INTRODUCTION
In schizophrenia, language deficits are prominent (Bokat & Goldberg, 2004). Crow (1998) proposed that, in schizophrenia, abnormal interhemispheric connectivity results in incomplete lateralization of language to the left hemisphere with consequent language impairments. Structural and functional abnormalities of the corpus callosum have been reported in many studies of patients with schizophrenia (Hulshoff Pol et al., 2004; Mohr et al., 2000), although a direct relationship between callosal and language deficits in schizophrenia has not been demonstrated. Abnormal development of the corpus callosum has been associated with language deficits in other disorders of neurodevelopmental origin. Children with developmental language disorder are impaired on tasks requiring the interhemispheric transfer of information (Beaton, 1997). Temple et al. (1989) reported that subjects with agenesis of the corpus callosum have persistent phonological processing deficits, which may be due to impairment in the “fine tuning of the extremes of lateralized functions” (p. 562). Also, Rushe et al. (2004) using functional magnetic resonance imaging (fMRI) reported young adults born very preterm with callosal damage have reduced lateralization of language function to the left hemisphere.
The present study investigates language and corpus callosum function in patients with schizophrenia and examines the hypothesis that the abnormalities are related. As a measure of callosal function, we used the Crossed Finger Localisation Task (CFLT; Satomi et al., 1991) as a measure of interhemispheric transfer of somatosensory information. In this task, a fingertip on one hand is touched by the experimenter and the subject's task is to respond by touching with their thumb the corresponding finger on the other hand. In humans, the digits of each hand are represented almost exclusively in the contralateral somatosensory cortex (Zaidel & Sperry, 1977), so the comparison of information presented to one hand is held to require transfer of sensory information across the corpus callosum. Patients with complete (Volpe et al., 1982) and partial resection of the corpus callosum (Geffen et al., 1985) are impaired on this task. This task was chosen because it is a task that is low in terms of cognitive processing demands, and as it does not involve the processing of linguistic stimuli, any relationship with language function is unlikely to be due to generalized linguistic processing deficits. Two measures of language function were selected. The FAS task of verbal fluency has reliably been shown to demonstrate language deficits in patients with schizophrenia (Bokat & Goldberg, 2004). The Perin's Spoonerisms task (Perin, 1983) was used as a measure of phonological processing, as previous research suggests that phonological processing is the aspect of language function that is preferentially disrupted by damage to the corpus callosum (Temple et al., 1989). A further aim of the study was to explore the relationship between language and callosal function to gender and motor dominance and the clinical features of schizophrenia.
METHODS
The study received full approval from the local research ethics committee.
Subjects
Thirty-five Diagnostic and Statistical Manual, Fourth Edition (DSM-IV), schizophrenia patients were recruited from the Home First Community and Mater Hospital Belfast Trusts. Mean duration of illness was 10 years, 5 months (SD, 96 months; range, 12–372 months), with a mean age at onset of 28 years (range, 14–53 years). Six patients were taking typical neuroleptic medication and 27 atypical medications. The Krawiecka Scale was used to measure severity of positive and negative symptoms (Krawiecka et al., 1977). Total scores for positive symptoms range from 0 (no symptoms) through to 12 (severe symptoms). The mean positive symptom score was 2.37 (SD, 2.31; range, 0–8), and the mean negative symptom score was 2.19 (SD, 2.17; range, 0–8).
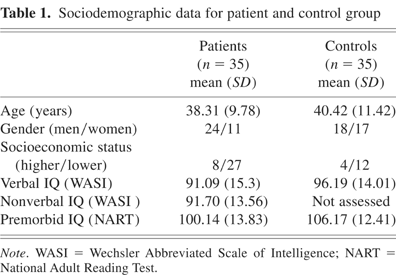
Thirty-five normal volunteers were recruited from the local population to serve as the comparison group. Table 1 shows the data for age, gender, socioeconomic status, current verbal IQ (Wechsler Abbreviated Scale of Intelligence), and premorbid IQ as assessed using the National Adult Reading Test (NART). Between-group comparisons using analysis of variance and χ2 yielded no significant results. Nonverbal IQ is presented for the patient group only. The mean score suggests average performance.
Sociodemographic data for patient and control group
Hand and foot preference was assessed using the Motor Dominance Demonstration Test (MDDT; Seisedos et al., 1999). This strategy differs from self-report measures in that subjects demonstrate their preferred hand through the performance of five one-handed actions and foot preference through the performance on two one-footed actions.
Exactly the same proportion of patients and controls (27 of 35) were rated as right handed (all tasks performed with the right hand) on the MDDT. Of the 35 patients, 21 were right footed (both tasks performed with the right foot) compared with 26 of the 35 controls. This difference was not significant (χ2 = 1.62, df = 1, p > .2). As the footedness measure yielded a more even distribution of scores in the patient group, it was selected as the measure of cerebral dominance for subsequent analysis. Furthermore, footedness is considered a better measure of functional asymmetry than handedness (Elias et al., 1998).
CFLT
The CFLT (Satomi et al., 1991) assessed the subjects' ability to transfer tactile information from one hemisphere to the other. Out of view of the subject, the experimenter touches the tip of a finger on one hand and the subject must then identify which finger was touched either on the same hand (SAME condition) or on the opposite hand (CROSSED condition) by bringing the thumb of that hand to touch the appropriate finger. After several practice trials (maximum of four) with vision, the test trials without vision began. In the first series of trials, only one finger was touched at a time, followed by a series of trials in which two fingers were touched consecutively. For the SAME condition, 8 trials on each hand on which only one finger was touched, were followed by 8 trials for each hand where two consecutive finger tips were touched, giving a total of 32 trials. The same number of trials was presented in the same order for the CROSSED condition. One point was given to each correct response, giving a maximum total score of 32 trials for the SAME condition and a maximum total of 32 for the CROSSED condition.
Assessment of Language Function
Verbal fluency was measured as the sum of words produced beginning with F, A, then S over three 1-min intervals. The Perin's Spoonerisms test was used as a measure of phonological processing (Perin, 1983). For this task, subjects switch the first sounds at the beginning of each of two words (e.g., Chuck Berry–Buck Cherry). A total of 16 pairs of words were presented and the total correct “spoonerisms” were recorded.
RESULTS
Crossed Finger Localization
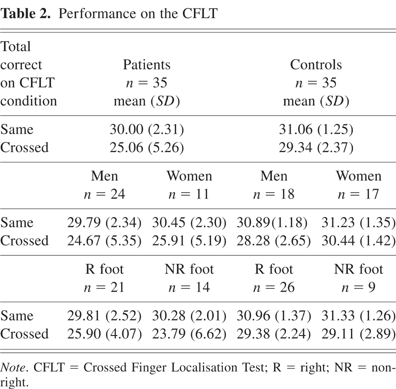
The descriptive statistics for patients and controls, men and women, right footers and non–right footers on the CFLT are presented in Table 2. Two separate analysis of variance tests with Group (Patient and Control), Gender (Men and Women), and Motor Dominance (Right, Non-Right Footedness) as between-subjects factors were conducted on data from the SAME condition and the CROSSED condition. The results revealed a main effect for Group for the CROSSED [F(7,62) = 12.00, p < .001; with patients performing worse] but not the SAME condition [F(7,62) = 2.81, p > .05]. The main effect for Gender or Motor Dominance was not significant for either the SAME or CROSSED conditions, nor were there any significant interactions.
Performance on the CFLT
Language Function
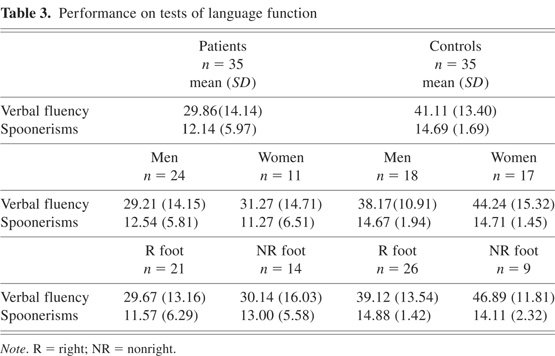
The descriptive statistics for patients and controls, men and women, right footers and non-right footers on the tests of language function are presented in Table 3. Analysis of variance with Group, Gender, and Motor Dominance as the between-subjects factors revealed a main effect for Group for the FAS test of verbal fluency [F(7,62) = 10.44, p < .005], but not the Spoonerisms test of phonological processing [F(7,62) = 2.84, p = .097]. The possibility of a ceiling effect limiting the power of parametric analyses to detect a significant effect prompted the use of nonparametric tests (the Mann–Whitney test), but still the difference was not significant. The effect of Gender or Motor Dominance was not significant for either test of language function, nor were there any significant interactions.
Performance on tests of language function
Relationship Between CFLT and Language Function
Only the patient group demonstrated significant correlations between Crossed CFLT and FAS performance (Pearson R = .46, p < .005) and between Crossed CFLT and spoonerism performance (Spearman r = .40, p < .05). These correlations remained significant after controlling for gender and motor dominance in partial correlations (R = .48, p < .01 and R = .39, p < .05, respectively). To preclude the possibility that correlations between performance on the language tests and CFLT in the patient group reflect a generalized performance deficit, partial correlations controlling for nonverbal IQ were conducted. The association between CFLT and verbal fluency remained (R = .50, p = .018), but the correlation between spoonerism and CFLT performance was only marginally significant (R = .40, p = .06). Figure 1 is a scatter plot showing the relationship between CFLT scores and verbal fluency scores. Finally, a series of correlations between performance on the CROSSED condition and on the language tests with background clinical variables (ratings of positive and negative symptoms, duration of illness, and age at onset) revealed no significant results.

Scatter plot of scores on the Crossed Finger Localisation Test (CFLT) and verbal fluency for the patient group.
DISCUSSION
Patients with schizophrenia were impaired on a measure of verbal fluency, but on the Spoonerism test, the difference was not significant. On the CFLT, they were impaired on the CROSSED but not the SAME condition, suggesting a particular difficulty in cross-callosal transfer of tactile information. CFLT crossed performance was significantly associated with performance on tests of language function in the patient group, but not the control group. This finding is consistent with our hypothesis that functional abnormalities of the corpus callosum are related to language deficits in schizophrenia.
That schizophrenia patients are impaired on measures of language production is not new (Bokat and Goldberg, 2004, for review). Phonological processing deficits were also expected, but the impairment failed to reach statistical significance. Inspection of the data revealed a ceiling effect and a much wider range of scores for the patient group. Thus, it would be premature, to conclude that patients with schizophrenia have normal phonological processing ability on this test, when the data suggest that for at least a proportion of such patients, clear impairments exist. It is possible that had we used a phonological processing task that did not impose a maximum score, for example a rhyming fluency task, a clear deficit would have been evident in the patient group.
The patients' deficit on the CFLT is consistent with other studies that suggest functional abnormalities of the corpus callosum in schizophrenia (Mohr et al., 2000). The significant correlations between CFLT performance and language function suggests that a similar mechanism may contribute to impaired performance on the tasks. It is unlikely this finding simply reflects a general impairment in cognitive processing, as the association remained significant after controlling for a measure of nonverbal IQ. However, other interpretations of the CFLT performance deficit exist. For example, Satomi et al. (1991) concluded that tactile anomia and limb apraxia accounted for impaired CFLT performance in their callosal patient. Our patient group showed no evidence of such impairments in their performance on the SAME conditions of the CFLT; however, performance was close to ceiling. A more difficult task may have elicited subtle deficits in the sensorimotor aspects of the CFLT. Subtle difficulties with the motor aspects of language production, and difficulties with the sensorimotor aspects of the CFLT may explain the positive correlation between CFLT and the language tasks. Alternatively, although selected as a nonverbal task, performance on the CFLT may have been facilitated by the use of verbal strategy, such as naming or counting the fingers (Beaton et al., 2006). Another possible confound is that of working memory load. In contrast to other studies that used three and four finger sequences trials (Beaton et al., 2006), we deliberately limited our trials to one and two finger sequences so as to reduce working memory load. Future studies should manipulate working memory load experimentally to assess whether working memory deficits contribute to performance on the CFLT and/or mediate the relationship with CFLT performance and language function in this group.
While we are mindful that correlations do not suggest causality, one interpretation of the association between CFLT performance and language function could be that both are due to disruption of white matter connectivity (including the corpus callosum) in schizophrenia. White matter abnormalities are frequently reported in schizophrenia (Hulshoff Pol et al., 2004); a disease that has become increasingly recognized as a disorder of disconnection. Crow (1998) hypothesized that, in schizophrenia, reduced lateralization of linguistic functions to the left hemisphere is due to abnormal connectivity between the hemispheres by means of the corpus callosum. Witelson and Nowakowski (1991) proposed that axon loss during pre- and postnatal brain development is the mechanism involved in determining cerebral dominance. Furthermore, a recent study by Powell et al. (2006) combined diffusion tensor imaging (DTI) and fMRI and reported that greater functional lateralization (measured using fMRI) is associated with greater leftward asymmetry in fractional anisotropy (i.e., greater organization of white matter fibers in the left compared with the right hemisphere). In schizophrenia, Hulshoff Pol et al. (2004) reported reduced density of the corpus callosum and anterior commissure, and these reduced interhemispheric tract densities were positively correlated with anterior cortical and subcortical gray matter densities. The authors speculated that developmental disturbance of the corpus callosum might be responsible for reduced hemispheric specialization of the frontal and temporal lobes in schizophrenia.
In contrast to many previous studies in patients with schizophrenia (Satz and Green, 1999), we found no evidence of an increase in non–right-sided motor dominance, and no effect of motor dominance CFLT performance. The lack of significant main effects may be due to the small number of participants who were right footed. It has been suggested that degree of motor dominance, as assessed by more continuous measures of performance (for example, finger tapping tests) may be a more reliable index of cerebral asymmetry than either hand or foot preference (Beaton, 1997). A more reliable measure of motor dominance would permit further testing of the hypothesis that reduced hemispheric specialization (of motor function) is related to callosal function in schizophrenia, as has been shown in disorders such as developmental dyslexia (Beaton, 1997). The lack of association with clinical variables was also unexpected, but might be explained by the fact that our sample of patients had moderately few symptoms, as is characteristic of a more chronic group.
The present data are offered tentatively as being consistent with the view that, in schizophrenia, deficits in language and callosal function have a common neurological basis. There are several limitations to this conclusion, not least the validity of our interpretation of CFLT performance as a callosal deficit. Furthermore, in the absence of reliable measures of working memory and motor function, we cannot conclude the presence of specific relationships between callosal and language function. Although we can hypothesize that certain neuropsychological (e.g., verbal) functions will have a common neuroanatomical basis (e.g., corpus callosum immaturity or white matter abnormalities more generally), a more direct test of the hypothesis requires the combination of functional and structural neuroimaging techniques, such as functional connectivity using fMRI and DTI to measure structural connectivity.
ACKNOWLEDGMENTS
We thank all the participants who took part in this study. This study was conducted without funding from any body external to the employing institutions of the authors.






