Introduction
Traumatic brain injury (TBI) is associated with declines in cognitive, affective, behavioral and physical domains (Ponsford, Reference Ponsford, Levin, Shum and Chan2014). It is also associated with decreased capacity to complete activities of daily living, live independently and return to productivity (Ponsford, Draper, & Schönberger, Reference Ponsford, Draper and Schönberger2008). Given that TBI leads to structural damage to anterior and inferior surfaces of frontal and temporal lobes, as well as secondary diffuse axonal injury (Gennarelli & Graham, Reference Gennarelli and Graham1998), researchers have endeavored to understand how measures of injury relate to daily life functioning post-TBI.
Studies have related cognitive functioning on standardized neuropsychological tests to structural imaging post-TBI (Levine et al., Reference Levine, Kovacevic, Nica, Schwartz, Gao and Black2013; Stuss & Alexander, Reference Stuss and Alexander2007). Understanding how structural damage relates to everyday outcomes is more complex, but is nonetheless important for developing better predictions of outcome in clinical settings. Interpretation of findings across prior studies of brain-behavior relationships in TBI outcome has been hampered by heterogeneity of neuroimaging methods and choice of outcome measures. Prior studies have focused on the role of focal lesions, yielding mixed results, with some studies showing a relation between lesion presence and affective (Schönberger et al., Reference Schönberger, Ponsford, Reutens, Beare, Clarke and O’Sullivan2011), psychosocial (Sherer, Hart, Whyte, Nick, & Yablon, Reference Sherer, Hart, Whyte, Nick and Yablon2005) and functional outcomes (Kesler, Adams, & Bigler, Reference Kesler, Adams and Bigler2000), while other studies show small or non-significant relationships (Lehtonen et al., Reference Lehtonen, Stringer, Millis, Boake, Englander, Hart and Whyte2005).
Diffuse injury, as quantified by volume loss at the chronic stage, is a primary neuropathology of TBI (Levine et al., Reference Levine, Kovacevic, Nica, Cheung, Gao, Schwartz and Black2008; Povlishock & Katz, Reference Povlishock and Katz2005). With the advent of quantified structural analysis of high resolution magnetic resonance images, more recent research has demonstrated that diffuse axonal injury alone leads to disruption of large-scale brain networks; and, that the degree of disconnection is related to cognitive and behavioral outcomes (Kim et al., Reference Kim, Parker, Hart, Pluta, Ingalhalikar, Coslett and Verma2014). For example, studies have shown that TBI leads to dysregulation of the default mode and fronto-parietal control networks (Bonnelle et al., Reference Bonnelle, Leech, Kinnunen, Ham, Beckmann, Boissezon and Sharp2011). Given the established role of these networks in supporting cognitive performance (e.g., Schwindt et al., Reference Schwindt, Chaudhary, Crane, Ganda, Masellis, Grady and Black2013), distributed volume loss affecting elements of these networks is expected to relate to post-TBI outcomes.
The current investigation took a data-driven, multivariate approach in relating structural damage to measures of functioning post-TBI to examine relationships across functional domains while simultaneously relating this to structural injury. In the present study, 60 individuals were assessed with a comprehensive battery of outcome measures spanning physical, cognitive, emotional, and independent functioning and scanned with high-resolution structural magnetic resonance imaging (MRI) at 1-year post-injury. Regional brain volumes were quantified with a standardized protocol that yielded measures of both focal and diffuse injury. Partial least squares (PLS) correlation—an assumption-free multivariate approach—was used to characterize the pattern of covariance between the measures of brain volume and outcome.
Methods
Participants
Participants were recruited from Sunnybrook Health Sciences Centre in Toronto, Canada as part of the Toronto TBI study (Levine et al., Reference Levine, Kovacevic, Nica, Cheung, Gao, Schwartz and Black2008). Average time since injury was 12.7 months (SD=4.6). The sample reported here is further described in Levine et al. Reference Levine, Kovacevic, Nica, Schwartz, Gao and Black2013, with the exception of three participants who were excluded because of missing data. Average age of the sample was 30.8 years (SD=10.5) with 14.4 years of education (SD=2.4). Injury severity was determined by the Glasgow Coma Scale (GCS; Teasdale & Jennett, Reference Teasdale and Jennett1974) at the time of discharge from critical care, augmented by duration of unconsciousness, and post-traumatic amnesia, or presence of focal lesions (see Levine et al., Reference Levine, Kovacevic, Nica, Schwartz, Gao and Black2013). Thirteen participants had sustained a mild injury (GCS 13–15), 26 had sustained a moderate injury (GCS 9–12) and 21 had sustained a severe injury (GCS<8). Severity classification was upgraded in six cases where extended loss of consciousness (>2 hr), post-traumatic amnesia (>48 hr) or focal lesions suggested more severe injury than indicated by GCS. Eighteen non-injured, age (F(3,77)=0.77; p=.52), and education (F(3,77)=1.92; p =.13) matched comparison participants recruited from TBI participants’ friends and family as well as the Rotman Research Institute participant pool completed the outcome measures (Table 1). These participants were not scanned. Data were collected in compliance with institutional ethics boards.
Table 1 Age, education, and outcome measures by group
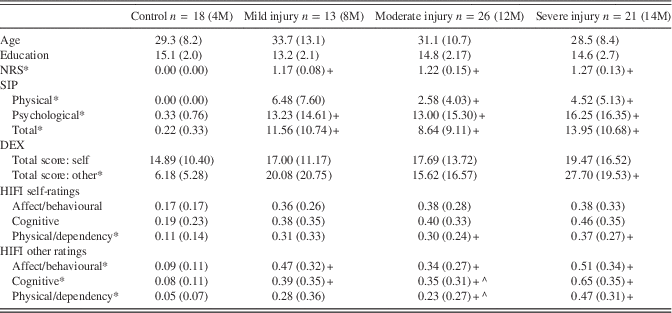
Note. NRS scores represent the average of all 29 items rated on a 0-4 scale. SIP scores are the percentage of items endorsed (present/not present) by scale and overall (136 items). DEX items (20 total) are rated on a 0-4 scale and totaled (max=80). HIFI scores represent the proportion of symptoms endorsed (present/not present) for each scale (affect/behavioural=14; cognitive=9; physical/dependency=8). Means (and standard deviations) are presented. M=males. Separate one-way ANOVAs were conducted for each measure and corrected for multiple comparisons using a false discovery rate procedure (.05 level; Benjamini & Hochberg, Reference Benjamini and Hochberg1995). *Significant ANOVAs were followed up with Tamhane T2 post-hoc comparisons (due to unequal variances). +Different from control group (p<.05); ^Different from severe injury group (p<.05).
Measures of Post-injury Functioning
Measures of post-TBI functioning included the Neurobehavioral Rating Scale (NRS; Levin et al., Reference Levin, Mattis, Ruff, Eisenberg, Marshall, Tabaddor and Frankowski1987), an examiner-rated symptom checklist capturing a broad range of domains: overall cognitive and behavioral deficits, insight, physical difficulties, mood disturbances, and language difficulties. Examiners were blinded to group. The participant’s total score was analyzed. The self-rated Sickness Impact Profile (SIP; Bergner, Bobbitt, Carter, & Gilson, Reference Bergner, Bobbitt, Carter and Gilson1981) includes a physical scale (ambulation, mobility, personal care, and fluidness of movement), a psychosocial scale (social engagement, reasoning, problem solving, level of disorientation, lability, irritability, and communication), and a total score. The total score consists of the physical and psychosocial scales as well as items querying sleep and rest, eating, ability to work, and engagement in household and recreational activities. The Head Injury Family Interview Problem Checklist (HIFI; Kay, Cavallo, Ezrachi, & Vavagiakis, Reference Kay, Cavallo, Ezrachi and Vavagiakis1995), completed by TBI participants and their informant (significant-other), includes an affect/behavioral subscale (emotion regulation, lability, depression, irritability, and anxiety), a cognitive subscale (planning, goal attainment, concentration, memory, and communication), and a physical/dependency subscale (apathy, initiative, speed, need for supervision along with physical symptoms such as dysarthria, poor balance, and vision problems). The Dysexecutive Function Questionnaire (DEX; Burgess, Wilson, Evans, & Emslie, Reference Burgess, Wilson, Evans and Emslie1997) consists of subscales measuring inhibition, intention, executive and memory functioning, positive and negative affect. Both self- and significant other ratings were included for the HIFI and the DEX. The Glasgow Outcome Scale (Jennett & Bond, Reference Jennett and Bond1975) was also administered to characterize gross level of recovery.
Image Acquisition and Processing
The procedure for image acquisition and processing is described elsewhere (Levine et al., Reference Levine, Kovacevic, Nica, Cheung, Gao, Schwartz and Black2008, Reference Levine, Kovacevic, Nica, Schwartz, Gao and Black2013). Briefly, all participants were scanned with a 1.5 Tesla MRI system at the time of data collection. T1-weighted, T2-weighted, and gradient echo T2 (in TBI individuals) sequences were obtained. Twenty participants (10 moderate, 10 severe) were identified as having focal cortical contusions largely in frontotemporal areas (8 right, 4 left, and 8 bilateral). These focal lesions, appearing on at least two slices, had a minimal diameter of 3 mm and were manually defined in the axial plane. A board-certified neuroradiologist specializing in TBI also reviewed the images for visible TBI neuropathology (e.g., contusions, diffuse axonal injury, etc.).
Brain MRI data were analyzed using a previously reported image processing pipeline (Levine et al., Reference Levine, Kovacevic, Nica, Cheung, Gao, Schwartz and Black2008, Reference Levine, Kovacevic, Nica, Schwartz, Gao and Black2013) for template matching, brain extraction, segmentation of gray matter, white matter, sulcal and ventricular cerebrospinal fluid (CSF), and lesion volumes. A modified Semi-Automated Brain Region Extraction (SABRE) method was used to derive 38 regions of interest (ROIs) customized to fit each patient’s brain anatomy. Regional volumes were adjusted for total intracranial capacity using a regression-based method (Arndt, Cohen, Alliger, Swayze, & Andreasen, Reference Arndt, Cohen, Alliger, Swayze and Andreasen1991). For the present study, a total of 36 CSF volumes (i.e., the inverse of total brain parenchyma, excluding the external capsule/corona radiata regions) were used to characterize volume loss. These provided the most stable patterns, with little additional information yielded by inclusion of gray and white matter volumes. PLS analyses were conducted using MATLAB and univariate analyses with SPSS.
Statistical Analyses
For pre-processing, a winsorization procedure was applied to outliers with scores greater than 2.5 SDs of the true mean. This affected 4.4% of the psychosocial data and 1.8% of the volumetric data. Missing data points (1.5%) were estimated based on the group mean according to severity. Table 1 includes conventional univariate statistics for descriptive purposes. Separate one-way analyses of variance, corrected for multiple comparisons (Benjamini & Hochberg, Reference Benjamini and Hochberg1995), were conducted and followed up with Tamhane T2 post hoc comparisons due to unequal variances (Table 1). However, we emphasize the multivariate (PLS) approach in interpreting the data.
The PLS correlation analysis (Krishnan, Williams, McIntosh, & Abdi, Reference Krishnan, Williams, McIntosh and Abdi2011) characterizes shared variance between two datasets. This is accomplished by identifying the correlation between two covariance matrices—in our case, volume loss data and outcome. Singular value decomposition (SVD) is then applied to the brain-behavior correlation matrix to identify latent variables (LVs) that express the maximal covariance common to both datasets. The latent variables identified are mutually independent. The statistical significance of each LV was assessed by 500 permutation tests with a threshold of p<.05, in which observations were shuffled within subjects to calculate the probability of each latent variable having occurred by chance. The stability of each brain region’s contribution to the LV is determined through bootstrap resampling (subjects were resampled 500 times with 50% of observations resampled with replacement). Brain regions were considered reliable if they had a ratio of salience to standard error (hereafter referred to as the bootstrap ratio, interpreted similar to a Z-score) greater than 3, corresponding to 99% confidence limits. PLS is a useful multivariate approach for when examining a large set of variables that are highly collinear. It also considers all brain region volumes in a single computational step so there is no requirement to correct for multiple comparisons.
We also used PLS to assess the relationship between TBI severity classification and outcome (i.e., without considering brain imaging data) to provide a comparison of acute injury severity data to chronic neuroimaging data in the prediction of outcome functioning. Finally, to assess for the impact of focal frontotemporal lesions on performance, the 20 patients with identified lesions were compared to the moderate/severe TBI participants without focal lesions (n=27).
Results
Injury Characteristics in Relation to Outcome
Nearly all TBI participants were classified as having good outcomes in gross terms, as evidenced by return to work or school and Glasgow Outcome Scale score (average of 4.69; SD=0.46). Raw scores on the 12 outcome measures of interest are presented by group in Table 1.
Two preliminary PLS analyses examined the effects of acute TBI injury severity and the presence of frontotemporal focal lesions on post-TBI functioning (Figure 1a). Prior studies have shown that acutely assessed injury severity does not clearly predict outcome at the chronic phase (Hoofien et al., 2002; Novack, Bush, Meythaler, & Canupp, Reference Novack, Bush, Meythaler and Canupp2001), and the usefulness of lesion presence for differentiating long-term outcomes has also been mixed (Lehtonen et al., Reference Lehtonen, Stringer, Millis, Boake, Englander, Hart and Whyte2005). Thus, we wished to examine the extent to which acute injury severity and lesion presence differentiate our groups in terms of outcome functioning. The analysis of GCS and outcome identified a single LV (p<.001, accounting for 91% of the cross-block covariance) that dissociated control participants from TBI participants in general (Figure 1). The severe group significantly contributed to the pattern whereas the other TBI groups did not, yet the three severity groups did not differ from each other. This is consistent with past literature showing little relation between acute injury severity and long-term outcomes. All outcome measures contributed to this LV with one exception, the DEX-self overall score, a measure that is often elevated in healthy adults (Burgess, Alderman, Evans, Emslie, & Wilson, Reference Burgess, Alderman, Evans, Emslie and Wilson1998).

Fig. 1 (a) Extent to which injury group status is related to outcome. Error bars reflect 99% confidence intervals. Error bars that do not cross the horizontal axis reflect a significant contribution to the latent variable. (b) Warm colors represent significant cerebrospinal fluid (CSF) volumes (p<.01) represented by the latent variable. ROIs extracted according to SABRE regional cortical divisions in axial and sagittal views. Abbreviations: LSF: lateral superior frontal, MSF: medial superior frontal, LMF: lateral middle frontal, MMF: medial middle frontal, LVF: lateral ventral frontal, MVF: medial ventral frontal, GCG: genual cingulate gyrus, ACG: anterior cingulate gyrus, MCG: middle cingulate gyrus, PCG: posterior cingulate gyrus, AT: anterior temporal, MT: medial temporal, PT: posterior temporal, O: occipital, ABGT: anterior basal ganglia/thalamus, PBGT: posterior basal ganglia/thalamus, EC: external capsule/corona radiata, IP: inferior parietal, SP: superior parietal. Right: right hemisphere, Left: left hemisphere. (c) Outcome measures related to volume loss.
When TBI participants with and without lesions were compared (moderate and severe injury only), the findings were similar to the above analysis. A single LV was identified (p<.001, accounting for 99% of the cross-block covariance) whereby both TBI groups were statistically differentiated from healthy comparison participants, again on all measures except the DEX-self (Figure 1). The lesion and no-lesion TBI groups did not differ.
Structural Volume Loss in Relation to Outcome
Our analysis of primary interest was a PLS correlation analysis identifying the shared covariance (as LVs) between whole-brain volume loss and outcome. The analysis yielded a single LV (p<.01, accounting for 51% of the cross-block covariance). Volume loss was greatest in the bilateral middle medial frontal regions and the cingulate gyrus (right greater than left; Figure 1b). The right inferior parietal, bilateral aspects of the basal ganglia/thalamus, bilateral medial temporal and right lateral frontal regions also showed significant volume loss that related to post-TBI outcome. The HIFI physical/dependency subscale as rated by the informants was the only significant outcome measure identified as defined by 99% confidence intervals: (Figure 1c). Other measures did not share significant variance with the pattern of volume loss observed.
Discussion
TBI is associated with declines in daily life functioning across cognitive, affective, behavioral and physical domains. The neuroanatomical correlates of such changes are poorly understood. In this investigation, a comprehensive battery of outcome measures was administered contemporaneously with high resolution structural MRI. We found that TBI in general was associated with elevated endorsement across the spectrum of functioning assessed by our battery. Although there was evidence that the severe TBI group was more impaired on some measures, there were no differences across severity groups when patterns across the battery were considered in a multivariate framework, replicating prior results showing that acutely assessed injury severity does not predict outcome at the chronic phase (Novack et al., Reference Novack, Bush, Meythaler and Canupp2001). In our sample, individuals with focal lesions were not differentiated from those without focal lesions who sustained a TBI of similar severity. This suggests that, in the chronic phase, those with and without focal frontotemporal lesions attain similar levels of daily life functioning.
A single outcome measure querying self-initiated behaviors and circumscribed physical symptoms (significant-other ratings on the HIFI physical/dependency scale) shared a significant amount of variance with a pattern of volume loss which was greatest over medial regions including bilateral middle medial prefrontal and cingulate gyrus. The right inferior parietal, bilateral basal ganglia, bilateral medial temporal and right lateral frontal regions also contributed to the pattern. Given previous literature, described below, demonstrating a relationship between medial inferior frontal and cingulate integrity and self-initiation behaviors (Cummings, Reference Cummings1993; Stuss & Alexander, Reference Stuss and Alexander2007) we speculate that the pattern of covariance captured by the latent variable may largely represent features of midline-frontal-subcortical dysfunction. These findings cannot be attributed to focal lesions, which were located ventral to the ROIs identified in this latent variable.
The cingulate gyrus and midline prefrontal regions are part of a frontal-subcortical circuit that mediates motivated behavior. Damage in these regions causes clinical syndromes of akinetic mutism at the acute stages, resolving to an apathetic presentation at the chronic phase (Cummings, Reference Cummings1993). More subtle deficits in apathy and initiation—also referred to as “energization”—have been detected in patients with chronic stable medial prefrontal lesions (Stuss & Alexander, Reference Stuss and Alexander2007). The identified brain pattern also overlaps with elements of the default mode network and fronto-parietal control networks, where TBI-related changes are associated with attentional lapses (Bonnelle et al., Reference Bonnelle, Leech, Kinnunen, Ham, Beckmann, Boissezon and Sharp2011).
The relationship between volume loss and outcome was observed in significant-other ratings; the contribution of TBI participants’ self-ratings on this measure fell just short of reliability based on our 99% confidence interval cut-off. Significant-other ratings may reflect a more objective assessment given awareness and insight deficits that are characteristic of TBI (Sherer et al., Reference Sherer, Boake, Levin, Silver, Ringholz and High1998). The self-report format of the measures, and/or other variables such as mental health problems (not formally assessed), may have contributed to the overall limited relation observed between outcome and volume loss. However, these possible limitations cannot easily explain the highly specific pattern consistent with known midline and cingulate functions.
Aside from the significant-other rated HIFI physical/dependency scale, it is striking that none of the outcome measures contributed significantly to the LV. This suggests that individual differences in regional brain volume do not relate to everyday changes in memory, attention, executive functioning, or emotional functioning (at least in this sample), even though such relationships were evident on neuropsychological assessment of these abilities (Levine et al., Reference Levine, Kovacevic, Nica, Schwartz, Gao and Black2013). The neural correlates of these abilities as assessed by questionnaires may be more diffusely represented than is the case for functions served by the medial-prefrontal circuit, and, therefore, less tractable given the ROI resolution of this study. It is also the case that compensation for these functions may be more readily attainable at 1 year in this relatively high functioning sample; inclusion of TBI participants with greater cognitive and behavioral deficits may have yielded more significant brain-behavior correlations.
Although our conclusions are supported by an established brain-behavior relationship between midline frontal regions and self-initiation, we acknowledge that these results were derived from a data-driven (as opposed to hypothesis-driven) analysis and, therefore, require replication. If replicated and further refined, future studies may be able to identify the combination of structural imaging and behavioral report that could identify individuals suited to therapies for improving self-initiation, such as pharmacological intervention, which has shown some efficacy in individuals with TBI and apathy (Wortzel & Arciniegas, Reference Wortzel and Arciniegas2012).
Acknowledgments
Ann Campbell, Catherine Hynes, Sabitha Kanagasabai, Charlene O’Connor, Colleen O’Toole, Marina Mandic, Karen Philp, Adriana Restagno, Jovanka Skocic, and Gary Turner are thanked for technical assistance. Natasa Kovacevic, Fuqiang Gao, Joel Ramirez, and Sandra Black are thanked for assistance with the neuroimaging pipeline. We gratefully thank the TBI participants and non-injured volunteers for participating in this research. This research was supported by grants from the Canadian Institutes of Health Research (Grant #s MT-14744, MOP-37535, and MOP-108540), and the NIH-NICHD (Grant #HD42385-01) to B.L. Conflicts of interest: Dr. Levine provides neuropsychological consultation in medicolegal cases involving TBI. Emma B. Guild is now at the Krembil Neuroscience Centre, University Health Network, Toronto, Ontario, Canada.