INTRODUCTION
The Weigl colour-form sorting test (Weigl, Reference Weigl1927) is a widely used clinical test of executive function, used to assess patients with frontal lobe damage (Crawford & Parker, Reference Crawford and Parker1992; Lezak et al., Reference Lezak, Howieson, Bigler and Tranel2012; Woods & Clare, Reference Woods and Clare2008). Executive functions are thought to be supported primarily by the frontal lobes and are generally considered to comprise a variety of general-purpose control mechanisms that modulate and organize more basic cognitive sub-processes (e.g., Stuss & Alexander, Reference Stuss and Alexander2007). Although several tests of executive function have been found to be specific to frontal lobe dysfunction (see Shallice & Cipolotti, Reference Shallice and Cipolotti2018), most are too time-consuming and complex for use with patients with severe impairments. In contrast, the Weigl is relatively simple, making it suitable for brief bedside assessment. The Weigl is considered to be a precursor to the, more complex, Wisconsin Card Sorting Test (Berg, Reference Berg1948) in requiring set shifting (MacPherson et al., Reference MacPherson, Della Sala, Cox, Girardi and Iveson2015).
The Weigl consists of 12 geometric shapes: a circle, a square, and a triangle, each in green, yellow, blue, and red. Without assistance, the patient is required to sort the pieces by colour or form and then to shift to the other sorting principle. Patients are considered to have passed the test if they are able to provide both sorting categories.
The Weigl has been used with patients with a range of aetiologies, including stroke (Chan et al., Reference Chan, Altendorff, Healy, Werring and Cipolotti2017; Gregoire et al., Reference Gregoire, Scheffler, Jäger, Yousry, Brown, Kallis and Werring2013; Leeds et al., Reference Leeds, Meara, Woods and Hobson2001), traumatic brain injury (Baird et al., Reference Baird, Papadopoulou, Greenwood and Cipolotti2005; De Luca et al., Reference De Luca, Maggio, Maresca, Latella, Cannavò, Sciarrone and Calabrò2019), dementia (Byrne-Davis et al., Reference Byrne-Davis, Bucks and Cuerden1998; Chiu et al., Reference Chiu, Papma, de Koning, Donker Kaat, Seelaar, Reijs and van Swieten2012; Tian et al., Reference Tian, Bucks, Haworth and Wilcock2003), Parkinson’s disease (Hobson et al., Reference Hobson, Meara and Taylor2007), psychiatric illness (Tamkin, Reference Tamkin1980; Tamkin & Kunce, Reference Tamkin and Kunce1982; Tamkin et al., Reference Tamkin, Kunce, Blount and Magharious1984), and alcoholism (Tamkin & Dolenz, Reference Tamkin and Dolenz1991). However, few studies have investigated the performance of patients with focal lesions.
In the first report on the test, Weigl (Reference Weigl1927) described the performance of a patient (L.R.) who suffered an unspecified “frontal lobe injury.” L.R. was able to correctly provide the colour sorting solution, however, when asked to provide a different sorting principle he sorted the blocks by colour again. Weigl concluded that, rather than sorting according to an abstract principle, L.R.’s responses were “…forced upon him by the sensorially manifest aspects of the situation” (p. 9). McFie and Piercy (Reference McFie and Piercy1952) conducted a group study of Weigl performance in 74 patients with left or right frontal, parietal, fronto-parietal, or temporal lesions of mixed aetiology. They reported that, while 22 out of 42 patients with left hemisphere lesions failed the test, only 2 of the 32 right hemisphere cases did so. Moreover, in patients with frontal lesions, the proportion of patients who failed was higher in patients with left (7/12) versus right (1/17) sided lesions. The authors only commented on the specificity of the test to frontal lobe damage in their patients with left hemisphere lesions. They noted that it appeared that a similar proportion of patients with left frontal (7/12) and left parietal (7/18) lesions failed the test, and that it seemed that a smaller proportion of left temporal patients (2/6) did so. Based on this, the authors argued that Weigl performance was sensitive to left hemisphere lesions rather than frontal damage. Crucially, modern methods of lesion localisation were unavailable at the time this study was conducted. In contrast to McFie and Piercy’s (Reference McFie and Piercy1952) findings, Hobson and colleagues (Reference Hobson, Meara and Taylor2007) found that, in a group on 105 stroke patients, there was no significant difference in Weigl performance between patients with left versus right hemisphere lesions. Unfortunately, this study did not include patients with focal frontal lesions. To the best of our knowledge, no further group studies of Weigl performance in patients with focal lesions have been reported. Indeed, MacPherson and colleagues (Reference MacPherson, Della Sala, Cox, Girardi and Iveson2015) did not discuss the Weigl in their Handbook of frontal lobe assessment due to a “…lack of evidence regarding specific prefrontal involvement when performing the task…” (p. 133). Thus, it remains unknown if the Weigl is a test specific to frontal lobe damage.
A further issue concerning the diagnostic significance of Weigl performance is whether performance is underpinned by fluid intelligence. One influential hypothesis is that impairments on tests of executive functioning following frontal lobe lesions can be best explained by disruption to general control processes, which match the requirements of the task being undertaken, independently of the type of information being processed (e.g., Duncan, Reference Duncan2001; Miller and Cohen, Reference Miller and Cohen2001). Evidence from neuroimaging has shown that the so-called multiple-demand network is associated with a wide range of cognitive operations, and it has been proposed that this putative network is the seat of general fluid intelligence (Woolgar et al., Reference Woolgar, Parr, Cusack, Thompson, Nimmo-Smith, Torralva and Duncan2010). In support of this, Roca et al. (Reference Roca, Parr, Thompson, Woolgar, Torralva, Antoun and Duncan2010) found that reduced fluid intelligence was sufficient to explain their frontal patients’ performance on several tests of executive functioning, including the Wisconsin Card Sorting Test. However, in contrast to this proposal, reduced fluid intelligence following focal frontal lobe lesions has been found to be insufficient to explain deficits on several tests of executive functioning, including the Stroop, phonemic fluency, design fluency, Proverbs, and Hayling Sentence Completion tests (Cipolotti et al., Reference Cipolotti, Molenberghs, Dominguez, Smith, Smirni, Xu and Chan2020; Cipolotti et al., Reference Cipolotti, Spanò, Healy, Tudor-Sfetea, Chan, White and Bozzali2016; Murphy et al., Reference Murphy, Shallice, Robinson, MacPherson, Turner, Woollett and Cipolotti2013; Robinson et al., Reference Robinson, Shallice, Bozzali and Cipolotti2012). Whether reduced fluid intelligence is sufficient to explain impaired Weigl performance following frontal lesions has yet to be established.
The aim of our retrospective study was to compare performance on the Weigl in a sample of patients with focal, unilateral, left or right, frontal, and non-frontal lesions. Both overall performance and errors were analysed. We also sought to investigate whether impaired Weigl performance could be explained by reduced fluid intelligence.
METHODS
Participants
Patients with brain lesions, who attended the Neuropsychology Department of the National Hospital for Neurology and Neurosurgery (Queen Square, London, UK), were retrospectively evaluated for eligibility. The following exclusion criteria were employed: (i) no Weigl data; (ii) non-focal unilateral brain lesion; (iii) current or previous psychiatric disorder; (iv) previous neurological disorders; (v) previous head trauma; (vi) chemotherapy previous to neuropsychological investigations; (vii) history of alcohol or drug abuse; (viii) inability to speak or understand any English; (ix) significant visual impairment; or (x) global aphasia.
A total of 83 patients (37 frontal, 46 non-frontal) with focal unilateral lesions met the inclusion criteria for the study. These patients were assessed between October 2004 and December 2019. The aetiology was stroke (27 frontal, 36 non-frontal patients) or tumour resection (10 frontal, 10 non-frontal patients). Diagnosis for all patients was confirmed by neurological investigations including neuroimaging (MRI or CT). Importantly, we have previously shown the grouping together of patients with focal, unilateral, and lesions caused by stroke or tumour resection to be methodologically justifiable (Cipolotti et al., Reference Cipolotti, Healy, Chan, Bolsover, Lecce, White and Bozzali2015; MacPherson et al., Reference MacPherson, Allerhand, Gharooni, Smirni, Shallice, Chan and Cipolotti2020). The study was carried out in accordance with the Declaration of Helsinki and approved by the NRES Committee London – Queen Square.
Neuropsychological Investigation
We retrospectively reviewed the performance of the 83 patients with Weigl data on a single neuropsychological assessment. This assessment comprised a series of well-known tests with published standardised normative data. Estimated premorbid function was assessed using the 50-item National Adult Reading Test (Nelson, Reference Nelson1982). Fluid intelligence was measured using performance IQ of the Wechsler Adult Intelligence Scale – third edition (WAIS-III; Wechsler, Reference Wechsler1997), known to load on fluid intelligence (Kaufman & Lichtenberger, Reference Kaufman and Lichtenberger1999). For 15 patients, fluid intelligence was assessed using Raven’s Advanced Progressive Matrices (Raven, Reference Raven1976), a test also known to load on fluid intelligence (Court & Raven, Reference Court and Raven1995). For these patients, Raven’s Advanced Progressive Matrices percentile scores were converted to IQ scores using a conversion table (Spreen et al., Reference Spreen, Sherman and Strauss2006). Thus, fluid intelligence was measured using WAIS-III performance IQ or Raven’s Advanced Progressive Matrices, depending on which test had been administered (see Cipolotti et al., Reference Cipolotti, Molenberghs, Dominguez, Smith, Smirni, Xu and Chan2020 for a similar method). Basic level visual perception was assessed using the 20-item Incomplete Letters test from the Visual Object and Space Perception Battery (Warrington & James, Reference Warrington and James1991). Visual and verbal recognition memories were assessed using the 25-item Short Recognition Memory Tests for Faces and Words (Warrington, Reference Warrington1996). Naming was assessed using the 30-item Graded Naming Test (GNT; McKenna & Warrington, Reference McKenna and Warrington1980). Executive functioning was measured using a test of phonemic fluency (number of words beginning with “s” generated within 60 s; Spreen & Strauss, Reference Spreen and Strauss1998).
The Weigl was administered as a part of the assessment, using the method described by Weigl (Reference Weigl1927). Patients were presented with the 12 blocks and asked to sort them. Following completion of the first sort, they were asked to group the blocks again but in a different way. After each sort, the clinician asked the patient to explain how they had grouped the blocks. We used the same scoring adopted by McFie and Piercy (Reference McFie and Piercy1952). Providing both the solutions of colour and form was classed as a pass. Providing only one of the two solutions, colour or form, or providing none of the two solutions was classed as a fail. When patients failed, we classified the error according to whether the patient provided the same solution twice (e.g., colour, colour) or produced a pattern that followed neither the principle of colour or form (e.g., pattern of mixed colour and form).
Neuroimaging
All patients were classified based on T2 or susceptibility weighted MRI (n = 77) or CT (n = 6) scans. Lesions were traced using MIPAV (https://mipav.cit.nih.gov/) and segmented and normalised to Montreal Neurological Institute (MNI) stereotaxic space using statistical parametric mapping-5 software (Wellcome Department of Imaging Neuroscience, London, England; www.fil.ion.ucl.ac.uk; see Cipolotti et al., Reference Cipolotti, MacPherson, Gharooni, van-Harskamp, Shallice, Chan and Nachev2018 for a description of the full procedure). Patients were classified as having frontal or non-frontal lesions using templates based on Brodmann area maps provided with MRIcron (http://www.sph.sc.edu/comd/rorden/mricron). Frontal patients were identified as those with a lesion in any part of the brain anterior to the central sulcus and superior to the lateral fissure (see Robinson et al., Reference Robinson, Shallice, Bozzali and Cipolotti2012 for a similar method). In patients classified as frontal, the average extent of overlap between the lesion and frontal areas was 90.51% (SD = 8.03). In patients classified as non-frontal, the average extent of overlap between the lesion and frontal areas was 4.36% (SD = 6.80). The distribution of the frontal and non-frontal patients’ lesions is displayed in Figure 1. In three cases, scans could be viewed but not processed for lesion segmentation due to a technical error. In these cases, patients were classified based on visual inspection of scans and radiological reports. Importantly, when all analyses were re-run with these three cases excluded the pattern of results remained unchanged. Aetiology and location of each of the frontal patients’ lesions are presented in Supplementary Table 1.

Fig. 1. Lesion distribution volume map for (a) Frontal and (b) Non-Frontal patients. Results are displayed on transversal slices (numbers indicate MNI coordinates) of the ch2better.nii.gz template in MRIcron (https://www.nitrc.org/projects/mricron). The colour code indicates in how many patients a given voxel was lesioned.
Three analyses were carried out: standard, laterality, and frontal-laterality. In the standard analysis, comparisons were made between patients with focal frontal (n = 37) or non-frontal (n = 46) lesions. In the laterality analysis, comparisons were made between patients with focal left (n = 41) or right (n = 42) hemisphere lesions. In the frontal-laterality analysis, comparisons were made between patients with left (n = 18) or right (n = 19) frontal lesions.
Statistical Analyses
All statistical analyses were conducted using SPSS. Skewness and kurtosis were assessed, by inspecting boxplots, and homogeneity of variances was assessed, using Levene’s test. In all cases where Levene’s test showed that the error variances between the groups differed significantly, data were transformed using square root or log transformation. If error variances remained unequal, non-parametric statistics were used (Mann–Whitney U tests).
For all three standard, laterality and frontal-laterality analyses, independent t tests were used to compare groups in terms of demographic variables (age, years of education, and chronicity—days between stroke/tumour resection and neuropsychological assessment) and neuropsychological performance. Fisher’s exact test was used to compare the groups in terms of gender. This test was also used to compare the proportion of patients who passed or failed the Weigl. In patients who failed the Weigl, Fisher’s exact test was used to compare the groups in terms of the proportion of patients who produced specific errors, for example, those who produced the same solution twice. When significant differences were found between groups in terms of the proportion of patients who passed or failed the Weigl or produced specific errors, the sensitivity and specificity of these variables were also calculated (e.g., sensitivity of Weigl pass/fail to Frontal vs. Non-Frontal group membership). The relationship between performance on the Weigl and fluid intelligence was investigated using point-biserial correlation.
RESULTS
Standard Analysis
In the standard analysis, the Frontal and Non-Frontal groups were well matched for age, gender, years of education, chronicity, and lesion volume (all p > .05; see Table 1). Similarly, there were no significant differences between the groups in terms of NART IQ, Fluid intelligence, Incomplete Letters, Short RMT Words and Faces, or GNT scores (all p > .05). However, patients with frontal lesions had significantly lower Phonemic Fluency scores than patients with non-frontal lesions, t(31) = −1.77, p < .05, d = −0.66.
Table 1. Demographics, neuropsychological, and Weigl performance
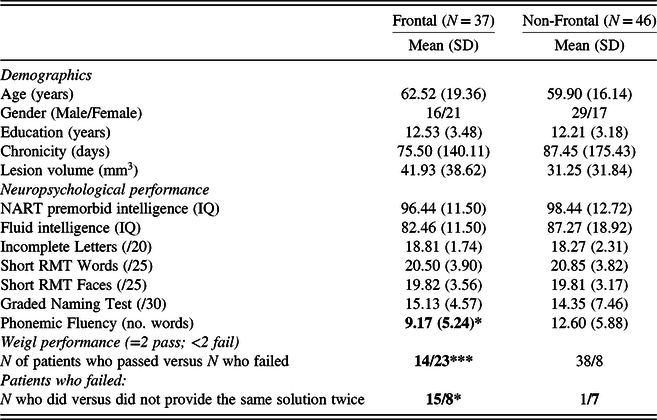
GNT = Graded Naming Test; RMT = Recognition Memory Test; NART = National Adult Reading Test; SD = Standard Deviation.
Note: Bold indicates significant difference between the Frontal and Non-Frontal groups.
* p < .05; *** p < .001.
There was a highly significant difference in the proportion of Frontal (23/37) versus Non-Frontal (8/46) patients who failed to produce both correct solutions on the Weigl, χ 2 (1, N = 83) = 17.57, p < 0.001, ϕ = 0.460. Failure to produce both correct solutions had modest sensitivity (.62) but good specificity (.83) when considering whether patients belonged to the Frontal group compared with the Non-Frontal group. Of the patients who failed to produce the two correct solutions, a significantly greater proportion of Frontal patients than Non-Frontal patients produced the same solution twice, χ 2 (1, N = 31) = 6.61, p < 0.05, ϕ = 0.462. Producing the same solution twice also had modest sensitivity (.65) but good specificity (.88) when considering whether patients belonged to the Frontal group compared with the Non-Frontal group. Only one (frontal) patient made the error of producing a pattern that followed neither the principle of colour or form; thus, it was not meaningful to analyse this error type.
Weigl performance (pass/fail) was not significantly correlated with Fluid intelligence in the whole sample (r pb = .168, n = 52, p = .12), or in the Frontal (r pb = .161, n = 28, p = .21), and Non-Frontal groups (r pb = .094, n = 24, p = .331).
Laterality Analysis
In the laterality analysis, there were no significant differences between the Left and Right Hemisphere groups for age, gender, years of education, chronicity, or lesion volume (all p > .05). There were no significant differences between the groups in terms of NART IQ, Fluid intelligence, Short RMT Words and Faces, GNT, or Phonemic Fluency scores (all p > .05). Patients with right hemisphere lesions had significantly lower Incomplete Letters scores than patents with left hemisphere lesions, U = 361.00, p < .01, r = −.32.
There was no significant difference in the proportion of Left (16/41) versus Right (15/42) Hemisphere patients who failed to produce both correct solutions on the Weigl nor between the Left and Right Hemisphere patients in terms of the proportion of patients who provided the same solution twice.
Frontal-Laterality Analysis
In the frontal-laterality analysis, Left and Right Frontal groups were well matched for age, gender, years of education, chronicity, and lesion volume (all p > .05). There were also no significant differences between the groups in terms of NART IQ, Fluid intelligence, Incomplete Letters, Short RMT Words and Faces, or Phonemic Fluency scores (all p > .05). Patients with left frontal lesions had significantly lower GNT scores than patents with right frontal lesions, U = 31.00, p < .05, r = −.45.
On the Weigl, there was no significant difference between the proportion of Left (11/18) and Right (12/19) Frontal patients who did not produce both correct solutions on the test nor between the proportion of Left and Right Frontal patients who provided the same solution twice.
DISCUSSION
To the best of our knowledge, this is the first group study that has investigated the specificity of the Weigl to frontal lobe lesions in a sample of patients with focal unilateral frontal or non-frontal lesions. We found two robust results. First, a highly significantly greater proportion of patients with frontal lesions failed to produce both solutions on the test than patients with non-frontal lesions. Second, a significantly greater proportion of frontal patients produced the same solution twice than did non-frontal patients. These results are unlikely to be explained by confounding factors, as patients with frontal and non-frontal lesions were well matched in terms of demographic variables and performance on neuropsychological tests, with the exception that frontal patients were impaired on another test of frontal executive dysfunction (Phonemic fluency). Thus, the current findings suggest that the Weigl is a reliable measure of frontal executive dysfunction.
Of relevance to clinical practice, we found that failure to produce both solutions on the Weigl and production of the same solution twice had modest sensitivity but good specificity to frontal lesions. Thus, although the Weigl may not be well suited as a screening measure, where higher sensitivity over specificity is desirable, the test may be useful as a reasonably specific diagnostic marker of frontal executive dysfunction.
We found no significant difference in Weigl performance between patients with left versus right hemisphere lesions. Moreover, a similar proportion of left (11/18) versus right (12/19) frontal patients failed the test. These results are consistent with the findings of Hobson and colleagues (Reference Hobson, Meara and Taylor2007), who also reported no significant difference in Weigl performance between stroke patients with left or right hemisphere lesions. In contrast, McFie and Piercy (Reference McFie and Piercy1952) found that a greater proportion of patients with left hemisphere lesions failed the Weigl. Furthermore, 7/12 of their left frontal patients and only 1/17 of their right frontal patients failed the test. The reason for this discrepancy is unclear and requires further investigation.
An issue of both clinical and theoretical importance is whether Weigl performance can be explained by a deficit in fluid intelligence. We found that, although frontal patients were more impaired on the Weigl than non-frontal patients, there were no significant differences between these groups in fluid intelligence. In addition, Weigl performance was not significantly correlated with fluid intelligence in the whole sample, or in patients with frontal or non-frontal lesions. Hence, our findings suggest that Weigl represents another example of an executive task for which impairment cannot be accounted for entirely by fluid intelligence (see Cipolotti et al., Reference Cipolotti, Molenberghs, Dominguez, Smith, Smirni, Xu and Chan2020 for further examples). This is notable, given that Roca and colleagues (Reference Roca, Parr, Thompson, Woolgar, Torralva, Antoun and Duncan2010) found that performance on the Wisconsin Card Sorting Test could be explained by reduced fluid intelligence in patients with focal frontal lesions. Although further investigation is required, this suggests that the Weigl may be a purer measure of executive functioning.
As the Weigl is generally administered to patients with relatively severe deficits (MacPherson et al., Reference MacPherson, Della Sala, Cox, Girardi and Iveson2015), it is important to acknowledge that our sample was somewhat biased toward such patients. Thus, the current findings may not necessarily generalise to all patients with focal lesions. Further investigation of Weigl performance in frontal patients with a wider range of abilities is warranted.
In conclusion, our findings suggest that the Weigl is a useful test to assess executive dysfunction following frontal lobe damage. Both a failure in producing both solutions and repetition of the same solution are useful to discriminate between frontal and non-frontal patients.
Acknowledgements
This project was funded by the Wellcome Trust (grant numbers 066763, 089231/A/09/Z) and supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre. JM was funded by the National Brain Appeal.
CONFLICT OF INTEREST
The authors have nothing to disclose.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/S1355617720000739




