INTRODUCTION
Neuroimaging studies of posttraumatic stress disorder (PTSD) suggest that PTSD is associated with functional and structural abnormalities in brain regions (e.g., prefrontal cortex, hippocampus, amygdala) relevant to fear and related emotions (Bonne, Grillon, Vythilingam, Neumeister, & Charney, Reference Bonne, Grillon, Vythilingam, Neumeister and Charney2004; Rasmusson, Vythilingam, & Morgan, Reference Rasmusson, Vythilingam and Morgan2003; Southwick, Rasmusson, Barron, & Arnsten, Reference Southwick, Rasmusson, Barron, Arnsten, Vasterling and Brewin2005; Yehuda, Reference Yehuda2002). Correspondingly, an emerging literature examining neuropsychological and information processing correlates of PTSD has revealed that PTSD in both military veterans and civilians is associated with cognitive abnormalities, particularly on tasks assessing learning, memory, and attention. Neuropsychological deficits appear to be circumscribed, with the most robust findings indicating impairment in acquiring new information (Gilbertson, Gurvits, Lasko, Orr, & Pitman, Reference Gilbertson, Gurvits, Lasko, Orr and Pitman2001; Jelinek, Jacobsen, & Kellner, Reference Jelinek, Jacobsen and Kellner2006; Koenen et al., Reference Koenen, Driver, Oscar-Berman, Wolfe, Folsom, Huang and Schlesinger2001), heightened sensitivity to interference on learning and memory tasks (Uddo, Vasterling, Brailey, & Sutker, Reference Uddo, Vasterling, Brailey and Sutker1993; Vasterling, Brailey, Constans, & Sutker, Reference Vasterling, Brailey, Constans and Sutker1998; Vasterling et al., Reference Vasterling, Duke, Brailey, Constans, Allain and Sutker2002; Yehuda et al., Reference Yehuda, Keefe, Harvey, Levengood, Gerber, Geni and Siever1995), and impairment in working memory, inhibition, and other executive aspects of attention (Gilbertson et al., Reference Gilbertson, Paulus, Williston, Gurvits, Lasko, Pitman and Orr2006; Jenkins, Langlais, Delis, & Cohen, Reference Jenkins, Langlais, Delis and Cohen2000; Koenen et al., Reference Koenen, Driver, Oscar-Berman, Wolfe, Folsom, Huang and Schlesinger2001; Leskin & White, Reference Leskin and White2007; McFarlane, Weber, & Clark, Reference McFarlane, Weber and Clark1993; Vasterling et al., Reference Vasterling, Brailey, Constans and Sutker1998). Deficits in memory consolidation and shift of attention have also been observed. On experimental tasks using emotionally valenced stimuli, PTSD is associated with attentional and memory biases to threat-relevant information (e.g., Constans, McCloskey, Vasterling, Brailey, & Mathews, Reference Constans, McCloskey, Vasterling, Brailey and Mathews2004; Dalgleish et al., Reference Dalgleish, Taghavi, Neshat-Doost, Moradi, Canterbury and Yule2003; Foa, Feske, Murdock, Kozak, & McCarthy, Reference Foa, Feske, Murdock, Kozak and McCarthy1991; McNally, Kaspi, Riemann, & Zeitlin, Reference McNally, Kaspi, Riemann and Zeitlin1990; McNally, Lasko, Macklin, & Pitman, Reference McNally, Lasko, Macklin and Pitman1995). Despite the vast gains in knowledge over the past decade regarding the neuropsychological correlates of PTSD, cross-sectional studies cannot address the direction of causation between neuropsychological compromise and PTSD. Although animal studies have suggested that stress may lead to neuropsychological compromise (Arnsten & Goldman-Rakic, Reference Arnsten and Goldman-Rakic1998; Birnbaum, Gobeske, Auerbach, Taylor, & Arnsten, Reference Birnbaum, Gobeske, Auerbach, Taylor and Arnsten1999; Ohl, Michaelis, Vollmann-Honsdorf, Kirschbaum, & Fuchs, Reference Ohl, Michaelis, Vollmann-Honsdorf, Kirschbaum and Fuchs2000; Shansky et al., Reference Shansky, Glavis-Bloom, Lerman, McRae, Benson and Miller2004), there is growing evidence that neurocognitive functioning may also serve as a risk/resilience factor for PTSD. Studies using archival pre-trauma estimates of general intellectual functioning derived from Army entrance examinations have generally revealed that the development of PTSD following trauma exposure is associated with lower pre-trauma estimates of intellectual functioning (e.g., Gale et al., Reference Gale, Deary, Boyle, Barefoot, Mortensen and Batty2008; Kremen, et al., Reference Kremen, Koenen, Boake, Purcell, Eisen and Franz2007; Macklin et al., Reference Macklin, Metzger, Litz, McNally, Lasko, Orr and Pitman1998), especially at lower levels of trauma exposure (Thompson & Gottesman, Reference Thompson and Gottesman2008).
Less is known, however, about the role that specific neurocognitive deficits, found to be commonly associated with PTSD (e.g., memory, inhibition, and attention deficits), play in either conferring risk or enhancing protection in developing PTSD following trauma. Using a sample of monozygotic twin pairs in which one twin was exposed to combat in Vietnam and the other was not, Gilbertson and colleagues found that twin pairs in which the exposed twin had PTSD showed smaller hippocampal volumes (Gilbertson et al., Reference Gilbertson, Shenton, Ciszewski, Kasai, Lasko, Orr and Pitman2002) and performed more poorly on measures of attention, executive functioning, and verbal memory (Gilbertson et al., Reference Gilbertson, Paulus, Williston, Gurvits, Lasko, Pitman and Orr2006) than twin pairs in which the exposed twin did not have PTSD. However, there were no significant differences in hippocampal volume or neurocognitive measures between brothers with PTSD and their nonexposed twins. Thus, hippocampal volume and neurocognitive functioning varied in part as a function of family membership, even when one brother had PTSD and the other did not. The results were interpreted to suggest that pre-exposure hippocampal volume and neurocognitive functioning may moderate the risk of developing PTSD following trauma exposure.
In a recent prospective investigation of civilian trauma survivors, Parslow and Jorm (Reference Parslow and Jorm2007) showed that more proficient pre-trauma performances on tasks of immediate and delayed verbal recall, verbal working-memory, visuomotor speed, and verbal intelligence were associated with lower post-trauma PTSD re-experiencing and arousal symptoms, with the strongest associations occurring in domains of immediate and delayed verbal recall, verbal intelligence, and working memory. The findings also revealed that participants with high levels of post-trauma PTSD symptoms showed a more attenuated improvement (i.e., showed less practice effects) on a word recall task from pre- to post-deployment than participants with low levels of post-trauma PTSD symptoms. Overall, their findings provided evidence that cognitive deficits on a number of tasks conferred additional risk of developing PTSD symptoms, but also suggested that more circumscribed deficits in verbal learning may result from PTSD symptoms. Because the study did not assess PTSD symptom levels prior to the trauma exposure, the extent to which baseline neurocognitive functioning was associated with the development of new PTSD symptoms versus any pre-existing symptoms is unclear. In addition, because PTSD avoidance and numbing symptoms were not measured, the degree to which cognitive functioning was associated with the full range of PTSD symptoms is unknown.
Nonetheless, these findings, when combined with those of Gilbertson and colleagues, provide provocative evidence that certain neurocognitive functions, perhaps in particular those reflecting prefrontal and hippocampal functions (e.g., sustained attention, inhibitory functions, and immediate and delayed memory, respectively), possibly moderate the relationship between trauma exposure and PTSD symptom development. This is especially intriguing when viewed in the context of the potential interplay between cognitive skills, coping, and the encoding and subsequent retrieval of trauma memories, which are thought to be necessary for adequate psychological resolution of the trauma (Ehlers & Clark, Reference Ehlers and Clark2000; Foa & Jaycox, Reference Foa, Jaycox and Spiegel1999; Foa & Riggs, Reference Foa, Riggs and Pynoos1994). For example, it is possible that less proficient memory and attention compromises the initial encoding of the trauma, resulting in poorly elaborated and fragmented trauma narratives that then promote impaired retrieval of the trauma event. Likewise, it is has been suggested that reduced cognitive ability to shift away from maladaptive cognitions or behaviors (Bremner et al., Reference Bremner, Innis, Southwich, Staib, Zoghbi and Charney2000; Shin et al., Reference Shin, Orr, Carson, Rauch, Macklin and Lasko2004) and decrements in the ability to gate or control trauma-related memories (Brewin, Reference Brewin2008; Vasterling & Brailey, Reference Vasterling, Brailey, Vasterling and Brewin2005) may promote the development of PTSD.
The primary goal of this article was to use data prospectively gathered before and after exposure to extreme stress (i.e., Iraq War deployment) to test the hypothesis that the integrity at baseline of cognitive processes linked to memory and reflective of prefrontal and hippocampal functioning (i.e., initial acquisition of information, memory retention, sustained attention, inhibition, working memory) would be associated with PTSD symptom outcome following stress exposure. Using a unique set of neuropsychological and PTSD self-report data collected on a large sample of U.S. Army soldiers before and after they deployed to Iraq, we extended the methodology of Parslow and Jorm (Reference Parslow and Jorm2007) by assessing PTSD symptoms both prior to and after stress exposure and by measuring the full range of PTSD symptoms.
Although our primary hypotheses centered on longitudinal relationships between pre-deployment neurocognitive functioning and post-deployment levels of PTSD symptoms, we also sought to extend prior cross-sectional findings by examining associations between PTSD symptoms and concurrent neurocognitive performances within a sample of active duty military personnel. This population extension facilitates examination of neurocognitive correlates of PTSD within a significantly briefer timeframe relative to stressor exposure than much of the prior research, in addition to allowing examination of a relatively healthy, nonclinically-recruited sample.
METHODS
Human subjects approvals were obtained from Human Subjects Research Review Boards of the Army, Tulane University Health Sciences Center, and Department of Veterans Affairs. All participants provided written informed consent prior to participation.
Participants
Participants were 668 active duty U.S. Army soldiers (n = 613 men; n = 55 women) deployed to Iraq between November 2003 and March 2005. These individuals were selected from 955 soldiers who deployed during the study period and who were enrolled in the Neurocognition Deployment Health Study (NDHS). Individuals were excluded from these analyses if they either did not perform the post-deployment assessment in person (n = 257; 27%) or demonstrated invalid response profiles (n = 21; 2%) or insufficient effort (n = 9; 1%) at any of the assessments. The predominant reason for nonparticipation at the post-deployment assessment was relocation to another military installation characteristic of standard military duty rotation (n =127; 49%). Other reasons included separation from the military (n = 62; 24%), leave or special assignments (n = 38; 15%), declined participation (n = 7; 3%), deployment at the time of assessment (n = 6; 2%), illness (n = 3; 1%), being deceased (n = 3; 1%), and unknown or unconfirmed relocation (n = 11; 4%).
Measures
Comprehensive descriptions of primary assessment data and secondary data obtained from automated military databases are provided elsewhere (Vasterling et al., Reference Vasterling, Proctor, Amoroso, Kane, Gackstetter, Ryan and Friedman2006a, Reference Vasterling, Proctor, Amoroso, Kane, Heeren and White2006b). Measures relevant to this report follow.
Demographic, neuromedical, and historical information
Each assessment documented current demographic and military information (e.g., age, gender, rank), risk factors for neuropsychological disorders (e.g., history of neurodevelopmental disorders, psychiatric disorders, brain injury), and factors potentially affecting neuropsychological performance. Brain injury incurred during the course of the study was queried by interview and defined as any self-reported head injury resulting in at least momentary loss of consciousness. Self-reported ethnicity data were gathered to help gauge the representativeness of the sample.
Performance-based neuropsychological tests
In line with the hypotheses, which centered on the prospective association between pre-deployment levels of neurocognitive performance and post-deployment reports of PTSD symptom levels, analyses included tests of immediate and delayed verbal and visual memory, sustained attention, working memory, and inhibitory functioning. To assess learning and memory, participants completed the Wechsler Memory Scale, 3rd edition (WMS-III; Wechsler, Reference Wechsler1997) Verbal Paired Associates and Wechsler Memory Scale (WMS; Wechsler, Reference Wechsler1945) Visual Reproductions tasks. These tasks were chosen to measure memory processes in the verbal-auditory and visual-spatial modalities, respectively. Verbal Paired Associates includes four cued recall learning trials and a delayed cued recall condition. Visual Reproductions requires reproduction of simple geometric designs after a single exposure followed by a delayed recall condition. Learning was measured on the Verbal Paired Associates task as the sum of items correctly recalled over the four learning trials. Percent retention was calculated as delayed recall/immediate recall × 100 for Visual Reproductions and delayed recall/Trial D × 100 for Verbal Paired Associates.
To assess sustained attention and inhibition, participants completed the Neurobehavioral Evaluation System, 3rd edition (NES3) Continuous Performance Task (CPT; Letz, Reference Letz2000), which requires detection of targets from distractor stimuli. Sustained attention impairment was measured by the number of omissions; disinhibition was measured by the number of false positive responses. All CPT error scores were log-transformed to adjust for nonnormal distributions. The NES3 vocabulary task was also administered to obtain an estimate of participants’ IQ. The NES3 vocabulary task is a computer-assisted 25-item multiple-choice test designed to estimate general verbal ability (Letz, Reference Letz2000) and is derived in part from the Armed Forces Qualifying Test – Verbal Subtest. Participants also completed the Trail Making Test, which assesses working memory and executive functioning (Reitan, Reference Reitan1958). Time to complete Part A (drawing lines between numerals in sequential order) was subtracted from time to complete Part B (drawing lines between sequential numbers and letters in alternation). The subtraction procedure parcels out basic attentional, psychomotor, and visual tracking skills, resulting in a better measure of working memory and cognitive flexibility.
All scores were free of subjective judgment except for Visual Reproductions, in which designs were scored by a primary rater according to set criteria. Reliability ratings performed on 10% of randomly selected drawings by a second rater blinded to unit and deployment status indicated high interrater reliability (Intraclass correlations: 0.75–0.95).
Combat and PTSD symptom severity
Combat severity was quantified by a modified version of the Deployment Risk and Resilience Inventory (DRRI; King, King, & Vogt, Reference King, King and Vogt2003) Combat Experiences module. The DRRI is a suite of self-report scales designed to assess risk and resilience factors important to modern military deployments. Evidence is available for the internal consistency reliability, test-retest reliability, discriminant validity, discriminative validity, and criterion-related validity of DRRI scales (King, King, Vogt, Knight, & Samper, Reference King, King, Vogt, Knight and Samper2006). The Combat Experiences module of the DRRI yields a continuous score that indexes combat intensity, with higher scores indicating greater combat intensity.
The PTSD Checklist (PCL; Weathers, Litz, Herman, Huska, & Keane, Reference Weathers, Litz, Herman, Huska and Keane1993) is a 17-item self-report scale that assesses posttraumatic stress symptom severity, providing a summary score of overall PTSD symptom severity. Higher scores indicate greater symptom severity. The scale items correspond directly to DSM-IV (American Psychiatric Association, 1994) symptom criteria, measuring the reexperiencing, avoidance and emotional numbing, and hyperarousal symptoms of PTSD. This widely used instrument has demonstrated coefficient alphas greater than .95, is highly correlated with other measures of PTSD, including the “gold standard” Clinician-Administered PTSD Scale (r = .93; Blake, Keane, Wine, & Mora, Reference Blake, Keane, Wine and Mora1990), and has demonstrated acceptable levels of discriminant validity relative to measures of other forms of psychopathology (Blanchard, Jones-Alexander, Buckley, & Forneris, Reference Blanchard, Jones-Alexander, Buckley and Forneris1996; Forbes, Creamer, & Biddle, Reference Forbes, Creamer and Biddle2001; Ruggiero, Del Ben, Scotti, & Rabalais, Reference Ruggiero, Del Ben, Scotti and Rabalais2003; Weathers et al., Reference Weathers, Litz, Herman, Huska and Keane1993).
Response validity
Validity of response profiles on questionnaires was assessed via inspection of scales with bidirectional items (e.g., “5” endorses pathological functioning on some items and intact functioning on others). If a respondent provided all extreme responses in the same direction on a scale with bidirectional items, that respondent’s data were not analyzed. The Test of Memory and Malingering (TOMM; Tombaugh, Reference Tombaugh1997) Trial 1 was administered to assess cognitive engagement. The data of participants scoring below 38, a cut-off found to show reasonable sensitivity and specificity in detecting insufficient effort on neurobehavioral tasks (O’Bryant, Engel, Kleiner, Vasterling, & Black, Reference O’Bryant, Engel, Kleiner, Vasterling and Black2007), were excluded from analyses.
Procedures
Participants completed paper-and-pencil survey and neuropsychological measures at both pre- and post-deployment assessments. Assessments were conducted at military installations by a civilian examiner team. All performance-based neuropsychological measures were individually administered according to scripted, standardized instructions. Participants completed paper-and-pencil surveys in small groups. Because soldiers participated in the study in a time of extraordinarily high operational demands, the study procedures were structured to optimize efficient use of their time, and the order of administration of the paper-and-pencil versus the neuropsychological tests was determined solely on the basis of immediate examiner availability and differed randomly across participants. Tests included in the current analyses were drawn from the larger set of neuropsychological tests administered as part of the primary study.
Statistical Analyses
Statistical analyses were performed using SPSS, v. 17.0. Descriptive statistics were computed for all study variables. When data distributions departed significantly from normal, raw scores were normalized via logarithmic transformation. Missing values for specific items on questionnaires were replaced by the mean value of the individual’s completed items for that measure if the participant responded to at least 50% of the items. If less than 50% of the items on a measure were completed, summary scores were not computed. Outliers were truncated at 3 standard deviations (SD) from the mean.
To examine the prospective and cross-sectional associations between PTSD symptom severity and neuropsychological functioning, a series of hierarchical linear regression analyses were conducted. Because of the high collinearity between immediate recall and retention memory measures, we performed two separate regression analyses: (1) one that included only the measures of immediate visual and verbal memory, and (2) another that included only the measures of delayed (retention) visual and verbal memory.
To answer the primary question of whether deficits in pre-deployment neurocognitive performances would negatively predict post-deployment PTSD symptom scores after taking into account pre-deployment PTSD symptoms, we entered pre-deployment PCL scores in the first step. Based on previous research demonstrating that female gender (Breslau, Kessler, &Chilcoat, Reference Breslau, Kessler and Chilcoat1998) and combat intensity (Orcutt, Erickson, & Wolfe, Reference Orcutt, Erickson and Wolfe2004; Perconte, Wilson, Pontius, Dietrick, & Spiro, Reference Perconte, Wilson, Pontius, Dietrick and Spiro1993) were associated with greater PTSD symptom severity, these constructs were included, along with participant age and test-retest interval, as covariates in the second regression step. Pre-deployment scores for the neurocognitive tests were entered in the third step.Footnote 1
To examine post-deployment cross-sectional associations between PTSD symptom severity and neuropsychological functioning, a series of hierarchical linear regressions were specified with post-deployment PCL score as the outcome variables for the analyses. In the first block, we entered covariates of participant age, gender, self-reported combat intensity, and test-retest interval. In the second regression block, we entered post-deployment performance scores for memory, response inhibition, working memory, and sustained attention.
RESULTS
Sample Characteristics
Table 1 presents pre-deployment characteristics of the final sample, as well as the characteristics of soldiers who participated at pre-deployment, but who were excluded or did not participate in Time 2 assessments. Participants in the final sample generally reflected the broader Operation Iraqi Freedom (OIF)-deployed Army population. Women were slightly underrepresented. Although enlisted personnel constitute the majority of deployers, commissioned officers were also underrepresented. Nonparticipants at post-deployment assessment reported less sleep and were more likely to be women, officers, have had a previous overseas deployment, used nonpsychoactive medication, and reported a history of neuromedical disorder than participants in the final sample. These groups, however, did not differ in pre-deployment PCL summary scores or estimated PTSD screening rates (i.e., PCL scores of 50 or greater and endorsement of the requisite number of symptoms in each of the DSM-IV symptom clusters). Furthermore, the participant and nonparticipant groups did not differ on pre-deployment performances of memory, inhibition, working memory, and sustained attention.
Table 1. Demographic and contextual sample characteristics at pre-deployment

Note
The sample size varies slightly across observations because of missing data. PCL = PTSD Checklist; WMS = Wechsler Memory Scale; WMS-III = Wechsler Memory Scale, 3rd edition; NES3 CPT = Neurobehavioral Evaluation System, 3rd edition, Continuous Performance Task.
Table 2 presents the means, standard deviations, and ranges for all continuous study variables among the participants. The standard deviation and range of the PCL scores and neurocognitive variables showed dispersion at each assessment. Inspection of the model residuals for the series of hierarchical regressions demonstrated normality and homoscedasticity.
Table 2. Means, standard deviations, and ranges for PTSD symptoms and neurocognitive performance measures at pre- and post-deployment (N = 668)

Note
PTSD = posttraumatic stress disorder; PCL = PTSD Checklist; WMS = Wechsler Memory Scale; WMS-3 = Wechsler Memory Scale, 3rd edition; NES3 CPT = Neurobehavioral Evaluation System, 3rd edition Continuous Performance Task; DRRI = Deployment Risk and Resilience Inventory.
Table 3 presents zero-order correlations computed among the variables of interest. Pre-deployment visual memory percent retention scores were significantly and negatively correlated with pre-deployment PCL scores; pre-deployment immediate visual memory scores were significantly and negatively correlated with post-deployment PCL scores. No other neuropsychological measures were correlated with either pre- or post-deployment PCL scores.
Table 3. Correlations for PTSD and neurocognitive performance measures

Note
N = 668, *p < .05, **p < .01; PTSD = posttraumatic stress disorder; 1 = PTSD Checklist pre-deployment; 2 = PTSD Checklist post-deployment; 3 = Wechsler Memory Scale-III, Verbal Paired Associates, immediate recall, number correct, trials 1–4; 4 = Wechsler Memory Scale-III Verbal Paired Associates, percent retention; 5 = Wechsler Memory Scale Visual Reproductions, immediate recall; 6 = Wechsler Memory Scale Visual Reproductions, percent retention; 7 = Neurobehavioral Evaluation System, 3rd edition, Continuous Performance Task, log-transformed false positives score; 8 = Neurobehavioral Evaluation System, 3rd edition, Continuous Performance Task, log-transformed negative responses score; 9 = Trail Making B – A (log-transformed seconds).
Prospective Analyses
Table 4 presents results of the regressions that explored associations between pre-deployment neuropsychological performances and post-deployment residualized change in PTSD symptom levels. Approximately 33% of the variance in residualized PCL scores was accounted for by each of the models. In each of the final models, higher levels of pre-deployment PCL scores, female gender, and higher levels of combat intensity were significantly associated with higher levels of residualized post-deployment PCL scores. Visual Reproductions immediate recall was the only pre-deployment neuropsychological performance indicator associated with residualized post-deployment PCL scores. The association was negative, suggesting that less proficient acquisition of visual information at pre-deployment was associated with less favorable PTSD outcomes at post-deployment.
Table 4. Longitudinal associations of residualized PCL scores with pre-deployment neurocognitive performances
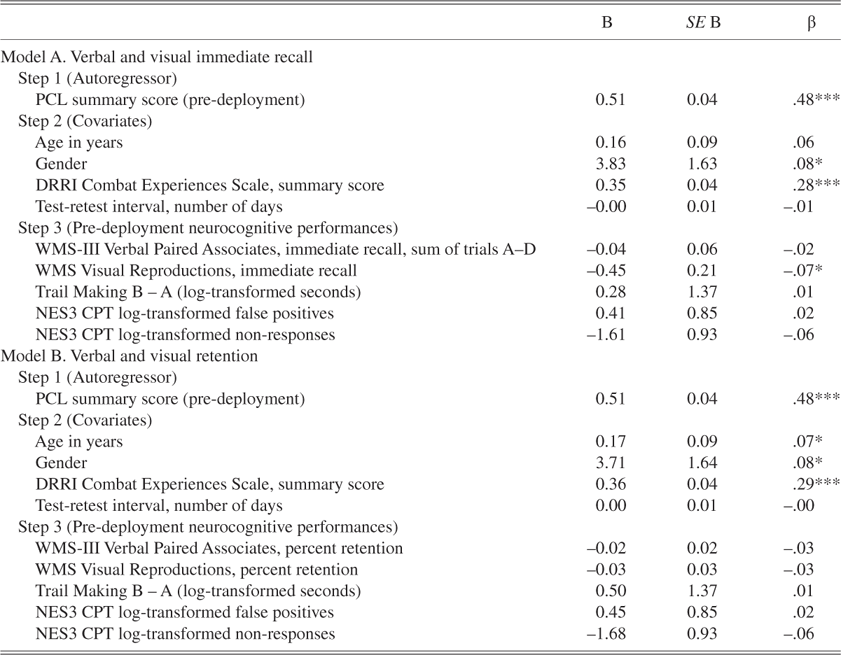
Note
For Model A: Step 1, R 2 = .24, F(1, 636) = 205.71, p < .00; Step 2, Δ R 2 = .08, Δ F(4, 632) = 17.99, p < .00; R 2 = .32, F(5, 632) = 59.93, p < .00; Step 3, Δ R 2 = .01, Δ F(5, 627) = 1.74, p = .12; final R 2 = .33, F(10, 627) = 31.01, p < .00. For Model B: Step 1, R 2 = .24, F(1, 636) = 205.71, p < .00; Step 2, Δ R 2 = .08, Δ F(4, 632) = 17.99, p < .00; R 2 = .32, F(5, 632) = 59.93, p < .00; Step 3, Δ R 2 = .01, Δ F(5, 627) = 1.13, p = .35; final R 2 = .33, F(10, 627) = 30.56, p < .00.
*p < .05, **p < .01, ***p < .001.
β = standardized parameter estimates; B = unstandardized parameter estimates; PCL = PTSD checklist; WMS = Wechsler Memory Scale; WMS-III = Wechsler Memory Scale, 3rd edition; NES3 CPT = Neurobehavioral Evaluation System, 3rd edition Continuous Performance Task. Parameter estimates are for the final model; DRRI = Deployment Risk and Resilience Inventory.
Exploratory post hoc regressions were conducted to examine whether the influences of pre-deployment neurocognitive performances on residualized PCL scores post-deployment differed by level of pre-deployment PCL scores. Interaction terms representing the product of pre-deployment PCL scores and each pre-deployment neurocognitive performance indicator were tested at the final regression step in separate models. Pre-deployment PCL scores moderated the associations of pre-deployment verbal, β = –0.01, t(626) = –2.14, p < .03, and visual, β = –0.04, t(626) = –2.52, p < .02, immediate recall performances with residualized PCL scores post-deployment. Specifically, the associations between lower pre-deployment proficiency in verbal and visual information acquisition and higher residualized PCL scores post-deployment were stronger at higher levels of pre-deployment PTSD symptoms. Interaction terms for the remaining pre-deployment neurocognitive performances were nonsignificant.
Cross-sectional Analyses
Table 5 presents the results of regression models that examined cross-sectional associations of post-deployment PCL summary scores with post-deployment neuropsychological performances. Approximately 12–15% of the estimated variance in post-deployment PTSD symptoms was accounted for by each of the final models. Gender and combat intensity were positively correlated with post-deployment PCL summary scores, suggesting that female gender and higher levels of combat intensity were related to greater PTSD symptom severity following deployment. Both post-deployment verbal and visual immediate recall performances were significantly associated with post-deployment PTSD levels, such that lower capacity for verbal and visual information acquisition following deployment was associated with more post-deployment PTSD symptomatology.
Table 5. Cross-sectional associations of post-deployment PCL scores with post-deployment neurocognitive performances

Note
For Model A: Step 1, R 2 = .11, F(4, 645) = 19.24, p < .00; for Step 2, Δ R 2 = .04, Δ F(5, 640) = 5.89, p < .00; final R 2 = .15, F(9, 640) = 12.15, p < .00. For Model B: Step 1, R 2 = .11, F(4, 643) = 19.66, p < .00; for Step 2, Δ R 2 = .01, Δ F(5, 638) = 1.41, p = .22; final R 2 = .12, F(9, 638) = 9.55, p < .00.
*p < .05, **p < .01, ***p < .001.
β = standardized parameter estimates; B = unstandardized parameter estimates; PCL = PTSD checklist; WMS = Wechsler Memory Scale; WMS-III = Wechsler Memory Scale, 3rd edition; NES3 CPT = Neurobehavioral Evaluation System, 3rd edition Continuous Performance Task. Parameter estimates are for the final model; DRRI = Deployment Risk and Resilience Inventory.
DISCUSSION
This study is the first to examine prospectively relationships between specific neurocognitive performances and the full range of PTSD symptoms after trauma exposure. Results showed that pre-trauma visual immediate memory performance was associated negatively with post-deployment PTSD symptom severity, even after controlling for pre-deployment PTSD symptom levels, combat intensity, test-retest interval, age, and gender. This finding suggests that the integrity of at least one component of visual memory may alter risk of PTSD symptom expression following exposure to extreme stress, contributing to variance in symptom outcomes above and beyond such potent factors as stressor severity (measured by combat intensity) and pre-existing symptom levels. Findings also cannot be attributed to age, gender, or the passage of time between the two assessments.
The finding of a relationship between pre-exposure visual immediate recall and post-deployment PTSD mirrors research documenting a link between Army entrance examination scores and subsequent PTSD (Gale et al., Reference Gale, Deary, Boyle, Barefoot, Mortensen and Batty2008; Kremen et al., Reference Kremen, Koenen, Boake, Purcell, Eisen and Franz2007; Macklin et al., Reference Macklin, Metzger, Litz, McNally, Lasko, Orr and Pitman1998; Thompson & Gottesman, Reference Thompson and Gottesman2008). It is also consistent with, but more circumscribed than, the prospective findings reported by Parslow and Jorm (Reference Parslow and Jorm2007), which showed that more proficient immediate and delayed verbal recall, verbal working memory, visuo-motor speed, and verbal intelligence measured prior to exposure to a natural disaster were associated with lower post-trauma PTSD re-experiencing and arousal symptoms. Our test, however, was more conservative than Parslow and Jorm (Reference Parslow and Jorm2007), in that we were able to examine the relationship between baseline functioning and change in PTSD symptoms, allowing us to make firmer inferences about the direction of causation.
We found an association of post-deployment PTSD with memory when memory was measured in the visual-spatial, but not in the verbal-auditory, modality. Autobiographical memory studies of non-trauma-exposed samples have demonstrated that decreased visual input reduces the recollection of autobiographical events (Rubin, Burt, & Fifeld, Reference Rubin, Burt and Fifeld2003), and damage to the occipital lobe impedes autobiographical memory (Greenberg & Rubin, Reference Greenberg and Rubin2003). Although speculative, it is possible that the ability to form a visual image allows for rehearsal and appropriate habituation to the traumatic event. Thus, proficiency in the initial acquisition of visual images may promote visual processing of traumatic experiences necessary for habituation and subsequent resilience and/or recovery. This possibility is consistent with research showing that visual imagery evokes stronger affective responses than verbal processing (Holmes, Wilson, Pontius, Dietrick, & Spiro, Reference Holmes, Mathews, Dalgleish and Mackintosh2006) and is more effective than verbal processing in reducing anxiety in the context of interpretation training (Holmes & Mathews, Reference Holmes and Mathews2005). It is also consistent with Gilbertson and colleagues (Gilbertson et al., Reference Gilbertson, Shenton, Ciszewski, Kasai, Lasko, Orr and Pitman2002; Reference Gilbertson, Williston, Paulus, Lasko, Gurvits, Shenton and Orr2007), who showed that, in monozygotic Vietnam veteran twins discordant for combat exposure, smaller hippocampi and correlated deficiencies in solving allocentric (i.e., configural relationships among distal environmental features) visuo-spatial processing problems constituted a risk for the development of PTSD. As the hippocampus has been shown to be important in the processing of configural relationships in one’s environment (Astur, Taylor, Mamelak, Philpott, & Sutherland, Reference Astur, Taylor, Mamelak, Philpott and Sutherland2002) and configural processing of environmental cues is pivotal in the extinction of conditioned fear responses (Frankland, Cestari, Filipkowski, McDonald, & Silva, Reference Frankland, Cestari, Filipkowski, McDonald and Silva1998), Gilbertson et al. (Reference Gilbertson, Williston, Paulus, Lasko, Gurvits, Shenton and Orr2007) interpreted their findings to suggest that smaller hippocampal volume before trauma exposure might promote the development of PTSD through a failure to support visually-mediated context-appropriate extinction of conditioned emotional responses. Interestingly, dissociation, a potent risk factor for PTSD (e.g., Ozer, Best, Lipsey, & Weiss, Reference Ozer, Best, Lipsey and Weiss2003) involves alterations of the visual and somatosensory modalities (Bernstein & Putnam, Reference Bernstein and Putnam1986; Bremner et al., Reference Bremner, Krystal, Putnam, Southwick, Marmar and Charney1998). Regardless of the mechanism, the prospective results suggest that a pre-deployment neurocognitive strength (immediate visual memory, in particular) buffers against the adverse effects of deployment to a war-zone noted by other studies (Dohrenwend et al., Reference Dohrenwend, Turner, Turse, Adams, Koenen and Marshall2006; Hoge et al., Reference Hoge, Castro, Messer, McGurk, Cotting and Koffman2004, Reference Hoge, McGurk, Thomas, Cox, Engel and Castro2008; Kang, Natelson, Mahan, Lee, & Murphy, Reference Kang, Natelson, Mahan, Lee and Murphy2003).
Prior cross-sectional research showed relative performance deficiency on verbal memory (Brewin, Kleiner, Vasterling, & Field, Reference Brewin, Kleiner, Vasterling and Field2007) and intellectual tasks (e.g., Vasterling et al., Reference Vasterling, Brailey, Constans and Sutker1998) among those with PTSD. In contrast, our prospective findings indicated that a visual, but not a verbal, memory task was significantly associated with residualized post-deployment PCL scores. As demonstrated by Gilbertson et al. (Reference Gilbertson, Williston, Paulus, Lasko, Gurvits, Shenton and Orr2007) and prior research demonstrating PTSD-related dissociations between global and local processing on visuo-spatial tasks (c.f. Vasterling, Brailey, Sutker, Reference Vasterling, Brailey and Sutker2000; Vasterling, Duke, Tomlin, Lowery, & Kaplan, Reference Vasterling, Duke, Tomlin, Lowery and Kaplan2004), it is likely that PTSD is associated with relative decrements in only select aspects of visuo-spatial processing that are not detected on all tasks. It is also probable that our measures of visual and verbal memory processes were not pure with respect to the processes invoked to complete the tasks. For example, geometric line drawings may be verbally elaborated, and verbal word pairs may be visualized. Inclusion of verbal and visual memory indices in each of the regression analyses permitted some control for overlapping processes; however, because we only included one test representative of each modality, and the two memory tests also varied on attributes other than modality, modality-specific interpretations of our findings should be made with caution.
Post hocanalyses revealed that the effects of pre-deployment levels of immediate verbal and visual memory on post-deployment PTSD symptom levels were moderated by pre-deployment PTSD symptom levels. At higher levels of pre-deployment PTSD symptoms, neurocognitive performance exerted a greater influence on post-deployment PTSD symptom levels, as demonstrated both by increased strength of associations and by a broader scope of associations (i.e., visual and verbal learning). Previous studies have shown that pre-existing psychopathology increases risk for PTSD following exposure to a traumatic event (Bowman, Reference Bowman1997; Brewin, Andrews, & Valentine, Reference Brewin, Andrews and Valentine2000; Kessler, Sonnega, Bromet, Hughes, & Nelson, Reference Kessler, Sonnega, Bromet, Hughes and Nelson1995; Kessler et al., Reference Kessler, Sonnega, Bromet, Hughes, Nelson, Breslau and Yehuda1999; Ozer et al., Reference Ozer, Best, Lipsey and Weiss2003; Schlenger et al., Reference Schlenger, Kulka, Fairbank, Hough, Jordan, Marmar and Weiss1992). The observation that pre-exposure psychopathology interacted with a pre-exposure neurocognitive factor represents an intriguing finding, suggesting that intact neurocognitive skills may be particularly important in facilitating successful coping with pre-existing psychological distress in the face of cumulative stress exposures.
The finding that immediate recall, but not retention, of verbal and visual-spatial information was associated with post-deployment PTSD symptoms raises the question of whether attention or strategic memory processes impacting initial registration of information are particularly important to psychological resilience and recovery following trauma exposure. Although we did not document an association with pre-deployment attentional or executive measures and post-deployment PTSD symptom change, this assertion is consistent with neuropsychological conceptualizations of PTSD that emphasize the role of the prefrontal cortex (e.g., Carrion et al., Reference Carrion, Weems, Eliez, Patwardhan, Brown, Ray and Reiss2001; De Bellis et al., Reference De Bellis, Keshavan, Shifflett, Iyengar, Beers, Hall and Moritz2002; Karl et al., Reference Karl, Schaefer, Malta, Dorfel, Rohleder and Werner2006; Rauch et al., Reference Rauch, van der Kolk, Fisler, Alpert, Orr and Savage1996; Shin et al., Reference Shin, Orr, Carson, Rauch, Macklin and Lasko2004) and prior cross-sectional work (e.g., Isaac, Cushway, & Jones, Reference Isaac, Cushway and Jones2006; Jenkins, Langlais, Delis, & Cohen, Reference Jenkins, Langlais, Delis and Cohen1998; Uddo et al., Reference Uddo, Vasterling, Brailey and Sutker1993; Vasterling et al., Reference Vasterling, Brailey, Constans and Sutker1998, Reference Vasterling, Duke, Brailey, Constans, Allain and Sutker2002; Yehuda et al., Reference Yehuda, Keefe, Harvey, Levengood, Gerber, Geni and Siever1995; Yehuda, Golier, Halligan, & Harvey, Reference Yehuda, Golier, Halligan and Harvey2004) documenting PTSD-related impairment of memory processes (e.g., sensitivity to interference) linked to frontal lobe integrity. Alternatively, the association with visual immediate recall and PTSD symptoms could reflect the contribution of primary visual-spatial processing deficits to PTSD risk. Because we did not include a task of visual-spatial processing without a memory component, however, the degree to which primary visual-spatial versus visual-spatial memory processing accounts for the relationship between visual immediate memory and PTSD symptoms remains unclear.
As an extension of the previous literature, we also examined the cross-sectional relationships of neurocognitive performances to PTSD symptom severity at post-deployment and found that immediate visual recall and verbal learning performances were negatively correlated with PTSD symptom severity. These findings are consistent with the cross-sectional literature, revealing both visual and verbal memory associations with PTSD symptoms (Brewin et al., Reference Brewin, Kleiner, Vasterling and Field2007), but do not directly address causal direction. However, in the absence of a longitudinal relationship between pre-exposure verbal learning and post-deployment PTSD symptoms (except when PTSD symptoms were already high at baseline), the post-deployment relationship of verbal learning to PTSD symptoms raises the question of whether PTSD may have led to a decline in verbal learning. This assertion is consistent with Parslow and Jorm (Reference Parslow and Jorm2007)’s finding of an interaction between time (pre- vs. post-trauma) and post-trauma PTSD on a word recall task. It may be that some cognitive weaknesses confer risk of PTSD development, whereas others are a result of PTSD.
There may be alternative explanations for the findings. For example, it could be argued that pre-deployment visual memory decrements are associated with baseline PTSD symptoms, and that the association between pre-deployment visual memory and post-deployment PCL scores simply reflects elevated baseline PCL scores. However, the correlation between pre-deployment immediate visual memory and pre-deployment PCL scores was not significant, and baseline PCL scores are parceled out of post-deployment PCL scores, via their inclusion in the models as a covariate. Another possibility is that the visual immediate memory test was particularly sensitive to insufficient effort or symptom exaggeration. If so, symptom feigning would be reflected by both poorer performance on the visual immediate memory test and elevated PCL scores. However, we excluded participants for insufficient effort based on their TOMM responses, and there is no evidence that visual immediate memory is more sensitive to insufficient effort/symptom exaggeration than other neuropsychological tasks administered.
There are several limitations to our study. We included only one measure of each of the neuropsychological constructs of interest, limiting the extent to which findings can be generalized beyond the specific parameters of the tasks administered. As discussed earlier, we did not include a control test of visual-spatial processing devoid of a memory component. The verbal and visual memory tasks were also not well-matched with respect to the specific task demands. Thus, we remain unable to exclude the possibility that specific task demands accounted for the dissociation of findings between verbal and visual immediate recall tasks.
The study design offered the advantage of allowing examination of the relationship between pre-trauma memory functioning and post-trauma PTSD symptoms prior to the PTSD becoming chronic. It will be beneficial, however, to conduct longer-term longitudinal studies in which the relationship between memory and PTSD symptoms is examined over time, permitting determination of whether pre-trauma memory is equally potent at all time points in the natural history of PTSD. Such an approach is consistent with previous findings showing that risk factors associated with developing PTSD may differ from risk factors associated with maintaining PTSD (Dunmore, Clark, & Ehlers, Reference Dunmore, Clark and Ehlers1999; Koenen, Stellman, Stellman, & Sommer, Reference Koenen, Stellman, Stellman and Sommer2003; Schnurr, Lunney, & Sengupta, Reference Schnurr, Lunney and Sengupta2004).
Brewin et al. (Reference Brewin, Andrews and Valentine2000) showed that sample type (i.e., civilian vs. military) may moderate the associations between risk factors and PTSD, with effects generally being stronger for military samples. Because our sample was comprised of active duty military personnel exposed to war-zone trauma, the extent to which results will generalize to nonmilitary trauma populations is unknown. Importantly, many of the individuals in our sample were not trauma naïve or free of PTSD symptoms prior to deployment. However, an advantage of the longitudinal design is that we were able to control statistically for pre-deployment symptom levels. Despite these limitations, the longitudinal design, performance-based neuropsychological measures, and a sizable sample of deployed participants provide unique information regarding the longitudinal trajectory of the relationship between baseline neuropsychological performance and PTSD symptom outcome following exposure to extreme stress.
ACKNOWLEDGMENTS
The authors of this article would like to thank Kevin Brailey for his invaluable comments and feedback, Helen MacDonald and Anna Graefe for their assistance with data scoring, and Roberta F. White and Robert Kane for their input on test selection in the broader Neurocognition Deployment Health Study. We additionally thank Dr. Kane for his on-going collaboration in the Neurocognition Deployment Health Study.
This work was supported by the U.S. Army Medical Research and Materiel Command (DAMD 17-03-0020; HSRRB Log No. A-11815) and VA Clinical Sciences Research and Development awards. The work also was supported in part by resources provided by the South Central Mental Illness Research, Education, and Clinical Center and U.S. Army Research Institute for Environmental Medicine. Some of the work was completed at the Southeast Louisiana Veterans Healthcare System and Tulane University. The U.S. Army Medical Research Acquisition Activity (Fort Detrick, MD) is the awarding and administering acquisition office for DAMD 17-03-0020







