Published online by Cambridge University Press: 12 February 2004
Performance on some neuropsychological tests is best expressed as an intra-individual measure of association (such as a parametric or non-parametric correlation coefficient or the slope of a regression line). Examples of the use of intra-individual measures of association (IIMAs) include the quantification of performance on tests designed to assess temporal order memory or the accuracy of time estimation. The present paper presents methods for comparing a patient's performance with a control or normative sample when performance is expressed as an IIMA. The methods test if there is a significant difference between a patient's IIMA and those obtained from controls, yield an estimate of the abnormality of the patient's IIMA, and provide confidence limits on the level of abnormality. The methods can be used with normative or control samples of any size and will therefore be of particular relevance to single-case researchers. A method for comparing the difference between a patient's scores on two measures with the differences observed in controls is also described (one or both measures can be IIMAs). All the methods require only summary statistics (rather than the raw data from the normative or control sample); it is hoped that this feature will encourage the development of norms for tasks that use IIMAs to quantify performance. Worked examples of the statistical methods are provided using data from a clinical case and controls. A computer program (for PCs) that implements the methods is described and made available. (JINS, 2003, 9, 989–1000.)
The assessment of neuropsychological deficits in the individual case normally involves comparing a patient's score on a neuropsychological test (e.g., number of items correct) with the distribution of scores obtained from an appropriate control or normative sample. However, for some neuropsychological constructs and their related measurement procedures, it is necessary, or at least preferable, to quantify performance through the use of an intra-individual measure of association (IIMA). These measures of association can either be parametric or non-parametric correlation coefficients, or the slopes of regression lines. Evaluation of an individual patient's performance is made by comparing the magnitude of the index of association observed for the patient with those of a normative or control sample.
The examination of memory for temporal order provides an obvious example of the use of an IIMA. Typically, performance on a temporal order memory task is quantified by computing the intra-individual (rank order) correlation between the actual order in which items (e.g., words or pictures) were presented, and the patient's memory for this order (Shimamura et al., 1990). The correlation computed from the patient's data can be compared with the average correlation obtained from a control or normative sample (a low correlation would suggest impairment); see Mayes et al. (2001) for a recent example.
As another example, in the area of motor control, there is a high intra-individual correlation between maximum grip aperture when reaching for objects and the size of the objects (Carey, 1996). An investigator may wish to determine whether this relationship breaks down in an individual with neurological disease. This could be examined by comparing the slopes of the intra-individual regression lines (relating object size and aperture size) obtained from healthy participants with the slope of the regression line obtained from the patient. Relatedly, there is a positive relationship between the peak velocity of manual reaching and the distance from a target; the further away the target, the faster the peak velocity (Jeannerod, 1984). Again, an investigator may wish to examine whether this relationship breaks down in cases with neurological disease; see Carey et al. (1998) for an example.
As a fourth example, in the area of object recognition, it has been found that there is a robust intra-individual relationship between the latency with which an object is named and the degree to which it is rotated away from its prototypical orientation (Turnbull et al., 1997). Failure to find such an association in a patient would suggest that mental rotation was not being used to achieve object identification; see Turnbull et al. (2002) for a recent single-case study. Distance estimation tasks provide yet another example from the area of visual perception; here the issue would be whether a patient exhibits an attenuation of the expected relationship between visually estimated distance and actual distance (Carey et al., 1998).
IIMAs have also been employed in the area of auditory perception. For example, Steinke et al. (2001) used measures of tonality to assess melody recognition in a patient with right hemisphere damage. Performance on each of four tests of tonality (Steinke et al., 1997) was quantified by computing the Spearman rank order correlation between a participant's ratings of tonality and music-theoretic predicted levels of tonality. Estimation of weight (Jones, 1986) provides another example where IIMAs can be used (to compare estimated against actual weight); estimation of weight has been found to be impaired in a number of neurological conditions (see, e.g., Heindel et al., 1991; Lafargue & Sirigu, 2002).
Finally, in the investigation of time estimation, accuracy can be assessed by examining the strength of the intra-individual relationship between the length of the actual time intervals and the estimated time intervals. There is evidence that, particularly when the time intervals exceed 20 s (Richards, 1973; Sherwin & Effron, 1980), amnesic patients exhibit deficits in time estimation as indicated by a weakening of the association between actual and estimated time (Nichelli et al., 1993; Venneri et al., 1998). Indeed, in severe cases, the direction of the relationship can be reversed (e.g., the correlation, or slope of the regression line, has a negative sign), thereby indicating that shorter intervals are estimated as longer.
In the foregoing examples the breakdown or weakening of an intra-individual association found in healthy participants would suggest impairment. However, in some circumstances an investigator may seek evidence of impairment through demonstrating that there is a strengthening of an intra-individual relationship in a patient. For example, it might be found that a patient's performance on a primary task is differentially sensitive to the degree of working memory load (e.g., number of digits retained) imposed by a secondary task. Were this the case, then one would expect to find a higher (negative) correlation between working memory load and primary task performance in the patient than in the healthy control participants.
In all of the foregoing examples, correlation coefficients, or slopes are treated as data. It is not uncommon for correlation coefficients to be used in this way; see Howell (1997) for a brief commentary. A particularly pertinent example is provided by the previously mentioned study by Shimamura et al. (1990) on temporal order memory. In this study, t tests and ANOVAs were used to compare the mean intra-individual correlations (between the actual and reported order of the stimulus items) obtained from healthy and amnesic samples. Venneri et al. (1998), in their study of time estimation in amnesic and control samples, used ANOVA to compare the mean slopes (relating actual elapsed time to estimated elapsed time) obtained from the amnesic and control participants.
In both these examples, inferential statistics were used to test differences between two samples. In contrast, both academic neuropsychologists who study single cases, and clinical neuropsychologists, are concerned with comparing an individual with a normative or control sample. However, just as the group researcher is concerned with whether group differences are statistically significant, so single case researchers or clinicians would wish to determine whether the observed difference between their patient and a normative or control sample was statistically significant. More generally, neuropsychologists have an interest in estimating the abnormality or rarity of a patient's performance; that is, they wish to estimate the proportion of the healthy population that would be more extreme than their patient. The remainder of this paper is concerned with developing statistical methods to address these needs.
The range of potential solutions to these problems is constrained by the fact that the normative or control samples, against which an individual is to be compared, will often be modest in size. Among the reasons for this is the fact that theoretical advances in neuropsychology continue to occur at a rapid rate, whereas the collection of large-scale normative data is a time-consuming and often arduous process (Crawford, 1996). Thus, neuropsychologists may continue to have access only to provisional normative data long after a new measure has been developed; these norms may be no more than control sample data from an experimental study. Second, when performance on a neuropsychological measure is best expressed as an intra-individual measure of association, a specific factor that may have discouraged collection of norms is the lack of explicit, practical guidance on how to analyse and interpret an individual's score when data are in this form. It is hoped that the methods presented here will help remove this obstacle.
The need to develop methods that are suitable for use with small control or normative samples is perhaps most apparent when one considers single-case research. Within academic neuropsychology there has been a resurgence of interest in single-case studies and this has led to significant advances in our understanding of normal and pathological cognitive function (Caramazza & McCloskey, 1988; Code et al., 1996; Ellis & Young, 1996; Humphreys, 1999; McCarthy & Warrington, 1990; Shallice, 1988). In many of these studies the theoretical questions posed cannot be addressed using existing instruments and therefore novel instruments are designed specifically for the study (Shallice, 1979). The sample size of the control or normative group recruited for comparison purposes in such studies is typically below 10 and often less than 5. In passing, it may be noted that the control group need not be healthy participants; for example, one can envisage many hypotheses that state that a particular patient will exhibit a breakdown in an association of interest in contrast to samples of patients having other clinical features in common.
For the reasons outlined above it is clear that statistical methods that treat the normative or control statistics as parameters (i.e., treat the normative sample as if it were a population rather than a sample) would have limited applicability. In this respect, the need for an appropriate method of dealing with intra-individual measures of association, is directly analogous to the simpler case where the researcher or clinician wishes to compare a conventional test score for a patient (e.g., number of items passed on a memory test) with a control or normative sample.
The ‘standard’ procedure for statistical inference in this latter situation is well known. When it is reasonable to assume that scores are normally distributed, the patient's score is converted to a z score, based on the mean and standard deviation in the normative sample, and evaluated using tables of the area under the normal curve (Howell, 2002; Ley, 1972). Thus, if the researcher or clinician has formed a directional hypothesis concerning the patient's score prior to testing (e.g., that the score will be below the mean), then a z score which fell below −1.64 would be considered statistically significant (using the conventional 5% level). More generally, the procedure provides the neuropsychologist with information on the rarity or abnormality of the individual's score. This method treats the normative sample statistics as if they were parameters. When the N of the normative sample is large this is not problematic. However, it is problematic when, for example, the sample consists of only 10 persons.
Drawing on work by Sokal and Rohlf (1995), Crawford and Howell (1998) presented a method of comparing an individual's score with a normative sample in which the sample statistics are used as sample statistics rather than treated as population parameters. The method is a modified independent samples t test in which the individual is treated as a sample of N = 1 and, therefore, does not contribute to the estimate of the within-group variance. The formula is

where X1 = the individual's score, X2 = the mean score of the normative sample,

= the standard deviation of the scores in the normative sample, and N2 = the sample size. The degrees of freedom for t are N2 + N1 − 2, which reduces to N2 − 1. This method can be used to determine if an individual's score is significantly different from that of the normative or control sample. More generally, it provides an unbiased estimate of the abnormality of the individual's score; i.e., if the p value (one-tailed) for t was calculated to be 0.03 then it can be estimated that only 3% of the healthy population would exhibit a score lower than that observed for the individual.
Crawford et al. (1998) extended this approach to cover circumstances where the neuropsychologist wishes to compare the difference between a pair of test scores (e.g., scores on verbal versus spatial short-term memory tasks) observed for an individual, with the distribution of differences observed in a control or normative sample. Crawford and Garthwaite (2002) also extended it to permit comparison of the differences between an individual's scores on each of k tests and the individual's mean score on the k tests with the differences between these quantities in a control or normative sample.
In the present paper we further extend this general approach to cover circumstances where the clinician or researcher wishes to compare an intra-individual measure of association obtained from a patient with a normative or control sample. Before presenting these methods it should be noted that an additional consideration in developing them was that they should only require summary statistics from the control or normative sample and the patient, rather than the raw data. This was motivated by three considerations. First, the summary statistics required are easily obtained from any standard statistical package. Second, working with the summary statistics is less time consuming for the user. Third, by requiring only summary data, this should encourage the development of norms for neuropsychological measures for which performance is best expressed as an IIMA. That is, publication of the summary statistics from a normative or control sample would be sufficient for independent researchers or clinicians to use the norms with their own patients.
The methods set out below can be used to compare an individual with a normative sample when performance is expressed as a Pearson correlation coefficient (i.e., a parametric coefficient) or a non-parametric coefficient (Spearman Rank Order Correlation Coefficient or Kendall's Tau). The only information required is N and the correlation coefficients for each of the members of the control or normative sample and the patient.
The original intention had been to also present methods for comparing the slope of a patient's regression line with those of controls. However, the procedures required in this latter case are more complicated (partly because more information is available when working with slopes). Prior to testing for a difference in slopes a sequence of tests has to be run. These examine, (1) if the error variances of the controls are homogenous, (2) if the error variance of the patient differs significantly from controls, and, if so, whether (3) there appears to be little variation between the controls in their slopes. The results from these pre-tests determine which of three formulae should be used to compare the patient's slope with controls. As these methods are very different from the methods for correlation coefficients, and require lengthy computations, we have prepared a follow-up paper that deals with slopes (Crawford & Garthwaite, 2003).
When performance on the task of interest is expressed as a Pearson (i.e., parametric) correlation coefficient, we let ρi denote the correlation coefficient for the ith person and let ri denote its sample estimate, based on k trials or items. The sampling distribution of correlation coefficients is not normally distributed and so we apply Fisher's transformation to the coefficients for the individuals in the normative or control sample and the patient:
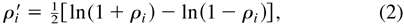
and,

then approximately,

We assume that ρ1′ … ρn′ are values from a normal distribution,

That is, we accept that the true values of the (transformed) correlations differ between individuals. We denote their mean value in the normative population by R and their variance by λ2. Also, we assume these true values (ρi′, i = 1… N) follow a normal distribution. We want to test the null hypothesis that ρn+1′ (the true correlation coefficient for the patient) is from the same distribution. Assuming k is sufficiently large (i.e., > 10) for the approximation in equation (4) to hold, then r1′…rn′ are a simple random sample from the normal distribution,

This last equation (6) shows that ri′ varies from R both because ri′ varies about ρi′ (with variance 1/(k − 3)), and because ρi′ varies about R (with variance λ2). The variance of ri′ is the sum of these components of variance. If the null hypothesis holds, then rn+1′ is also from the distribution in (6). We calculate the mean and standard deviation of the transformed correlations in the control sample, i.e.,

and

Then, if rn+1′ (the patient's observed correlation) comes from the same normal distribution as ri′…rn′,

has a t distribution on N − 1 degrees of freedom. If the null hypothesis is rejected at the conventional .05 level, then the patient's correlation is significantly different from that of the controls. Furthermore, if the precise one-tailed probability for t is multiplied by 100 then we have an estimate of the percentage of the population that would obtain a correlation lower than the patient's.
It can readily be appreciated that formula (9) is directly equivalent to the formula employed by Crawford and Howell (1998). In Equation (1) the difference between a patient's score on a neuropsychological test (e.g., number of items passed) and the mean score of the control or normative sample is divided by the standard error of the mean of the control sample. In the present case, where performance on the neuropsychological task is represented not by a conventional score but by a correlation coefficient, the difference between the patient's transformed coefficient and the mean transformed coefficient of the control sample is divided by the standard error of the mean coefficient.
A potential alternative means of testing the difference between the patient's transformed correlation and the mean correlation in the controls would be simply to convert the patient's transformed correlation to z, based on the mean and SD of the controls, and refer this z to a table of the area under the normal curve. (Such a method could also be used with the other forms of correlation coefficients dealt with in the present paper.) However, as when comparing a patient's performance on a conventionally scored test with controls, this method is inappropriate, since it treats the control sample statistics as if they were population parameters. The practical effect of using this alternative method would be to exaggerate the abnormality of the patient's performance and to spuriously inflate the chance of finding statistically significant effects. A comparison of the results from these methods is provided in the worked example (see later section).
The above method is designed to yield a point estimate of the rarity or abnormality of a patient's IIMA when the IIMA employed is a correlation coefficient. However, it would also be desirable to obtain confidence limits on the abnormality of the patient's IIMA. That is, using (9) we obtain a point estimate of the percentage of the population that will perform more poorly than the patient but now we wish to obtain confidence limits on this percentage. Such an aim is in keeping with the contemporary emphasis in statistics, psychometrics, and biometrics on the use of confidence limits (American Psychological Association, 2001; Daly et al., 1995; Gardner & Altman, 1989; Zar, 1996). Gardner and Altman (1989), for example, in discussing the general issue of the error associated with sample estimates note that “these quantities will be imprecise estimates of the values in the overall population, but fortunately the imprecision itself can be estimated and incorporated into the findings” (p. 3). Similarly the American Psychological Association (2001) take the view that confidence limits or intervals represent, “in general the best reporting strategy. The use of confidence intervals is therefore strongly recommended” (p. 22). The use of confidence limits is particularly appropriate when results are obtained from small samples.
To generate confidence limits on the abnormality of this form of IIMA we use a result obtained by Crawford and Garthwaite (2002). Let P denote the percentage of the population that will fall below a given individual's transformed correlation (rn+1′), and suppose we require a 100(1 − α)% confidence interval for P. As in the formula for the point estimate, let (rn+1′ − r′) represent the difference between the individual's score and the mean score of the normative or control sample, let

be the standard deviation in the normative sample, and let N be the size of the normative sample. We assume scores for the control population are normally distributed. If we put

then c is an observation from a non-central t distribution on N − 1 degrees of freedom. Non-central t-distributions have a non-centrality parameter that affects their shape and skewness. We find a value of this parameter, δU, such that the resulting non-central t-distribution has

as its 100α/2 percentile. Then we find the value δL such that the resulting distribution has

as its 100(1 − α/2) percentile. From tables for a standard normal distribution we obtain

These probabilities depend upon α, c and N and we denote them by h(α/2;c; N) and h(1 − α/2;c; N), respectively. Then a 100(1 − α)% confidence interval for P may be written as

From equation (3), ri′ is a monotonically increasing function of ri, so (h(α/2;c; N), h(1 − α/2;c; N)) is also a 100(1 − α)% confidence interval for the percentage of the population that will fall below a given individual's untransformed correlation, rn+1. Details of the derivation of h are given in Crawford and Garthwaite (2002) and a worked example of obtaining 95% confidence limits on the rarity of an IIMA is provided in a later section.
When performance on the task of interest is expressed as a non-parametric correlation coefficient, i.e., Spearman Rank Order Correlation Coefficient or Kendall's Tau, we suggest that, as was done with Pearson correlations, Fisher's transformation is applied to the coefficients of the control or normative sample and the coefficient of the patient. Kraemer (1974), in reviewing inferential methods for rank order coefficients notes that Fisher's transformation “has proved successful as a transformation which, when applied to several forms of correlation coefficients, is approximately normally distributed with variance independent of ρ” (p. 114). Fieller et al. (1957) and Fieller and Pearson (1961) showed that the approximation is good for Spearman's Rank Order correlation coefficient and Kendall's Tau for k > 10 and |ρ| < .9. Therefore, if comparing the non-parametric coefficient obtained for a patient with a control or normative sample, the procedure is identical to that for parametric correlation coefficients. That is, the mean and SD of the transformed coefficients are calculated and entered into formula (9) along with the transformed coefficient for the patient. Similarly, if confidence limits on the abnormality of the patient's score are required, the procedure described for parametric coefficients should be followed.
To illustrate the methods we use the example of the test of temporal order memory developed by Shimamura et al. (1990). In this task participants are read a list of 15 words and are then given the words on individual cards and asked to attempt to put them in the order in which they were originally presented. A 34-year-old female patient with a left frontal lesion was administered this test and the Spearman Rank Order correlation between the actual order of the words and the order produced by the patient was .210; the transformed correlation was .213. It will be noted that k in this example (the number of items used to compute the coefficient) is 15 and is thus sufficiently large for Fisher's transformation to give approximately normal distributions.
In the course of studying the effects of ageing on memory and executive functions, Crawford et al. (2000) obtained data for this task from 111 healthy persons. Because we wish to illustrate the use of this method when the control or normative sample is modest in size, we selected 14 of these participants to match the patient in terms of gender, age and years of education. Mean age in the control sample was 34.2 (SD = 2.69) and mean years of education was 12.0 (SD = 1.51); the patient had 13 years of education. Table 1 presents the Rank Order Correlation Coefficients and the transformed correlations for the controls. The mean of the transformed correlations in the controls is .637 with a SD of .180. Entering these data into formula (9) yields the following result,

Correlation (r) and transformed (r′) correlation between order and reported order on a temporal order memory task in 14 control participants (the correlations are Spearman Rank Order Correlation Coefficients); the final column presents raw scores on a free recall task, used in the example of testing for dissociations.

As the hypothesis tested by the researcher or clinician in this example is directional, that is, that the patient's performance will be significantly lower than matched controls, a one-tailed test is appropriate. The one-tailed critical value for t at the 5% level on 13 degrees of freedom is 1.77. The individual's score is, therefore, significantly different from the controls at the 5% level. The exact one-tailed probability for t in this example is .0202 and so the expectation is that only 2.02% of individuals in the population from which the normative sample was drawn would obtain a score as low as that observed for the patient. To obtain 95% confidence limits on this percentage we proceed as follows:

We want a non-central t distribution on N − 1 = 13 degrees of freedom that has −8.815 as its 0.975 quantile. This determines the non-centrality parameter to be −12.652 so we put δL = −12.652. We also want a non-central t distribution on 13 df that has −8.815 as its 0.025 quantile. This gives δU = −4.892. Then,

Hence the 95% lower confidence limit for P is 0.04% and the upper limit is 9.56%. To summarize the results for this case: the patient's memory for temporal order was significantly poorer (p < .05) than controls and it is estimated that only 2.02% of the population would exhibit a score poorer than that observed; the 95% confidence interval on this percentage is .04% to 9.56%.
As noted in a previous section, a potential alternative means of testing the difference between the patient and controls would be simply to convert the patient's transformed correlation to z. It is informative to compare this alternative with the proposed method for the present example. The patient's correlation expressed as a z score is −2.36. Referring this z to a table of the normal curve reveals that the estimated percentage of the population that would obtain a correlation lower than this is 0.9%. This exaggeration of the abnormality of the patient's performance would be even more pronounced with smaller control samples. Furthermore, in the present example, the conclusion from application of both the t test and z is that the patient is significantly impaired (p < .05). However, obviously these methods need not be in agreement. For example, if the patient's transformed correlation had been .335, then z (−1.68) would be significant (p < .05). However, this would be a spurious result arising from treating the sample as a population; the t-test would not be significant (t = 1.62, p > .05).
As another example of the use of these methods, Steinke et al. (1997) conducted a single-case study of a patient, C.N., who had suffered bilateral temporal lesions. Steinke et al. administered a series of tests of music perception to C.N. and 6 matched controls. Performance on the tests of music perception was quantified by the rank order correlation between an individual's ratings of the stimulus items (e.g., of tonality) and music-theoretic predicted levels. C.N. performed poorly on these tasks. No inferential method was employed to test whether C.N. differed significantly from controls but this can be readily achieved with the present method. For example, on a test of novel melodies the correlation obtained for C.N. was .46 (hence r′ is .497). The mean of the Fisher transformed correlations (r′) in the 6 controls was 1.29 with a SD of .305. Entering these data into formula (9) yields a t of 2.406 and a one-tailed probability of .031. Therefore, C.N.'s performance was significantly poorer than controls. The confidence interval on the percentage of the population that would obtain a lower score (i.e., a lower correlation) than C.N. was from .008% to 20.3% (the point estimate was 3.1%).
The emphasis in the foregoing discussion of these methods has been on their use with modest control or normative samples. However, it should be stressed that these methods are applicable to comparison of an individual with normative samples of any size. Indeed methods such as these (i.e., methods that use the t distribution rather than the standard normal distribution) are in fact technically the correct way to make a comparison against any normative sample because our normative samples are always just that, samples rather than populations. Of course, if the normative sample is large, then methods that use the t distribution and those that treat the sample statistics as population parameters will converge; that is, z can be used to provide an adequate approximation to t with large Ns.
The amount of normative data on IIMA tasks is currently limited, at least in part, because there has been little guidance on how to use such data to draw inferences concerning an individual's performance. It is to be hoped that the development of these methods will help to remedy this situation. To illustrate, suppose a researcher standardized a temporal order memory task on a large sample of the general adult population. To make this available as normative data the researcher would simply need to report the mean and SD of the Fisher transformed correlations between actual and reported order (separate means and SDs could be reported for different age groups etc. if required). The end user would then enter these statistics into formula (9) along with the transformed correlation for their patient (most statistics texts provide a table for converting between r and r′). In order to make this process as convenient as possible, the program that accompanies this paper (see later section) offers the option of entering the mean and SD of the transformed correlations for the normative or control sample and the raw (i.e., untransformed) correlation obtained from the patient.
We hope that the availability of these methods will encourage the development of normative data for tasks in which performance is quantified using an IIMA. In the interim (i.e., prior to development of large-scale normative data) the methods allow researchers and clinicians to use IIMAs with the modest data that are already available (including, in the case of single-case studies, data collected from a control sample).
Up to this point we have been concerned with methods of testing for a significant deficit on a single task. Although the ability to identify a deficit in the individual case is fundamental, the presence of a deficit in a given cognitive function often only acquires theoretical importance when it is accompanied by the absence of a deficit in other related functions. That is, a central aim in many neuropsychological case studies is to fractionate the cognitive system into its constituent parts, and this aim is pursued by attempting to establish the presence of dissociations of function. Typically, if a patient obtains a score in the impaired range on a test of a particular function and is within the normal range on a test of another function, this is regarded as evidence of a dissociation. However, this evidence in isolation may not be at all convincing (Crawford & Garthwaite, 2002). For example, a patient's score on the “impaired” task could lie just below the cut-point for defining impairment and the performance on the other test lie just above it. Therefore, a more stringent test for the presence of a dissociation would also involve a comparison of the difference between tests observed for the patient with the distribution of differences between these same tests in the control sample (Crawford et al., 2003).
As previously noted, Crawford et al. (1998) devised a method that can be used to test whether the difference between an individual's score on two tasks is significantly different from the differences observed in a control sample. This method can, therefore, provide an additional test for the presence of a dissociation. However, it is also useful in the converse situation where a patient's scores are within the impaired range on both tasks. When this pattern is observed, the researcher can still test whether the magnitude of the difference between the two tasks is abnormal; i.e., evidence can be sought for the presence of a differential deficit on the test of one of the functions.
The method was developed for use with tasks in which performance is quantified by conventional means (e.g., number of items correct). For example, Crawford et al. (1998) use the example of testing whether the difference between a patient's performance on a verbal short-term memory task and a spatial short-term memory was significantly larger than the differences in a control sample. However, their method can be just as applicable when performance on one or both of the tasks is expressed as an IIMA. The formula for this test, which is essentially a modified paired samples t test, is

where ZX and ZY are the scores of an individual on Test X and Test Y expressed as z scores formed using the means and SDs of the control sample, rxy is the correlation between Tests X and Y in the control sample, and N2 is the number of participants in the control sample. The test statistic follows a t distribution on N2 − 1 degrees of freedom. Multiplying the one-tailed probability of t by 100 gives the point estimate of the abnormality of the individual's score. A derivation for the formula can be found in Appendix 1 of Crawford et al. (1998).
The use of this method is best illustrated with an example. Let us suppose that the patient whose temporal order memory performance was used to illustrate the method for correlations had also been administered a conventional verbal free recall task involving recall of 15 words. Given the evidence that temporal order memory is differentially impaired following frontal lesions in comparison with free recall (e.g., Shimamura et al., 1991), we could test the directional hypothesis that the patient's temporal order memory will be significantly poorer than her performance on free recall.
The healthy participants (N = 14) selected from Crawford et al.'s (2000) sample to serve as controls for this patient had in fact been administered the verbal free recall task described above. Their mean score (i.e., number of words correctly recalled) was 9.07 with a SD of 2.46 (range = 5–14). The raw scores of the controls on this task are presented in the final column of Table 1 (thereby providing potential users of this method with all the data required to work the example from scratch). Suppose that the patient's score on the free recall task was 8. As recorded earlier, the patient's score on the temporal order task (i.e., the correlation between the actual and reported order of items) was .213 after applying Fisher's transformation. Also as reported earlier, the mean and SD of the transformed correlations in the control sample was .637 with a SD of .180. The only remaining statistic required to test for a dissociation between free recall and temporal order memory is the correlation between performance on the two tasks in the control sample; this (Pearson) correlation was .51 (note that this correlation is computed from the free recall scores and the transformed correlations of the controls).
Using the means and SDs of the controls, the patient's score on the free recall task expressed as a z score is −0.435 and the z score for temporal order is −2.356. We will designate the free recall task as Test X and the temporal order task as Test Y (the choice is arbitrary). Entering these data into formula (12) we obtain t:

The one-tailed probability for a t of 1.87 on 13 degrees of freedom is .042. We would conclude, therefore, that the patient's performance on the temporal order task was significantly poorer than performance on free recall; that is, there is evidence of a dissociation between memory for temporal order and free recall of equivalent stimuli. In addition, by multiplying this p value by 100 we have an estimate of the percentage of the healthy population that would exhibit a discrepancy in favor of recall larger than that observed for the patient (4.2%); that is, discrepancies of this magnitude are fairly rare. A confidence interval on this percentage can be obtained using a method devised by Crawford and Garthwaite (2002). In the interests of brevity we do not provide a worked example here but the 95% confidence interval for this example is from 0.23% to 15.3%.
As another example, we return to Steinke et al.'s (1997) study of impaired music perception in case C.N. An important aim of Steinke et al.'s case study was to test whether music perception could be dissociated from non-musical cognitive abilities. To examine this, C.N. and controls were also administered a series of other tests, including subtests of the WAIS–R (Wechsler, 1981) and the Wisconsin Card Sorting Test (Heaton, 1981). Steinke et al. did not use an inferential method to test whether C.N.'s performance on music perception was significantly poorer than performance on these other tasks but this can readily be achieved using the present method. For example, C.N.'s score on the Wisconsin was 96 and the mean and SD in controls was 89.2 and 3.49, respectively (the correlation between performance on the novel melodies task and the Wisconsin in the controls was 0.83). Entering these data, together with the previously recorded data for the novel melodies task, into formula (12), yields a t of 7.22 and a one-tailed probability of .0004. Therefore, the discrepancy between C.N.'s performance on the music perception and non-musical task was highly significant and there is strong evidence for a dissociation.
The foregoing examples involved comparing the difference between a patient's performance on two tasks with the differences in controls when performance on only one of the tasks was expressed as an IIMA. However, as noted, Crawford et al.'s (1998) method is just as applicable when performance on both tasks is expressed as an IIMA. Therefore, the method can be used whenever there is a need to examine performance under different experimental conditions (i.e., comparison of a patient's correlation obtained under Condition A versus the correlation obtained under Condition B).
For example, Carey et al. (1998) examined the ability of a patient (D.F.) to visually estimate distance; they reported the correlations between actual and estimated distance for D.F. and controls under monocular and binocular conditions. Milner et al. (1991) hypothesized that cases such as D.F. should be markedly more impaired when distance is estimated using monocular versus binocular vision. The present method could be used to test this hypothesis by comparing the difference between the correlations under monocular versus binocular conditions for a patient against the differences between the correlations observed in controls. The indications are that, in the case of D.F., application of such a test would reveal a significant difference. Carey et al. (1998) found that the correlation between actual and estimated distance for D.F. was markedly higher under binocular conditions, whereas the correlations for two controls were very similar under monocular and binocular conditions.
As another example, Venneri et al. (1998) examined time estimation under single and secondary task conditions (the secondary tasks imposed demands on either working memory or attention). The method outlined could be used to examine whether a patient's performance is more sensitive than controls to the effects of these secondary tasks.
Furthermore, memory for temporal order can be tested with verbal and non-verbal stimuli (Mayes et al., 2001); in both cases performance is quantified by computing the correlation between actual and reported order. The method described could be used to test for a significant difference in performance on these two tasks in the individual case. Finally, it was noted that Steinke et al. (2001) have developed four tests of tonality and that performance on each of these tasks is quantified by computing the Spearman rank order correlation between a patient's ratings of tonality and music-theoretic predicted levels of tonality. If a researcher wished to test for possible dissociations between two of these different aspects of tonality the method described above could be used to test for their presence.
Before leaving this topic it should be noted that, in the illustrative examples, the concern was with testing whether there was a statistically significant dissociation between performance on the two tasks. However, in the search for dissociations, the aim is to uncover dissociations between functions, not the tests used as (imperfect) indicators of these functions. Therefore, it can be of some value to demonstrate that tests of a particular putative function provide similar indications of impairment (i.e., that they do not differ significantly). This is perilously close to attempting to prove the null hypothesis and therefore such evidence can play only a supporting role. That is, when there is evidence that was regarded as a unitary function, which can be fractionated into two new putative functions A and B (as indicated by the presence of significant differences between measures of A and B), the lack of significant differences among the tasks assessing each of these putative functions lends support to the view that these new constructs possess internal consistency.
The present methods test whether a patient's score (specifically, an IIMA) or score difference came from the same population as a control or normative sample and are distinct from a test on the reliability of differences. A reliable difference between an individual's test scores is one unlikely to have arisen from measurement error in the tests. However, many healthy individuals will have reliable differences among their abilities in different cognitive domains. Indeed, if the tests concerned have high reliability, reliable differences will be very common and therefore cannot be taken as indicating acquired impairment (see, e.g., Crawford, 2003; Crawford & Allan, 1996). Therefore, methods that quantify the probability that a patient's score difference was drawn from the distribution of score differences in the healthy population are more germane to the needs of clinicians and single-case researchers (Crawford & Garthwaite, 2002; Crawford et al., 1998).
Similarly, the confidence limits presented in the present paper are confidence limits on the estimated rarity or abnormality of a given score (specifically, an IIMA) or difference between scores. As noted, they allow the user to quantify the effects of error arising from using a sample in place of the population: they quantify the fallibility of normative or control sample data. These confidence limits are therefore distinct from confidence limits that quantify the effect of measurement error in a test instrument (or instruments) on an individual's score (or score differences). These latter limits are obtained by multiplying a test's standard error of measurement (or the standard error of measurement of the difference when concerned with score differences) by a standard normal deviate (i.e., 1.96 for 95% limits)
The tests developed in the present paper involve assumptions about the underlying distributions from which the normative data were sampled. In the case of the simple comparison of a patient's IIMA with a control mean, the assumption is that the control data were sampled from a normal distribution. In the case of testing for a dissociation, it is assumed that the differences between the two tasks follow a normal distribution. This latter assumption holds if scores on the two tasks follow a bivariate normal distribution but this condition is not essential. These same assumptions apply to the corresponding methods for obtaining confidence limits on the abnormality of an IIMA or difference (i.e., potential dissociation) between an IIMA and another task.
It follows that these procedures should be avoided when it is known or suspected that the control or normative data are markedly skewed or platykurtic–leptokurtic. It should be noted that the possible alternative method discussed in a previous section (i.e., treating the sample as a population and using z to evaluate a patient's performance) makes exactly the same assumption of normality and is equally compromised by nonnormality. When the control or normative samples are small, the neuropsychologist should also be alert to the presence of outliers. For example, in elderly control or normative samples it is not uncommon to observe occasional cases who perform very poorly despite the absence of any other evidence that suggests the presence of a brain pathology (e.g., early stage dementia).
When testing for differences between parametric (i.e., Pearson) correlations, a crucial assumption is that relationships between the variables involved are linear. A visual check of this assumption can be made using scatterplots for each individual case. If non-linearity is apparent, the neatest solution, if it works, is to transform the variables; for example, by taking their logarithms, square roots or reciprocals. Transformation of variables is discussed in all comprehensive statistics textbooks; for example, see Tabachnick and Fidell (1996) or Howell (2002). It must be emphasized that, if any of the aforementioned transformations are employed, the same transformation must be applied to the data from all cases (i.e., all control cases and the patient). However, if the aim is to detect dissociations between two tasks, where performance on one or both of which are expressed as an IIMA, it is perfectly acceptable to apply different transformations to the data from the two tasks (provided that, within each task, the same transformation is applied to all cases).
Finally, it will be appreciated that the statistical power of any method of statistical inference will decline as sample size decreases. Thus with the small Ns with which we are concerned it is inevitable that power will be low. The most obvious way of increasing power is to increase the size of the control or normative sample against which the individual's score is to be compared. Power can also be increased by adopting a more liberal significance level, e.g., 15% rather than 5%, but although this more liberal strategy will increase Type I errors (false positives), it will decrease Type II errors (false negatives). The decision to depart from the conventional 5% level should be based on the relative risks the researcher or clinician attaches to the occurrence of these two types of errors. The reasons for departing from the 5% level must be strong, as the 5% level has proved a good choice in general.
With the exception of the methods for finding confidence limits on the abnormality of a patient's IIMA, the calculations involved in the tests presented in the present paper are relatively straightforward. Armed with a set of basic statistical tables, they could be performed by hand or with the aid of a calculator or spreadsheet. However, as we are aware of the time pressures under which many neuropsychologists operate, we have written a computer program for PCs to accompany this paper. Apart from saving time, use of the program reduces the chance of arithmetic or clerical error. Furthermore, it provides a precise probability value for the tests, whereas tabled values of t only record the t value that must be exceeded to achieve a given level of significance. An exact probability is more useful in many situations as the emphasis will be on the rarity or abnormality of the individual's level of performance. For example, it is still of some value to have an estimate of the percentage of healthy individuals expected to exhibit a coefficient as extreme as the patient's when the t value for a given test does not exceed the most liberal tabled value (e.g., .10 in many tables). It also does away with the need for interpolation when a value falls between two tabled critical values.
The program IIMA.EXE compares the parametric or non-parametric correlation coefficient obtained for a patient with the coefficients of a control or normative sample. The program prompts for the number of cases in the control or normative sample (N), the correlation coefficient for the patient (rN+1) and the correlation coefficients for each of the controls. The program then applies Fisher's transformation to the coefficients. The output consists of the one and two-tailed significance of the difference between the patient and controls, the abnormality of the patient's coefficient (e.g., the estimated percentage of the population that would obtain a coefficient that was lower than the patient's) and 95% confidence limits on the abnormality. The output can be viewed on the screen, printed, or saved to a file.
As noted, the program incorporates an option of carrying out the test using the mean and SD of the transformed coefficients for controls rather than requiring that the user enters the untransformed correlations individually. This is useful if a researcher or clinician wishes to examine the performance of additional patients against the same controls at a later point (the M and SD of the transformed correlation is included in the output to enable users to use the program in this way). This option also makes it easy for a researcher to use normative data obtained from a third party.1
A compiled version of this program can be downloaded from the following web site address: www.psyc.abdn.ac.uk/homedir/jcrawford/iima.htm
If a researcher wishes to test for a dissociation between two tasks when performance on one or both of the tasks is expressed as an IIMA, then a program (DIFFLIMS.EXE) previously provided by Crawford and Garthwaite (2002) can be used.2
This program can be downloaded from http://www.psyc.abdn.ac.uk/homedir/jrawford/abnolims.htm
Computation of the mean and SDs and the correlation between tasks in the controls could be carried out in a statistics or spreadsheet package. If the package used does not offer Fisher's transformation then the correlations could be entered into the IIMA.EXE program as it provides the transformed correlations for each of the controls and patient as output. (It is likely that these data would be run through the IIMA.EXE program in any case as the researcher would normally wish to test whether the patient's IIMA was significantly different from controls before testing if there is evidence of a dissociation between the IIMA and performance on another task.) The transformed correlations could then be entered into the statistics or spreadsheet package so that the correlation between the IIMA scores and scores on the other task can be computed for the controls. If any doubts remain over how to conduct this test then the data from the worked example for dissociations could be run through the DIFFLIMS.EXE program to verify that the procedure is being followed correctly.
The single case approach in neuropsychology has made a significant contribution to our understanding of the architecture of human cognition (Caramazza & McCloskey, 1988; Code et al., 1996; Ellis & Young, 1996; Humphreys, 1999; McCarthy & Warrington, 1990; Shallice, 1988). However, as Caramazza (1988) notes, if advances in theory are to be sustainable they “must be based on unimpeachable methodological foundations” (p. 619). The statistical analysis of single case data is an aspect of methodology that has been relatively neglected. This is to be regretted. Other methodological (and logical) considerations may have compelled many researchers to abandon group-based research, but it is clear that the statistical problems associated with drawing inferences from single cases significantly exceed those of the former approach.
Very useful and elegant methods have been devised for drawing inferences concerning an individual patient's performance on fully standardized neuropsychological tests, that is, on tests that have been normed on a large representative sample of the population (e.g., Capitani, 1997; Willmes, 1985). However, in neuropsychology, new tests are constantly being devised to measure new theoretical constructs. Understandably, these tests are not fully standardized when employed with single cases; instead they are administered to a control sample that, typically, has a very modest N. Therefore, methods that treat the control sample statistics in such studies as population parameters are not appropriate. Although there remains much to do, we believe that the methods presented here make a useful contribution to the process of developing valid, optimal, and practical statistical methods for single-case research. To our knowledge the specific problems addressed in the present paper are not covered in any existing textbooks or papers in neuropsychology or psychological statistics. However, it is clear from the list of examples provided (which is by no means exhaustive) that intra-individual measures of association have a wide range of applications in neuropsychological research and practice. Finally, we reiterate our hope that these methods will encourage the development of larger scale normative data for tasks in which performance is best expressed as an IIMA.
We are grateful to Dr. Sytse Knypstra of the Department of Econometrics, University of Groningen, The Netherlands, for providing an algorithm that finds the non-centrality parameter of a non-central t distribution given a quantile, its associated probability, and the degrees of freedom. The algorithm is incorporated into the computer program IIMA.EXE that accompanies this paper. We are grateful to Dr. Willi R. Steinke (Halton District School Board, Toronto) and Professor Lola L. Cuddy (Queen's University, Ontario) for providing the raw data for their case C.N. and controls. Thanks also to Dr. David P. Carey (University of Aberdeen) for commenting on a draft version of this paper.

Correlation (r) and transformed (r′) correlation between order and reported order on a temporal order memory task in 14 control participants (the correlations are Spearman Rank Order Correlation Coefficients); the final column presents raw scores on a free recall task, used in the example of testing for dissociations.