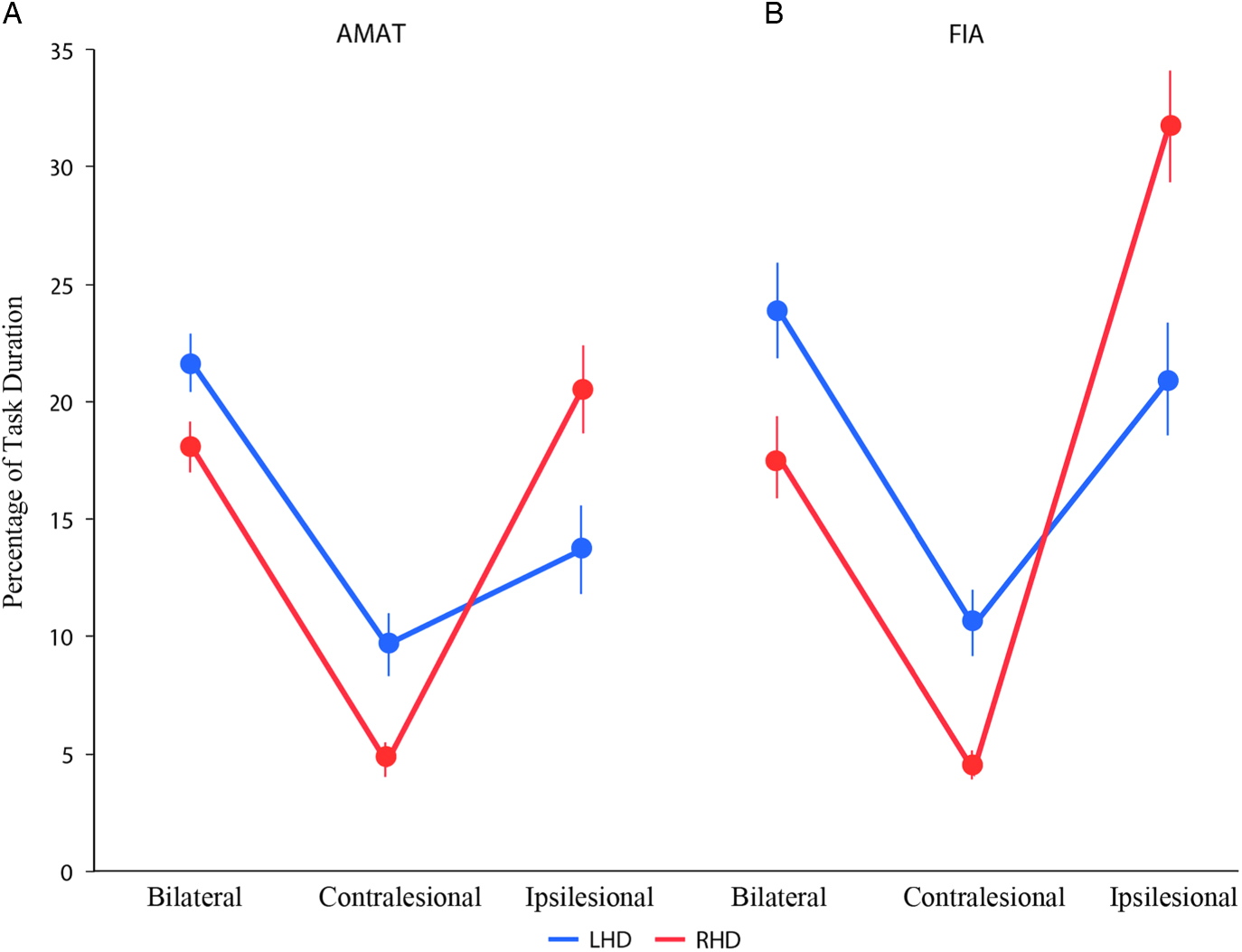
Fig. 2 Mean percentage of arm usage (Bilateral, Contralesional and Ipsilesional) on the two tasks, AMAT (A) and FIA (B) for the two stroke groups. LHD and RHD groups are shown in blue and red color, respectively.
Post hoc tests then revealed clear group differences in bilateral, ipsilesional, and contralesional arm use patterns. RHD patients used their ipsilesional arm substantially more than the LHD patients (p<.0001; Cohen’s d=0.948), while LHD patients used their contralesional arm significantly more than the RHD patients (p=.0013; Cohen’s d=0.6025). The LHD group also showed significantly greater bilateral arm use compared to the RHD group (p=.0046; Cohen’s d=0.5301). In other words, both stroke groups preferred to use their right arm (ipsilesional arm for RHD and contralesional arm for LHD) more than their left arm, consistent with the prior suggestion (Haaland et al., Reference Haaland, Mutha, Rinehart, Daniels, Cushnyr and Adair2012; Rinehart et al., Reference Rinehart, Singleton, Adair, Sadek and Haaland2009) of a pre-morbid, right arm preference that was responsible for this pattern of arm use after stroke.
Relationship Between Arm Use and Impairment Level
The main question of interest in this study was whether and how the differential arm use patterns noted above for the two stroke groups varied with the degree of impairment. To this end, we found a strong group×FM score interaction in a regression model for contralesional arm use (F1,112=12.0081; p=.0008, partial eta squared=0.09683). This suggested that the amount of contralesional arm use for different levels of impairment was dependent on the hemisphere of damage. LHD patients tended to use their contralesional, right arm substantially more as their impairment levels decreased (FM score increased) relative to the RHD group’s usage of their contralesional, left arm (Figure 3A).

Fig. 3 Relationship between contralesional (A), ipsilesional (B), and bilateral (C) arm use and impairment level (FM score) for the two stroke groups. LHD and RHD groups are shown in blue and red color, respectively.
In contrast, contralesional arm use appeared fairly similar, and very low, for both groups at greater impairment levels (smaller FM scores). Interestingly, no such relationship was noted for ipsilesional (group×FM score interaction: F1,112=0.8030; p=.3721; partial eta squared=0.0071) or bilateral arm use (group×FM score interaction: F1,112=1.80; p=.0741; partial eta squared=0.0282). Ipsilesional arm use was greater for both stroke groups for greater levels of impairment (Figure 3B, main effect of impairment: F1,112=131.69; p<.0001, partial eta squared=0.5404), while bilateral arm use increased for both stroke groups as impairment levels decreased (Figure 3C, main effect of impairment: F1,112=39.63; p<.0001; partial eta squared=0.2614). Thus, only contralesional arm usage patterns consistently differed depending on the hemisphere of damage and impairment levels.
In summary, we found that in a pre-morbidly right-handed patient population: (1) Greater contralesional arm use was observed in LHD but not RHD patients as levels of impairment decreased; (2) For higher levels of impairment, contralesional arm use was low in both LHD and RHD groups; and (3) Hemisphere of damage did not influence the relationship between ipsilesional arm use and degree of impairment. Rather, greater ipsilesional arm use was seen for RHD compared to LHD patients regardless of impairment level.
DISCUSSION
It is well recognized that post-stroke patterns of arm use change with the level of impairment. It has been demonstrated that both the amount and quality of contralesional, affected arm use decrease as impairment levels increase (Lang et al., Reference Lang, Wagner, Edwards and Dromerick2007; Thrane et al., Reference Thrane, Emaus, Askim and Anke2011). Here we show that the strength of this association depends on the side of brain damage: as impairment levels decreased, LHD patients tended to use their contralesional, right arm more than RHD patients used their contralesional, left arm.
The most parsimonious explanation for this is a preference to use the dominant, right arm in these pre-morbidly right-handed subjects; like past work (Haaland et al., Reference Haaland, Mutha, Rinehart, Daniels, Cushnyr and Adair2012; Rinehart et al., Reference Rinehart, Singleton, Adair, Sadek and Haaland2009), we observed that both LHD and RHD subjects showed a strong preference to use their right arm. However, for RHD patients, this was their ipsilesional arm. Given their preference to use this arm, the amount of contralesional, left arm use did not change much despite a reduction in impairment. Thus, arm preference effects strongly drove the change in contralesional arm use patterns with changing impairment levels. Importantly, these differences were unrelated to the degree of arm preference in the two groups because the LHD and RHD groups were not different in terms of their (pre-stroke) right-arm preference. Similarly, these differences also appear unrelated to any difference in absolute impairment levels since these were also matched across the two stroke groups.
Another insight from the present results was that group differences in the association between arm use and impairment were not seen in the ipsilesional or bilateral conditions. Ipsilesional arm use was greater in RHD compared to LHD patients, and generally decreased with lesser impairment. There was, however, no change in the pattern of group differences in ipsilesional arm use as impairment levels changed. In other words, greater ipsilesional arm use was seen in the RHD patients for all impairment levels.
Why did ipsilesional arm use not increase in LHD patients at lower impairment levels (increasing FM scores) to at least the extent of the RHD patients? This could also be attributed to arm preference effects. As impairment levels decreased, LHD subjects preferred to use their contralesional, right arm to a greater extent, thereby keeping use of the non-preferred, left arm low even though it was this arm that was unaffected. It is also plausible that, when LHD subjects did indeed use their left arm at low impairment levels, they preferred to use it with their right arm (bilateral movement). Indeed, bilateral arm use was greater in LHD patients than RHD patients and this difference was more pronounced at lower impairment levels, though the interaction between FM score and group did not reach statistical significance (see the Results section). Future work with greater subject numbers can firmly establish this.
In our work, we used direct measures of arm use duration (expressed as a percentage of total FIA and AMAT duration) to understand the relationship between arm motor patterns and impairment post-stroke. This was similar to the work of Lang et al. (Reference Lang, Wagner, Edwards and Dromerick2007), who used actual duration measures, but different from Uswatte et al. (Reference Uswatte, Giuliani, Winstein, Zeringue, Hobbs and Wolf2006) and Thrane et al. (Reference Thrane, Emaus, Askim and Anke2011) who used a composite “ratio” between ipsilesional and contralesional arm use for examining such associations. The use of a composite measure, however, precludes the ability to examine the change in individual arm use with different impairment levels. The use of a single measure also precludes examination of bilateral arm use, which is the most preferred motor pattern for everyday tasks (Rinehart et al., Reference Rinehart, Singleton, Adair, Sadek and Haaland2009; Vega-Gonzalez, Bain, Dall, & Granat, Reference Vega-Gonzalez, Bain, Dall and Granat2007).
Additionally, the ratio measure overestimates the strength of the association between arm use and impairment. For instance, Thrane et al. (Reference Thrane, Emaus, Askim and Anke2011) observed a correlation of ~0.85 when considering the arm movement ratio, but this correlation dropped to ~0.6 when only contralesional arm use was considered. While we did not specifically examine the association between contralesional arm use as a whole and impairment (since our focus was on LHD and RHD differences), our results indicate that the biggest driver of group differences in the association of arm use and impairment was the contralesional arm usage pattern alone. It may, therefore, be better to consider just individual arm use measures when addressing issues related to changes in motor patterns post-stroke rather than composite measures that include both arms.
Our findings have some implications for upper extremity rehabilitation post-stroke. First, like past work, they suggest that quantifying upper arm use has important benefits because it can potentially suggest motor patterns to target during therapy. More importantly, the current results suggest that LHD and RHD patients may benefit by following distinct rehabilitation approaches, at least at lower impairment levels. If contralesional, left arm use continues to be low in RHD patients despite lower impairment (at least when compared to right arm usage for LHD patients as seen in the current data), rehabilitation approaches may want to emphasize unilateral, contralesional arm training in these patients. It is in such situations where approaches such as constraint induced movement therapy (CIMT) (Chiu & Ada, Reference Chiu and Ada2016; Grotta et al., Reference Grotta, Noser, Ro, Boake, Levin, Aronowski and Schallert2004; Kwakkel, Veerbeek, van Wegen, & Wolf, Reference Kwakkel, Veerbeek, van Wegen and Wolf2015; Taub et al., Reference Taub, Miller, Novack, Fleming, Nepomuceno, Connell and Crago1993) may be more beneficial. Once contralesional arm usage is optimized, rehabilitation could then focus on bilateral arm training (Coupar, Pollock, van Wijck, Morris, & Langhorne, 2010; McCombe Waller & Whitall, Reference McCombe Waller and Whitall2008; Stoykov, Lewis, & Corcos, Reference Stoykov, Lewis and Corcos2009).
In contrast, for LHD patients, particularly those with lower levels of impairment, approaches such as CIMT may carry smaller benefits because of their natural preference to use their contralesional, right arm. Instead, rehabilitation for such patients could focus on overcoming ipsilesional deficits through bilateral motor training. In contrast, for higher levels of impairment, rehabilitation may focus on training the contralesional arm regardless of the hemisphere of damage. In any case, precise quantification of arm use would be the ideal first step toward determining the training strategy to be developed.
Study Limitations
Our study is not free of limitations; several of these can be addressed in future work. First, the patient population in our study was predominantly right-handed and, therefore, caution must be exercised while generalizing this pattern of findings to left-handers. Second, we used only the FM score as a measure of impairment. The FM assessment largely interrogates the ability to control individual joints and only minimally tests strength. Given that hemiparesis can result in both loss of control and reduction in strength, it would be useful to examine other, “purer” measures of impairment that separate these two components.
Additionally, functional tasks other than the AMAT and the FIA, particularly those that are primarily used to examine rehabilitation outcomes such as the Wolf Motor Function Test (Hodics et al., Reference Hodics, Nakatsuka, Upreti, Alex, Smith and Pezzullo2012) and the Action Research Arm Test (Nijland et al., Reference Nijland, Van Wegen, Verbunt, Van Wijk, Van Kordelaar and Kwakkel2010; Ninković, Weissenbacher, Pratschke, & Schneeberger, Reference Ninković, Weissenbacher, Pratschke and Schneeberger2015) could also be used to derive arm movement patterns post stroke. Finally, studies such as the current one can always benefit from greater subject numbers and greater power. This may help to more robustly establish the relationship between impairment and arm use patterns post-stroke.
CONCLUSIONS
We found that the relationship between contralesional arm use and motor impairment post-stroke depends on the hemisphere of damage. When impairment was low (FM score was high), contralesional arm use was greater for LHD than RHD patients, suggesting that the side of brain damage impacted arm use patterns at these impairment levels. This may be driven by pre-morbid arm preference effects, and holds significant implications for rehabilitation in which the hemisphere of damage is often ignored.
ACKNOWLEDGMENTS
We appreciate the support provided by Lee Stapp in preparing the lesion overlay. We acknowledge HealthSouth Rehabilitation Hospital, Lovelace Medical Center, and Lovelace Rehabilitation Hospital for patient referral. This work was funded by the Biomedical Laboratory Research and Development Service (101BX007080) and the Rehabilitation Research and Development Service (B4125R) grants from the VA Office of Research and Development to Kathleen Y. Haaland. This work was also supported by the Ramanujan Fellowship, and grants from the Department of Science and Technology (Government of India) to Pratik K. Mutha. The authors declare no conflict of interest.






