Introduction
Over the past 20 years there has been a steady rise in preterm birth worldwide (World Health Organization, 2012). In 2010, an estimated 14.9 million babies were born preterm (<37 weeks’ gestational age), and approximately 20% of these are born very preterm (VPT), defined as birth before 32 weeks’ gestational age (GA; Goldenberg, Culhane, Iams, & Romero, Reference Goldenberg, Culhane, Iams and Romero2008). Many VPT children experience later cognitive (Anderson & Doyle, Reference Anderson and Doyle2003), academic (Aarnoudse-Moens, Weisglas-Kuperus, van Goudoever, & Oosterlaan, Reference Aarnoudse-Moens, Weisglas-Kuperus, van Goudoever and Oosterlaan2009), and behavioral (Bhutta, Cleves, Casey, Cradock, & Anand, Reference Bhutta, Cleves, Casey, Cradock and Anand2002) problems, and up to 60% will show diffuse and focal structural brain injury (Inder, Anderson, Spencer, Wells, & Volpe, Reference Inder, Anderson, Spencer, Wells and Volpe2003; Ment, Hirtz, & Huppi, Reference Ment, Hirtz and Huppi2009). In addition to high rates of brain injury, brain growth has been reported to be delayed in VPT infants and children (Taylor et al., Reference Taylor, Filipek, Juranek, Bangert, Minich and Hack2011; Thompson et al., Reference Thompson, Wood., Doyle, Warfield, Lodygensky, Anderson and Inder2008). In particular, hippocampal volume has been found to be significantly reduced in VPT children compared with their term born counterparts (Gimenez et al., Reference Gimenez, Soria-Pastor, Junque, Caldu, Narberhaus, Botet and Mercader2008; Nosarti et al., Reference Nosarti, Al-Asady, Frangou, Stewart, Rifkin and Murray2002; Thompson et al., Reference Thompson, Wood., Doyle, Warfield, Lodygensky, Anderson and Inder2008). The hippocampi have a well-established role in memory and learning (Moscovitch & Umilta, Reference Moscovitch and Umilta1990; Nadel, Samsonovich, Ryan, & Moscovitch, Reference Nadel, Samsonovitch, Ryan and Moscovitch2000), and children born VPT often show impairments in these domains (Omizzolo et al., Reference Omizzolo, Scratch, Stargatt, Kidokoro, Thompson, Lee and Anderson2013; Rose, Feldman, & Jankowski, Reference Rose, Feldman and Jankowski2005; Taylor, Klein, Minich, & Hack, Reference Taylor, Klein, Minich and Hack2000; Woodward, Edgin, Thompson, & Inder, Reference Woodward, Edgin, Thompson and Inder2005). This raises the question whether there may be a relationship between reduced hippocampal volume and memory and learning impairments.
The hippocampal formations comprise a group of several related brain areas within the left and right medial temporal lobes, including the dentate gyrus, hippocampus, subiculum, presubiculum, parasubiculum, and entorhinal cortex (Amaral & Lavenex, Reference Amaral and Lavenex2007). They are known to be particularly vulnerable to many of the stressors and medical complications associated with VPT birth, including infection, hypoxia-ischemia, poor nutrition, hypoglycemia, hypothyroidism, and stress hormones (Gadian et al., Reference Gadian, Aicardi, Watkins, Porter, Mishkin and Vargha-Khadem2000; Isaacs et al., Reference Isaacs, Lucas, Chong, Wood, Johnson, Marshall and Gadian2000; Khwaja & Volpe, Reference Khwaja and Volpe2008; Sizonenko et al., Reference Sizonenko, Borradori-Tolsa, Bauthay, Lodygensky, Lazeyras and Huppi2006). These complications can result in reductions in gray matter volumes (Inder, Warfield, Wang, Huppi, & Volpe, Reference Inder, Warfield, Wang, Huppi and Volpe2005; Volpe, Reference Volpe2009), including pyramidal cell death, a slowing of neural migration (Rees, Breen, Loeliger, McCrabb, & Harding, Reference Rees, Breen, Loeliger, McCrabb and Harding1999) and neuronal injury (Volpe, Reference Volpe2001) to the hippocampi.
In vivo measurement and volumetric analyses of the brain using magnetic reasonance imaging (MRI) have become key components of neuroimaging research (Konrad et al., Reference Konrad, Ukas, Nebel., Arolt, Toga and Narr2009). Manual segmentation and automated measures, such as voxel-based morphometry, have been used to investigate the hippocampal formation. However, manual segmentation is still considered the gold standard (Konrad et al., Reference Konrad, Ukas, Nebel., Arolt, Toga and Narr2009), as voxel-based morphometry has been reported to produce significantly larger estimates of volume (Cherbuin, Anstey, Réglade-Meslin, & Sachdev, Reference Cherbuin, Anstey, Réglade-Meslin and Sachdev2009). Most studies within the VPT literature have used manual segmentation, and have reported reduced hippocampal volume in VPT infants (Lodygensky et al., Reference Lodygensky, Seghier, Warfield, Tolsa, Sizonenko, Lazeyras and Hüppi2008; Thompson et al., Reference Thompson, Wood., Doyle, Warfield, Lodygensky, Anderson and Inder2008), children (Isaacs et al., Reference Isaacs, Lucas, Chong, Wood, Johnson, Marshall and Gadian2000; Lodygensky et al., Reference Lodygensky, Rademaker, Zimine, Gex-Fabry, Lieftink, Lazeyras and Huppi2005), and adolescents (Abernethy, Palaniappan, & Cooke, Reference Abernethy, Palaniappan and Cooke2002; Nosarti et al., Reference Nosarti, Al-Asady, Frangou, Stewart, Rifkin and Murray2002). Although fewer studies have used voxel-based morphometry, reduced hippocampal volumes in adolescents (Gimenez et al., Reference Gimenez, Soria-Pastor, Junque, Caldu, Narberhaus, Botet and Mercader2008) and young adults (Lawrence et al., Reference Lawrence, McGuire, Allin, Walshe, Giampietro, Murray and Nosarti2010) born VPT have also been noted using this methodology.
Positron emission tomography (PET) studies indicate that the medial-temporal lobe, particularly the hippocampi, and the prefrontal cortex are vital for learning and long-term memory function (Cabeza & Nyberg, Reference Cabeza and Nyberg1997). Memory is a complex system, involving the initial registration of information into immediate memory, followed by the manipulation of this information in working memory. Material is then transferred to long-term memory using processes such as articulatory rehearsal (Baddeley, Reference Baddeley1996) and attentional refreshment (Barrouillet & Camos, Reference Barrouillet and Camos2001), where it is stored for later retrieval. Impairments to memory and learning have been reported in VPT infants as young as 12 months of age on a paradigm involving the reproduction of action sequences and recognition of pictures (Rose et al., Reference Rose, Feldman and Jankowski2005). Deficits to immediate/working memory in older VPT cohorts (Böhm, Smedler, & Forssberg, Reference Böhm, Smedler and Forssberg2004; Sansavini et al., Reference Sansavini, Guarini, Alessandroni, Faldella, Giovanelli and Salvioli2006; Vicari, Caravale, Carlesimo, Casadei, & Allemand Reference Vicari, Caravale, Carlesimo, Casadei and Allemand2004) and verbal list learning in very low birth-weight children (Taylor et al., Reference Taylor, Klein, Minich and Hack2000) have also been reported.
Several studies have investigated hippocampal volume and its relationship to general intellectual abilities and neurodevelopmental outcomes in preterm populations. Reduced hippocampal volumes in VPT neonates have been associated with poorer performance on the Mental Development and Psychomotor Development Indices of the Bayley Scale of Infant Development (BSID-II) at 2 years’ corrected age (Thompson et al., Reference Thompson, Wood., Doyle, Warfield, Lodygensky, Anderson and Inder2008). In VPT children at 8 years of age, reduced hippocampal volumes have been associated with poorer full-scale IQ (Lodygensky et al., Reference Lodygensky, Rademaker, Zimine, Gex-Fabry, Lieftink, Lazeyras and Huppi2005; Peterson et al., Reference Peterson, Vohr, Staib, Cannistraci, Dolberg, Schneider and Ment2000). Moreover, left hippocampal volume reduction at 15–16 years of age has been associated with low IQ in adolescents born with very low birth weight (VLBW; <1250 g) (Abernethy et al., Reference Abernethy, Palaniappan and Cooke2002). Isaacs and colleagues (Reference Isaacs, Edmonds, Chong, Lucas, Morley and Gadian2004) also reported a relationship between reduced hippocampal volumes and poorer Performance IQ in a cohort of adolescents born VPT and/or VLBW. In contrast, Abernethy, Cooke, and Foulder-Hughes (Reference Abernethy, Cooke and Foulder-Hughes2004) found little evidence for a relationship between hippocampal volumes in VPT children at 7 years and full-scale IQ.
Fewer studies have specifically examined the relationship between hippocampal volume and memory and learning in those born VPT, with published reports having a narrow focus. This is surprising given evidence for the important role of the hippocampi in memory and learning (Cabeza & Nyberg, Reference Cabeza and Nyberg1997; Moscovitch & Umilta, Reference Moscovitch and Umilta1990; Nadel et al., Reference Nadel, Samsonovitch, Ryan and Moscovitch2000). While the hippocampi have typically been related to episodic long-term memory function, evidence suggests that they may also play a significant role in immediate/working memory (Olson, Page, Moore, Chatterjee, & Verfaellie, Reference Olson, Page, Moore, Chatterjee and Verfaellie2006; Piekema, Kessels, Mars, Petersson, & Fernandez, Reference Piekema, Kessels, Mars, Petersson and Fernandez2006). In the VPT population, Beauchamp et al. (Reference Beauchamp, Thompson, Howard, Doyle, Egan, Inder and Anderson2008) found that reduced neonatal hippocampal volumes were associated with poorer visual working memory on a delayed alternation task in VPT 2 year olds. Reduced hippocampal volumes in adolescents aged 13 years who were born VPT/VLBW has been associated with poorer everyday memory (Isaacs et al., Reference Isaacs, Lucas, Chong, Wood, Johnson, Marshall and Gadian2000, Reference Isaacs, Vargha-Khadem, Watkins, Lucas, Mishkin and Gadian2003), and left hippocampal volume reduction in those born VPT has been reported to correlate with reduced verbal recognition memory and learning performances at age 10–18 years (Giménez et al., Reference Giménez, Junqúe, Narberhaus, Caldú, Salgago-Pineda, Bargalló and Botet2004).
In summary, research investigating the association between hippocampal volume and multiple components of memory and learning in a large, representative and contemporary VPT cohort is needed. This is especially important given the significance of these skills for academic progress and success (Bull, Epsy, & Wiebe, Reference Bull, Espy and Wiebe2008; Hornby & Woodward, Reference Hornby and Woodward2009; Sansavini et al., Reference Sansavini, Guarini, Alessandroni, Faldella, Giovanelli and Salvioli2006), highlighting the need for early detection and intervention. Using this framework, we recently reported that VPT children performed more poorly on measures of memory and learning in both verbal and visual domains compared with term controls at 7 years of age, with approximately 30% of the VPT group performing at least 1 SD below the control group mean (Omizzolo et al., Reference Omizzolo, Scratch, Stargatt, Kidokoro, Thompson, Lee and Anderson2013). Using children from the same cohort of VPT and term children, this study aims: 1) to compare hippocampal formation volume between VPT and term controls at age 7 years, and 2) to evaluate the association between hippocampal volume and memory and learning functioning in VPT children at 7 years.
Methods
Participants
Participants were part of the Victorian Infant Brain Studies (VIBeS) cohort, a prospective, longitudinal study examining brain abnormality and development in VPT children. Recruitment occurred from July 2001 to December 2003 at the Royal Women's Hospital in Melbourne, Australia; 224 VPT infants with either a GA of <30 weeks or a birth weight of <1250 g were recruited. Infants with severe congenital abnormalities that would impair neurological function were excluded. A concurrent control group of 46 children born full-term (37 to 42 weeks’ GA) and of normal birth weight (≥2500 g) was also recruited from the Royal Women's Hospital. An MRI brain scan was conducted on all infants without sedation, and at term equivalent age for VPT subjects. Follow-up assessment occurred at ages 2, 5, and 7 years (corrected for prematurity). At the latest follow up at 7 years of age, 198 VPT (88%) and 43 (93%) term children had a neuropsychological assessment, and 160 VPT (81%) and 36 (84%) term children had a MRI brain scan. The main reasons children did not have imaging data at age 7 years were that they were too anxious or unsettled (VPT, n = 18; term, n = 3), did not consent (VPT, n = 6, term, n = 1) or were too impaired (VPT, n = 6). Neuropsychological assessments and MRI brain scans were completed over 2 days. Of the 160 VPT and 36 term children with scans, 145 (91%) VPT and 34 (94%) term children had scans that were suitable for analysis after scans with movement artifact were excluded.
Procedure and Measures
This study was approved by the Human Research Ethics Committees of the Royal Women's Hospital and the Royal Children's Hospital. Parents gave written informed consent for their child to participate. MRI scanning took place at the Children's MRI Centre at Melbourne's Royal Children's Hospital with a 3 Telsa Trio Siemens MRI machine (Siemens, Erlangen, Germany). Before the MRI scan, each child underwent a mock MRI scanning session that was aimed to familiarize the child with the scanning procedure. Scans were conducted without sedation or anesthesia, and both T1 (0.85 mm sagittal slices, flip angle = 9°, repetition time = 1900 ms, echo time = 2.27 ms, field of view = 210 × 210 mm, matrix = 256 × 256) and T2 weighted (0.90 mm sagittal slices, repetition time = 3200 ms, echo time = 447 ms, field of view = 240 × 215 mm, matrix =256 × 230) structural images were acquired.
Hippocampal segmentation
A single operator (C.O.) manually outlined left and right hippocampal formations in the coronal view of the T1 scan using ITK-SNAP 2.2.0 (see Figure 1), and was blinded to all perinatal data (including to which birth group the images belonged). Tracing always proceeded from the posterior to anterior sections in a sequential manner (i.e., beginning at the hippocampal tail, and followed by the hippocampal body and head). Each slice of the hippocampal formation was traced from the superiomedial edge to the lateral edge, downward to the inferior aspect, and finally to the medial edge. In general, anatomical boundaries proposed by Watson and colleagues (Reference Watson, Andermann, Gloor, Jones-Gotman, Peters, Evans and Leroux1992) and Pruessner and colleagues (Reference Pruessner, Li, Serles, Pruessner, Collins, Kabani and Evans2000) were followed, with reference to anatomical atlases by Woolsey, Hanaway, and Gado (Reference Woolsey, Hanaway and Gado2003). While tracing occurred in the coronal view, reference was made to the sagittal and horizontal views to provide more reliable identification of structural boundaries. The dentate gyrus, four cornu ammonis regions, alveus, and the fimbria were included within the hippocampal formation measurement.

Fig. 1 T1 image showing left (green) and right (red) hippocampal boundaries as traced on (a) the coronal plane from anterior to posterior, (b) the axial plane, (c) the sagittal plane, and (d) the three-dimensional representation.
Boundaries of the hippocampal tail
The most posterior slice of the hippocampal tail (HT) was defined as the slice where the crus of the fornix was clearly visible surrounding the hippocampus inferiomedially to the trigone of the lateral ventricle (TLV). Consistently, the lateral border was defined as the boundary between the white matter of the fimbria and the TLV. Medially, the border was where the quadrigeminal cistern met the hippocampal sulcus. Initially, an arbitrary border was used to help define the superior border of the HT. This arbitrary border was a horizontal line from the superior border of the quadrigeminal cistern to the TLV. This was to help define and separate the HT from the fasciolar gyrus, which sits above the HT. Further anteriorly, the fimbria was used as the superior border. The border between the hippocampus and the parahippocampal gyrus provided the inferior border (an upward slant medially). Although these procedures led to some exclusion of the medial and superior sections of the HT, this method appeared to produce the most consistent approach for defining the HT.
Boundaries of the hippocampal body
The fimbria formed the superior border of the hippocampal body (HB). The lateral border of the HB was defined by the inferior horn of the lateral ventricle, and the most visible inferiolateral gray matter was included. The medial border was where the subiculum joined with the hippocampal sulcus.
Boundaries of the hippocampal head
The hippocampal head (HH) can be identified as the most anterior portion of the hippocampal formation. The first coronal slice of the HH was defined when the uncal recess appeared in the superomedial portion of the hippocampus. This formed a distinct protuberance or “hook” which was used to identify the superomedial border. The inferior border was defined by the uncus, and the border between the entorhinal cortex and the hippocampus. When the gray matter at the superior part of the hippocampal formation became interspersed with amygdala, hippocampus, and basal ganglia (putamen and globus pallidus; Pruessner et al., Reference Pruessner, Li, Serles, Pruessner, Collins, Kabani and Evans2000), the fimbria was used to separate the hippocampal formation from amygdala, evident as a line of white matter intermingling with cerebrospinal fluid. The lateral border was the border between the alveus and the temporal horn of the lateral ventricle. Further anteriorly, the medial edge was the most medial point of the temporal lobe. The last slice was identified as the most anterior slice where the temporal horn of the lateral ventricle was still seen laterally to the hippocampus.
Repeat segmentations to assess intra-rater reliability were conducted 1 week apart on 10 subjects. Intra-class correlation coefficients were 0.97 (p < .01) for the right and 0.96 (p < .01) for the left hippocampal formations. Inter-rater reliability was carried out by an experienced operator (D.K.T.) on 10 subjects. Intra-class correlation coefficients were 0.96 (p < .01) for the right hippocampus and 0.97 (p < .01) for the left hippocampus.
Neuropsychological Assessment
Children underwent extensive neuropsychological assessment as part of the 7-year follow-up. Selected measures from the test battery were used to assess IQ as well as memory and learning within the verbal and visual modalities and are outlined below.
Wechsler Abbreviation Scale of Intelligence (WASI; Wechsler, Reference Wechsler1999)
IQ was estimated using the four-subtest version of the WASI.
Working Memory Test Battery for Children (WMTB-C; Pickering & Gathercole, Reference Pickering and Gathercole2001)
Three subsets from the WMTB-C were administered. Forward Digit Recall was used to assess immediate verbal memory and involved each child being read a sequence of numbers which they were required to recall in the same order. Number sequences began at one digit and increased to nine digits (or until ceiling was reached), with six trials per sequence. Digits were read at a rate of one per second. Backward Digit Recall measured verbal working memory and had a similar process, although children were required to repeat sequences in the reverse order. Block Recall assessed immediate visual memory. The examiner tapped an array of three-dimensional blocks in a sequence (also at a rate of one per second), and participants were required to tap them in the same sequence. Responses on all tasks were scored 0 or 1 for each trial, and a total score reflected the number of trials completed correctly.
California Verbal Learning Test – Children's Version (CVLT-C; Delis, Kramer, Kaplan, & Ober, Reference Delis, Kramer, Kaplan and Ober1994)
The CVLT-C (Delis et al., Reference Delis, Kramer, Kaplan and Ober1994) was used to measure verbal memory and learning. Children were read a 15-item word list (List A) and asked to immediately recall the list. This was repeated another 4 times, which in total derived a learning score. Next, a second or distracter list (List B, also of 15 words) was administered, which children were also required to immediately recall, followed by a short-delay recall of List A. After a 20- to 30-min delay, long-delay recall and recognition trials of List A were administered. Outcome measures were immediate verbal memory (number of words recalled on trial 1 of list A), verbal learning (total number of words recalled across trials 1–5), and memory after short and long delays.
Dot locations subset from the Children's Memory Scale (Cohen, Reference Cohen1997)
Dot locations was used to assess visual memory and learning. Children were presented with an array of 6 dots on a 3 × 3 grid and required to immediately recall their spatial location. This procedure was repeated two more times, and was followed by a second (or distractor) dot array. A short-delay recall of the original dot array was then administered, which was followed by a long-delay recall 20 to 30 min later. Outcome measures included immediate visual memory (trial 1), visual learning (total trials 1–3), and visual memory after short and long delays.
Neonatal Brain Abnormality Score
A neonatal neurologist rated cerebral abnormality on T1 and T2 structural scans using a system described by Kidokoro, Neil, and Inder Reference Kidokoro, Neil and Inder(2013), which is an adaptation of a procedure applied previously (Inder et al., Reference Inder, Anderson, Spencer, Wells and Volpe2003; Woodward, Anderson, Austin, Howard, & Inder, Reference Woodward, Anderson, Austin, Howard and Inder2006). This scoring system rates presence and severity of white matter abnormality (cystic lesions, signal abnormality, myelination delay, callosal thinning, lateral ventricular volume, white matter volume), cortical gray matter abnormality (extracerebral space, signal abnormality, gyral maturation), deep gray matter abnormality (signal abnormality, deep gray matter volume) and cerebellar abnormality (signal abnormality, cerebellar volume) on a scale from 0 to 4. These four subscales are summed to produce a global neonatal brain abnormality score (scores ranged from 0 to 40).
Social Risk
Social risk was defined as a high score on a parent questionnaire based on family structure, language spoken at home, education of primary caregiver, occupation and employment status of primary income earner, and maternal age at birth (overall score 0–12; Roberts et al., Reference Roberts, Howard, Spittle, Brown, Anderson and Doyle2008).
Statistical Analyses
Data were analyzed using Stata 12 (StataCorp, 2011). Raw scores were used to report test results, and scores were recorded as “missing” when children were too impaired to complete tasks. Linear regression was used to examine differences between birth groups (VPT and term) in left and right hippocampal volumes (measured in cubic centimeters; cc), with separate models applied to each hemisphere. Each model was fitted using generalized estimating equations (GEEs) with an exchangeable correlations structure and robust standard errors to allow for correlations between twins/triplets in the study. Subsequent analyses controlled for gender, intracranial volume (ICV), and neonatal brain abnormality score.
Linear regression was also used to determine the relationships between left and right hippocampal volumes and memory and learning outcomes in the VPT cohort. Again, models were fitted with GEEs and robust standard errors. Secondary analyses adjusted for the effects of gender, ICV, and neonatal brain abnormality score.
Results
Sample Characteristics
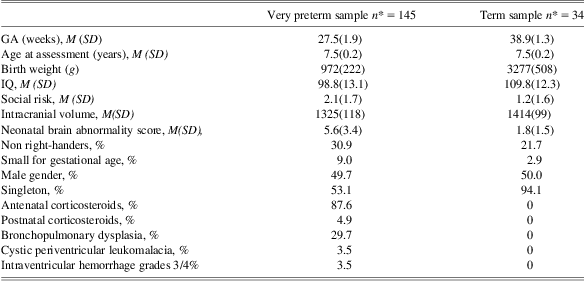
Sample characteristics of the VPT and term cohorts are outlined in Table 1. ICV was smaller on average in the VPT group (6.3% reduction compared with term born peers, p < .01). Groups also differed on neonatal brain abnormality score (p < .01), IQ (p < .01), the percentage of singletons (p < .01) and social risk (p < .01). In contrast, there was little difference in gender, handedness, and age at assessment between the VPT and term groups.
Table 1 Demographic and perinatal characteristics of the sample assessed at 7 years of age for the current study

*Some sample sizes are less than the total sample due to missing data (social risk [very preterm = 139, term = 33]. Intracranial volume [very preterm = 144], and postnatal corticosteroids [very preterm = 144]).
M = mean; SD = standard deviation; GA = gestational age; IQ = general intellectual functioning.
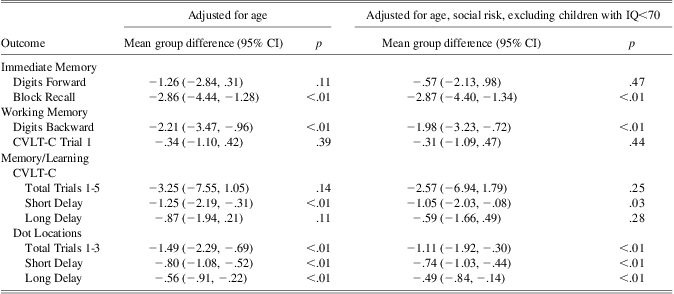
Table 2 displays the mean group differences for the VPT and term control groups on the memory and learning measures, and illustrates the generalized memory deficits of this VPT group.
Table 2 Mean group differences on memory and learning outcomes at age 7 years

Note: lower scores reflect poorer performance in the VPT group. N ranges from 179 to 164 depending on the outcome.
VPT = very preterm; CVLT-C = California Verbal Learning Test – Children's Version; CI = confidence interval.
Hippocampal Volume Analysis
Table 3 shows that VPT children had reduced right (p < .01) and left (p < .01) hippocampal volumes compared with term controls. The VPT group displayed a reduction of 5.9% to the right hippocampus, and 6.8% to the left hippocampus relative to mean hemispheric volume in the term controls. However, the evidence for these differences reduced for the right and left hippocampi when gender, ICV, and neonatal brain abnormality were added to the model (see Table 3). The proportion of variance (R2) accounted by birth group (i.e., VPT and term) was 4.8% for right hippocampal volume and 7.2% for left hippocampal volume. R2 increased to 10.3% for the right hippocampus and 14.1% for the left hippocampus when gender was added to the model, 23.1% (right hippocampus) and 25.4% (left hippocampus) when ICV was added, and 25.4% (right hippocampus) and 26.8% (left hippocampus) when neonatal brain abnormality was added. Of interest, there was little evidence that the association between group and hippocampal volume differed between genders (interaction; left hippocampal volume p = .81, right hippocampal volume p = .89).
Table 3 Associations between birth group and hippocampal volume at 7 years

VPT = very preterm; ICV = intracranial volume ; M = mean; SD = standard deviation; CI = confidence interval; b = coefficient for group from the linear regression model representing the difference in means between the VPT and term groups.
Hippocampal Volume as a Predictor of Memory and Learning Outcomes in Children Born VPT
Figure 2 displays the relationship between left (a) and right (b) hippocampal volumes and performance on memory and learning outcomes in the VPT group. Unadjusted and analyses adjusted for gender, ICV, and neonatal brain abnormality score showed that neither left or right hippocampal volumes were associated with performance on our memory or learning outcomes. Right and left hippocampal volumes did not predict IQ in the VPT group in unadjusted or adjusted (i.e., gender, ICV, and neonatal brain abnormality score) models (see Figure 2).

Fig. 2 Regression coefficients and 95% confidence intervals for the association between hippocampal volume and memory and IQ measures at 7 years of age in the VPT children for (a) the left hippocampus, and (b) the right hippocampus. Estimates represent the difference in outcome are per cc change in hippocampal volume from an unadjusted analysis (dotted lines) and an analysis adjusted for gender, ICV and neonatal brain abnormality (solid lines).
Discussion
This prospective, longitudinal study investigated hippocampal volume, and memory and learning outcomes in 7-year-old children born VPT. Children born VPT had smaller hippocampi compared with their term peers, but not after adjusting for gender, ICV and neonatal brain abnormality. Contrary to expectation, we found little evidence of a relationship between hippocampal volume and memory or learning outcomes within the VPT group.
Previous research has reported hippocampal volume reductions in VPT and/or VLBW cohorts when compared to term born peers in the neonatal period as well as later childhood (Giménez et al., Reference Giménez, Junqúe, Narberhaus, Caldú, Salgago-Pineda, Bargalló and Botet2004; Isaacs et al., Reference Isaacs, Lucas, Chong, Wood, Johnson, Marshall and Gadian2000, Reference Isaacs, Vargha-Khadem, Watkins, Lucas, Mishkin and Gadian2003; Nosarti et al., Reference Nosarti, Al-Asady, Frangou, Stewart, Rifkin and Murray2002; Peterson et al., Reference Peterson, Vohr, Staib, Cannistraci, Dolberg, Schneider and Ment2000; Thompson et al., Reference Thompson, Wood., Doyle, Warfield, Lodygensky, Anderson and Inder2008). Whereas some of these studies report that hippocampal volume reductions in these children persist after adjusting for overall brain size (Giménez et al., Reference Giménez, Junqúe, Narberhaus, Caldú, Salgago-Pineda, Bargalló and Botet2004; Isaacs et al., Reference Isaacs, Vargha-Khadem, Watkins, Lucas, Mishkin and Gadian2003; Nosarti et al., Reference Nosarti, Al-Asady, Frangou, Stewart, Rifkin and Murray2002; Peterson et al., Reference Peterson, Vohr, Staib, Cannistraci, Dolberg, Schneider and Ment2000), others do not (Isaacs et al., Reference Isaacs, Lucas, Chong, Wood, Johnson, Marshall and Gadian2000; Thompson et al., Reference Thompson, Wood., Doyle, Warfield, Lodygensky, Anderson and Inder2008). Like previous studies, our unadjusted analyses showed reduced hippocampal volumes in the VPT group, however subsequent analyses indicated that these differences were largely related to overall smaller brain size (in the case of the right hippocampus) and neonatal brain injury (in the case of the left hippocampus). For example, hippocampal volume reductions in the VPT cohort (right = 5.8%, left = 6.8%) were similar in magnitude to whole brain volume reduction (ICV = 6.3%).
Although the hippocampi resemble adult morphology at approximately 5 years of age (Insausti, Cebada-Sanchez, & Marcos, Reference Insausti, Cebada-Sanchez and Marcos2010), they continue to grow with further organization, dendritic branching, and myelination into adolescence (Insausti et al., Reference Insausti, Cebada-Sanchez and Marcos2010). Given this study examined hippocampal volumes in VPT children at 7 years of age, the full effects of VPT birth on normal hippocampal growth may not be apparent until later in development. In support of this argument, studies examining hippocampal volumes in older children and adolescents born VPT show larger hippocampal reductions in comparison to whole brain volume (Giménez et al., Reference Giménez, Junqúe, Narberhaus, Caldú, Salgago-Pineda, Bargalló and Botet2004; Isaacs et al., Reference Isaacs, Vargha-Khadem, Watkins, Lucas, Mishkin and Gadian2003; Nosarti et al., Reference Nosarti, Al-Asady, Frangou, Stewart, Rifkin and Murray2002; Peterson et al., Reference Peterson, Vohr, Staib, Cannistraci, Dolberg, Schneider and Ment2000). For example, Nosarti and colleagues (Reference Nosarti, Al-Asady, Frangou, Stewart, Rifkin and Murray2002) reported a 6.0% decrease in whole brain volume in a group of VPT 15 year olds compared with term controls, but found a 15.6% and 12.1% decrease in the right and left hippocampi, respectively. Furthermore, Giménez and colleagues (Reference Giménez, Junqúe, Narberhaus, Caldú, Salgago-Pineda, Bargalló and Botet2004) reported a whole brain volume reduction of approximately 8% in their cohort of VPT children and adolescents aged 10–18 years compared with term controls, but found a 16.7% and 15.5% reduction in left and right hippocampal volumes, respectively.
Several methodological factors may help explain the differences in hippocampal volumes between our study and the aforementioned studies. Nosarti et al. (Reference Nosarti, Al-Asady, Frangou, Stewart, Rifkin and Murray2002) recruited their VPT cohort during the late 1970s and early 1980s, whereas our cohort was recruited in the early 2000s. The substantial advances in perinatal care that have occurred over the past two decades, and particularly in the 1990s (Horbar et al., Reference Horbar, Badger, Carpenter, Fanaroff, Kilpatrick, LaCorte and Soll2002), might have protective effects on the hippocampi. Furthermore, Giménez and colleagues (Reference Giménez, Junqúe, Narberhaus, Caldú, Salgago-Pineda, Bargalló and Botet2004) used voxel-based morphometry for hippocampal analysis, whereas our study used manual segmentation, which is arguably a more precise and reliable estimate (Cherbuin et al., Reference Cherbuin, Anstey, Réglade-Meslin and Sachdev2009).
Previous studies have shown that reduced neonatal hippocampal volume is associated with several intellectual and neurodevelopmental outcomes in VPT children and adolescents. For example, neonatal hippocampal volume reductions have been linked to developmental and motor delays at 2 years of age (Thompson et al., Reference Thompson, Wood., Doyle, Warfield, Lodygensky, Anderson and Inder2008), and reduced hippocampal volumes at 8 years of age has been associated with poorer full-scale IQ (Lodygensky et al., Reference Lodygensky, Rademaker, Zimine, Gex-Fabry, Lieftink, Lazeyras and Huppi2005; Peterson et al., Reference Peterson, Vohr, Staib, Cannistraci, Dolberg, Schneider and Ment2000). In this study, we found no evidence for an association between hippocampi volume and IQ. Possible explanations for this discrepancy include the younger age of children in the current study, varying measures of IQ used across studies, and differences in segmentation protocols for hippocampal formations between studies.
Additionally, previous research has linked reduced hippocampal volumes during adolescent years to everyday memory impairment (Isaacs et al., Reference Isaacs, Lucas, Chong, Wood, Johnson, Marshall and Gadian2000, Reference Isaacs, Vargha-Khadem, Watkins, Lucas, Mishkin and Gadian2003), and verbal learning and recognition memory impairment (Giménez et al., Reference Giménez, Junqúe, Narberhaus, Caldú, Salgago-Pineda, Bargalló and Botet2004) in VPT cohorts. Although VPT children in our study have been found to perform poorer than term controls in IQ and memory measures (Omizzolo et al., Reference Omizzolo, Scratch, Stargatt, Kidokoro, Thompson, Lee and Anderson2013), we were unable to associate memory functioning or IQ with hippocampal volumes measured at 7 years. Thus, our findings suggest that the effects of VPT birth on memory and learning at age 7 years are not confined to hippocampal volumes alone.
The VPT brain is not a typically developing brain. A recent account of functional localization suggests that when a task is sufficiently difficult, and therefore exceeds the resources of a particular brain area, other areas will be recruited to assist with the excess workload (Just & Varma, Reference Just and Varma2007). When the development of a particular brain structure is altered during VPT birth, such as the hippocampi, resources in this area may be reduced and additional regions recruited (Lawrence et al., Reference Lawrence, McGuire, Allin, Walshe, Giampietro, Murray and Nosarti2010). This theoretical account may explain why hippocampal volume itself was insufficient to explain memory and learning deficits in our VPT cohort, and suggests the involvement of more complex networks and neural systems.
The hippocampal formations have extensive connections across multiple brain regions (Rolls, Reference Rolls2000; Thierry, Gioanni, Degenetais, & Glowinski, Reference Thierry, Gioanni, Degenetais and Glowinski2000). Studies investigating neural networks underlying memory and learning highlight the important role of the prefrontal and parietal cortices (Cabeza & Nyberg, Reference Cabeza and Nyberg1997), and show hippocampal-prefrontal interactions (Hasselmo, & Sarter, Reference Hasselmo and Sarter2011). For example, the right anterior hippocampus and the right dorsolateral prefrontal cortex have been implicated with successful verbal memory processing in adults (Johnson, Saykin, Flashman, McAllister, & Sparling, Reference Johnson, Saykin, Flashman, McAllister and Sparling2001). Furthermore, prefrontal regions have been associated with general cognitive ability and memory in VPT children (Woodward et al., Reference Woodward, Edgin, Thompson and Inder2005). Early damage to the hippocampi associated with VPT birth, such as neonatal brain injury and the effects of corticosteroids, may secondarily influence memory and learning abilities by disrupting the underlying neural and functional circuitry of these areas.
The current study has several strengths. First, measures that assess multiple components of memory and learning were used to investigate the relationship with hippocampal volume in childhood. This contrasts previous literature that reports a limited number of neuropsychological measures. Second, each child participated in a mock MRI scan which exposed them to the scanning environment to ensure best quality images. Finally, our study was the first to have neonatal brain imaging in a VPT cohort, and therefore, allowed us to control for the effect of early neonatal brain abnormalities on later outcome.
While the current study provides insight into the integrity of the hippocampal formations following VPT birth, one methodological limitation remains to be addressed. Although manual tracing is considered the gold standard in measuring hippocampal volume (Konrad et al., Reference Konrad, Ukas, Nebel., Arolt, Toga and Narr2009), it is prone to human error, especially when defining the boundaries of the hippocampal and entorhinal regions and the borders between the hippocampus and amygdala (Konrad et al., Reference Konrad, Ukas, Nebel., Arolt, Toga and Narr2009). Furthermore, there are a large number of different anatomical protocols for delineating the hippocampal formation, which provide a possible source of variance and inconsistency in findings between studies and conditions (Geuze, Vermetten, & Bremner, Reference Geuze, Vermetten and Bremner2005; Konrad et al., Reference Konrad, Ukas, Nebel., Arolt, Toga and Narr2009; Van Leemput et al., Reference Van Leemput, Bakkour, Benner, Wiggins, Wald, Augustinack and Fischl2009). In the current study, however, there was excellent intra-observer and inter-observer agreement in measurement of hippocampal volumes.
In conclusion, findings from this study demonstrate that hippocampal volume alone does not give sufficient insight into the role that this vital region plays in memory and learning in children born VPT at 7 years of age. Future research might examine whether particular regions of the hippocampus are more affected by VPT birth, and whether specific regions are more strongly associated with memory and learning outcomes. Research investigating the neural substrates and networks which foster memory and learning in this population are also needed, ideally using techniques such as tractography. Establishing the functional consequences of altered hippocampal development will help us understand and identify at-risk children early in their development.
Acknowledgments
We acknowledge the input of the entire VIBeS research team, and all the families who participated in this study. This study was funded by Australia's National Health & Medical Research Council (Project Grants (237117 & 491209), Early Career Award (1012236 to D.T.), Senior Research Fellowship (628371 to P.A.)), National Institutes of Health (HD058056), and the Victorian Government's Operational Infrastructure Support Program. The information in this manuscript and the manuscript itself has never been published either electronically or in print, and there are no conflicts of interest.