Introduction
Advancing age is associated with decline in multiple cognitive domains, including episodic memory (Tulving, Reference Tulving1984), particularly the encoding of recent events (Grady, McIntosh, & Craik, Reference Grady, McIntosh and Craik2003; Morcom, Good, Frackowiak, & Rugg, Reference Morcom, Good, Frackowiak and Rugg2003). Recognition memory, the ability to recognize previously encountered events, objects, or people, has also been shown to display an age-related decline (Duverne, Motamedinia, & Rugg, Reference Duverne, Motamedinia and Rugg2009; Grady et al., Reference Grady, Grady, McIntosh, McIntosh, Craik and Craik2005). Recent studies have identified a network of cortical regions – each interconnected with the medial temporal lobe – that are consistently engaged during successful episodic retrieval (Rugg & Vilberg, Reference Rugg and Vilberg2013). Older age has been associated with alterations to this network, including greater frontal recruitment in conjunction with decreased occipital and parietal activation during episodic memory performance, (Anderson et al., Reference Anderson, Iidaka, Cabeza, Kapur, McIntosh and Craik2000; Cabeza, Reference Cabeza2004; Cabeza, Anderson, Houle, Mangels, & Nyberg, Reference Cabeza, Anderson, Houle, Mangels and Nyberg2000; Davis, Dennis, Daselaar, Fleck, & Cabeza, Reference Davis, Dennis, Daselaar, Fleck and Cabeza2008).
Other studies have found age-related decreases in prefrontal cortex activity, resulting in a more bilateral prefrontal activation pattern in older adults (Anderson et al., Reference Anderson, Iidaka, Cabeza, Kapur, McIntosh and Craik2000; Cabeza et al., Reference Cabeza, Grady, Nyberg, McIntosh, Tulving, Kapur and Craik1997, Reference Cabeza, Anderson, Houle, Mangels and Nyberg2000; Schacter, Savage, Alpert, Rauch, & Albert, Reference Schacter, Savage, Alpert, Rauch and Albert1996; Schiavetto, Köhler, Grady, Winocur, & Moscovitch, Reference Schiavetto, Köhler, Grady, Winocur and Moscovitch2002). Likewise, functional connectivity—the temporal correlation of neural activity between spatially remote regions—also shows age-related changes during episodic memory, including decreased functional coupling between the hippocampus and the retrosplenial and parietotemporal cortices (Daselaar, Fleck, Dobbins, Madden, & Cabeza, Reference Daselaar, Fleck, Dobbins, Madden and Cabeza2006).
A postulated mechanism underlying age-associated changes in episodic memory is structural alterations in cerebral white matter, with subsequent changes in functional brain activity, particularly coordinated activity among distal regions. Older age has long been associated with structural deterioration of white matter (Tang, Nyengaard, Pakkenberg, & Gundersen, 1997), a process which in turn is likely associated with disruption of neural networks underlying normal cognitive function, including episodic memory function (Grady, Reference Grady2008; Greenwood, Reference Greenwood2007; O’Sullivan et al., Reference O’Sullivan, Jones, Summers, Morris, Williams and Markus2001; Pfefferbaum, Adalsteinsson, & Sullivan, Reference Pfefferbaum, Adalsteinsson and Sullivan2005). Age-related reductions in white matter fractional anisotropy (FA) have been found to correlate with memory decline (Charlton, Schiavone, Barrick, Morris, & Markus, Reference Charlton, Schiavone, Barrick, Morris and Markus2010; Gunning-Dixon & Raz, Reference Gunning-Dixon and Raz2000; O’Sullivan et al., Reference O’Sullivan, Jones, Summers, Morris, Williams and Markus2001; Persson et al., Reference Persson, Nyberg, Lind, Larsson, Nilsson, Ingvar and Buckner2006), and recent diffusion MRI tractography studies (Lockhart et al., Reference Lockhart, Mayda, Roach, Fletcher, Carmichael, Maillard and DeCarli2012; Metzler-Baddeley, Jones, Belaroussi, Aggleton, & O’Sullivan, Reference Metzler-Baddeley, Jones, Belaroussi, Aggleton and O’Sullivan2011) have found age-related decline in recall to be associated with microstructural degradation in several white matter tracts including the fornix, which connects the hippocampal formation to the prefrontal cortex (Poletti and Creswell, Reference Poletti and Creswell1977), the uncinate fasciculus, connecting the anterior temporal lobe with the orbital and medial prefrontal cortex (Crosby, Reference Crosby1962; Schmahmann & Pandya, Reference Schmahmann and Pandya2007), and the inferior longitudinal fasciculus, a major fiber bundle linking the anterior temporal lobe to the occipital lobe (Catani, Howard, Pajevic, & Jones, Reference Catani, Howard, Pajevic and Jones2002; Schmahmann & Pandya, Reference Schmahmann and Pandya2007). Changes to myelin and axonal fibers in aging may cause less stable flow of electrical currents in dendrites and axons, resulting in disruptions in conduction of action potentials and degradation of coordinated activity across the brain.
The importance of white matter microstructure for cognitive function is rooted in the spatial distribution of cognitive processes in the brain, involving a complex interplay between multiple areas (Gläscher et al., Reference Gläscher, Rudrauf, Colom, Paul, Tranel, Damasio and Adolphs2010). Thus, declines in white matter microstructure are associated with disrupted network functional connectivity between brain regions interconnected with compromised structural fibers (Antonenko, Meinzer, Lindenberg, Witte, & Flöel, Reference Antonenko, Meinzer, Lindenberg, Witte and Flöel2012; Davis, Kragel, Madden, & Cabeza, Reference Davis, Kragel, Madden and Cabeza2012; Steffener, habeck, & Stern, Reference Steffener, Habeck and Stern2012). In contrast, greater integrity of white matter fibers is associated with stronger functional connectivity (Davis et al., Reference Davis, Kragel, Madden and Cabeza2012). However, no studies have yet investigated whether the relationship between white matter microstructure, behavior, and task-related functional connectivity explains episodic recognition performance and whether these relationships are modulated by a common influence of age.
In this study, we examine whether intact tract connectivity between cortical regions within the recognition network would be associated with better memory performance and greater task related functional connectivity among cortical regions in the episodic recognition network. Combining task-related connectivity analyses with measures of white matter microstructure may provide greater insight into the mechanisms underlying age-related differences in memory function.
Methods
Participants
Two hundred ninety-two healthy, right-handed adults aged 44–75 years (mean±SD=61.15±6.71 years) participated in one MRI session [functional MRI (fMRI) and diffusion tensor imaging (DTI)] as part of brain imaging studies on memory and aging. All participants were from the Wisconsin Registry for Alzheimer’s Prevention (WRAP) (Sager, Hermann, & La Rue, Reference Sager, Hermann and La Rue2005), which is a registry of healthy middle-aged adults who either have no parental family history of Alzheimer’s disease (AD) or at least one parent with late onset AD. Positive parental family history of AD classification was defined as having one or both parents with AD as determined by a validated interview (Kawas, Segal, Stewart, Corrada, & Thal, Reference Kawas, Segal, Stewart, Corrada and Thal1994), autopsy-confirmed, or probable AD as outlined by research criteria (McKhann et al., Reference McKhann, Drachman, Folstein, Katzman, Price and Stadlan1984, Reference McKhann, Knopman, Chertkow, Hyman, Jack, Kawas and Phelps2011), and was reviewed by a multidisciplinary diagnostic consensus panel. Absence of family history of AD required that the participant’s father survive to at least age 70 years and the mother to age 75 years without diagnosis of dementia or cognitive deterioration. Family history was classified as a binary variable. APOE ε4 genetic testing was performed by the Wisconsin Alzheimer’s Disease Research Center. Participants were categorized using a binary variable as an APOE ε4 carrier or non-carrier.
Inclusion criteria for all subjects consisted of the following: normal cognitive function determined by neuropsychological evaluation, no current diagnosis of major psychiatric disease or other major medical conditions (e.g., diabetes, myocardial infarction, or recent history of cancer), no history of head trauma, and no contraindications for a MRI scan. Three participants were removed from the analysis based on Mini-Mental State Exam (MMSE) scores below 28, and seven participants were removed due to abnormal findings on radiological read of the MRI. A final sample size of 282 participants (94 males and 188 females) was included in the analyses. Study procedures were approved by the University of Wisconsin Health Sciences Institutional Review Board and were in accordance with U.S. federal regulations. All participants provided written informed consent.
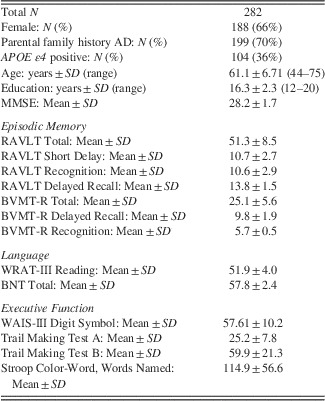
In addition to MRI, participants received a neuropsychological assessment (Table 1). The MMSE (Folstein, Folstein, & McHugh, Reference Folstein, Folstein and McHugh1975) was used as a global measure of cognition. Episodic memory was assessed with the Rey Auditory Verbal Learning Test (RAVLT) (Schmidt, Reference Schmidt1996) and Brief Visuospatial Memory Test (BVMT) (Benedict, Reference Benedict1997). Verbal generation and fluency were measured with the Wide Range Achievement Test-III reading subtest (WRAT-III) (Jastak & Wilkinson, Reference Jastak and Wilkinson1984) and the Boston Naming Test (BNT) (Kaplan, Goodglass, & Weintraub, Reference Kaplan, Goodglass and Weintraub1983). Different aspects of executive functions included: response speed and focused attention, measured with the Digits Symbol Substitution test from the Wechsler Adult Intelligence Scale – third Edition (WAIS-III) (Baddeley, Reference Baddeley1996), attention switching, examined with the Trail Making Test A and Trail Making Test B, which requires alternation between letters and digits (TMT-A, TMT-B) (Reitan & Wolfson, Reference Reitan and Wolfson1993), and suppression of response-incongruent information, measured with the Stroop test (Trenerry, Crosson, Deboe, & Leber, Reference Trenerry, Crosson, Deboe and Leber1989).
Table 1 Demographic features and neuropsychological measures

Note. Neuropsychological scores reported above are raw mean scores and standard deviations (SD). AD=Alzheimer’s disease; APOE ε4=Apolipoprotein E, ε4; BNT=Boston Naming Test (Kaplan et al., Reference Kaplan, Goodglass and Weintraub1983); BVMT-R=Brief Visuospatial Memory Test-Revised (Benedict, Reference Benedict1997); Digit Symbol (from WAIS-III) (Baddeley, Reference Baddeley1996); MMSE=Mini Mental State Examination (Folstein et al., Reference Folstein, Folstein and McHugh1975); RAVLT=Rey Auditory Verbal Learning Test (Schmidt, Reference Schmidt1996); TMTA/B=Trail Making Test A and B (Reitan & Wolfson, Reference Reitan and Wolfson1993); WRAT-III=Wide Range Achievement Test-III reading subtest (Jastak & Wilkinson, Reference Jastak and Wilkinson1984).
MRI Acquisition
Participants were imaged on a General Electric 3.0 Tesla Discovery MR750 (Waukesha, WI) MRI system with an eight-channel head coil. During the fMRI task, for each participant and for each run, 167 whole-brain volumes were acquired to measure the T2*-weighted blood oxygenation level dependent (BOLD) effect with the following parameters: gradient-recall echo-planar imaging (EPI), repetition time (TR)=2000 ms, echo time (TE)=30 ms, flip angle=90°, 64×64 matrix, field of view (FOV)=240 mm, thirty 4-mm-thick sagittal slices with a 1-mm gap acquired within each TR, resulting in voxel dimensions of 3.75×3.75×5 mm3. Three additional volumes were acquired at the beginning of the first run to allow for steady-state magnetization (discarded from analysis). Head movement during scanning was minimized with padding. Parallel imaging was employed using an array spatial sensitivity encoding technique (ASSET).
DTI was acquired using a diffusion-weighted, spin-echo, single-shot, EPI pulse sequence in 40 encoding directions at b=1300 s/mm2, with eight non-diffusion weighted (b=0) reference images. The cerebrum was covered using contiguous 2.5-mm-thick axial slices, FOV=240 mm, TR=8000 ms, TE=67.8 ms, 96×96 mm matrix, resulting in 2.5 mm isotropic voxels. High order shimming was performed before the DTI acquisition to optimize the homogeneity of the magnetic field across the brain and to minimize EPI distortions.
Behavioral Task
A schematic diagram of the fMRI task is provided in Figure 1. This paradigm and a variant has been previously published by our group in adults with memory decline (Nicholas et al., Reference Nicholas, Okonkwo, Bendlin, Oh, Asthana, Rowley and Johnson2014; Xu et al., Reference Xu, McLaren, Ries, Fitzgerald, Bendlin, Rowley and Johnson2009). Thirty minutes before fMRI, participants completed one run (48 trials) of an implicit encoding task where images of neutral faces were visually presented for 3000 ms with a 500 ms interstimulus interval. Using a labeled keyboard, participants rated each image on a Likert scale of 1 (“not at all”) to 4 (“very much”) based on the following encoding contexts: age, energy, distinctiveness, attractiveness, and likeability. The order of the encoding contexts and the presentation of the 48 faces were randomized for each participant. During fMRI, using an event-related design, participants completed two runs (96 trials, 11 min and 8 s) of a recognition task where images from the encoding phase, randomly mixed with novel images, were each presented for 2200 ms. Images presented in one run were not presented in the other run. Participants discriminated via button press between images previously viewed during the encoding phase or novel images resulting in their respective selection being highlighted on-screen. The average stimulus onset asynchrony was 6.8 s (range 4–11 s) with most stimulus onset asynchrony being 5 s, 6 s, or 7 s. The order of the runs was counterbalanced across participants.

Fig. 1 Outside the scanner, participants completed an implicit encoding task (a) where neutral faces were presented. Images were rated on a Likert scale of 1–4 based on the following characteristics: age, energy, distinctiveness, attractiveness, and likeability. During scanning, participants completed the recognition task (b) that consisted of discriminating via button press between images from the encoding task and randomly mixed novel images.
Reaction time for correctly identified images previously viewed and novel images was calculated. Sensitivity was defined as the statistic d’, which is a measure of the distance between the signal and the signal plus noise. d’ is interpreted as a measure of discrimination sensitivity and was calculated according to signal detection theory (Harvey, Reference Harvey1992): d’=ZHit Rate–ZFalse Alarm Rate, where the raw hit rate and raw false alarm rate are Z-score transformed from probabilities. Participants who performed at a hit rate <50% were excluded. Moreover, trials that received either no response or multiple responses were not included in any of the analyses.
fMRI Data Preprocessing and Analysis
fMRI echo-planar imaging data were slice-time corrected using Analysis of Functional NeuroImages (AFNI). The first three fMRI volumes acquired while the participant viewed the task instructions were discarded from the analysis. Motion correction to the first functional scan was performed using a six-parameter rigid-body transformation using Statistical Parametric Mapping (SPM8) (http://www.fil.ion.ucl.ac.uk/spm/software/spm8). For each individual, the mean of the functional images was spatially normalized to the Montreal Neurological Institute (MNI) template by applying a 12–parameter affine transformation followed by nonlinear warping (Ashburner & Friston, Reference Ashburner and Friston1999). The computed transformation parameters were applied to all of the functional images, interpolated to a final voxel size of 3×3×3 mm3. Images were subsequently spatially smoothed with an 8 mm Gaussian kernel.
A mixed-effects, event-related statistical analysis was performed in a two-level procedure. At the first-level, a separate general linear model was specified for each participant. BOLD responses time-locked to the onset of previously viewed images, novel images, and error trials were modeled separately, by convolving the onset vectors with a synthetic hemodynamic response function as implemented by SPM8. Trials were modeled for two TRs duration or 4000 ms duration. At the model estimation, the data were high-pass filtered (128 s cut-off) to remove low-frequency drifts, and serial correlations were accounted for by an autoregressive model of the first order. Other regressors entered into the first-level models included six parameters that represented the motion-related variance in the data (3 for rigid-body translation and 3 for rotation). Contrast images comparing activity associated with identifying previously viewed images to activity associated with identifying novel images were created. The individual contrast images were then entered into a second-level random-effects analysis, using a one-sample t test, to assess the group effect. The resulting summary statistical map was thresholded at p<.05 (topological FDR-corrected for multiple comparisons across the whole brain, voxel extent=20) (Figure 2) and was used as an inclusive mask for the functional connectivity analysis.

Fig. 2 Regions recruited during recognition (previously viewed images>novel images). Areas included bilateral posterior cingulate, precuneus, cuneus, angular gyrus, middle frontal gyrus, and bilateral hippocampus. p<.05, corrected for multiple comparisons. The statistical map is overlaid on a structural template. The color bar represents t values, ranging from 0 to 13.
Task-Related Functional Connectivity Analysis
We conducted generalized form of context-dependent psychophysiological interaction (gPPI) analyses with the Generalized PPI toolbox (McLaren, Ries, Xu, & Johnson, Reference McLaren, Ries, Xu and Johnson2012) (http://www.nitrc.org/projects/gppi/), which was implemented in MatLab R2011b and using functions in SPM8. Six regions of interest (ROIs) were defined as seed regions. We selected ROIs based on brain regions known to be part of the recognition network (Rugg & Vilberg, Reference Rugg and Vilberg2013) and showing group peak activation during recognition (previous viewed images>novel images). Seed ROIs were created as 6-mm spheres centered on peak activations in the left precuneus (MNI coordinates: −8 −62 28), left posterior cingulate (MNI coordinates: −8 −5432), bilateral hippocampus (MNI coordinates: 34, −34, 10; −30 −18 −22), right cuneus (MNI coordinates: 12 −9022), left angular gyrus (MNI coordinates: −40 −74 42), and left middle frontal gyrus (MNI coordinates: −44 32 4).
The gPPI model involved general linear modeling of the seed timecourse (physiological regressors), the task conditions (psychological regressors), and an interaction term for each task condition×the seed time course. Although errors were modeled at the first level univariate analysis, due to the low percentage of errors (accuracy on the task was 89.4%) they were not included in the gPPI model. For each subject, the physiological activity was computed as the deconvolved mean time series of all voxels within each ROI. The psychological regressors were created by separately convolving 2 task vectors: (1) correct previously viewed images and (2) a regressor for correct novel images, with the canonical hemodynamic response function. The interaction terms (PPIs) were then computed by multiplying the time series from the psychological regressors with the physiological activity. A whole-brain analysis (single-subject level) was performed using the general linear model in SPM8 with five regressors modeling the BOLD signal per run: two PPI regressors, two psychological/task regressors, and the mean time course in the seed region. Additional nuisance covariates included the same motion and session parameters that were also entered into the mean signal analyses, and the data were again subjected to a high-pass filter with a cutoff of 128 s.
For each of the six seed regions and for each subject, a PPI contrast was created and identified recognition-related changes in connectivity. Subsequently, these first-level PPI contrast images were entered into second-level analyses.
DTI Data Preprocessing and Analysis
The processing stream is reported in Adluru et al. (Reference Adluru, Destiche, Lu, Doran, Birdsill, Melah and Bendlin2014) and is briefly repeated here. A robust processing pipeline was used, based on methods in Zhang, Avants, and others. (Reference Zhang, Avants, Yushkevich, Woo, Wang, McCluskey and Gee2007). First, head motion and image distortions (stretches and shears) due to eddy currents were corrected with affine transformation in the FSL (FMRIB Software) package (http://www.fmrib.ox.ac.uk/fsl/). Geometric distortion from the inhomogeneous magnetic field applied was corrected with the b=0 field map and PRELUDE (phase region expanding labeler for unwrapping discrete estimates) and FUGUE (FMRIB’s utility for geometrically unwarping EPIs) from FSL. Brain tissue was extracted using FSL’s BET (Brain Extraction Tool). Tensor fitting was performed using a nonlinear least squares method in Camino (http://cmic.cs.ucl.ac.uk/camino/).
Individual maps were registered to a population specific template constructed using Diffusion Tensor Imaging Toolkit (DTI-TK, http://www.nitric.org/projects/dtitk/), which is an optimized DTI spatial normalization and atlas construction tool (Wang et al., Reference Wang, Gupta, Liu, Zhang, Escolar, Gilmore and Styner2011; Zhang, Yushkevich, Alexander, & Gee, Reference Zhang, Yushkevich, Alexander and Gee2006; Zhang, Yushkevich, Rueckert, & Gee, Reference Zhang, Yushkevich, Rueckert and Gee2007). A subset of 80 diffusion tensor maps was used to create a common space template. All diffusion tensor maps were normalized to the template with a rigid, affine, and diffeomorphic alignments and interpolated to 2×2×2 mm3 voxels. With DTI-TK, FA maps were calculated in the normalized space and were visually inspected to rule out inclusion of maps with missing data in ROIs or other artifacts. White matter alignment was performed using a diffeomorphic (topology preserving) registration method (Zhang, Avants, et al., Reference Zhang, Avants, Yushkevich, Woo, Wang, McCluskey and Gee2007) that incrementally estimates the displacement field using a tensor-based registration formulation (Zhang et al., Reference Zhang, Yushkevich, Alexander and Gee2006).
The Johns Hopkins International Consortium for Brain Mapping (ICBM) FA template was warped to the study’s template space using Advanced Normalization Tools (ANTS). Using ANTS, the Johns Hopkins ROIs (Wakana, Jiang, Nagae-Poetscher, van Zijl, & Mori, Reference Wakana, Jiang, Nagae-Poetscher, van Zijl and Mori2004) were individually warped to the study’s template space. Seven bilateral white matter ROIs were chosen for analysis: the genu and splenium of the corpus callosum, the fornix (body and column), the cingulum bundles adjacent to the cingulate cortex (cingulum-CC, left and right averaged), the cingulum bundle projections to the hippocampus (cingulum-HC, left and right averaged), the superior longitudinal fasciculi (SLF, left and right averaged), and the uncinate fasciculi (left and right averaged). The FA map for each subject in normalized template space was masked by the chosen ROIs. The resulting FA ROIs were thresholded at 0.2 to reduce inclusion of gray matter voxels in the white matter masks. The thresholded FA ROIs were binarized and used as ROI masks to isolate the white matter ROIs of each subject’s standard space FA maps. Finally, the average values of FA within each subject’s ROI-masked maps were calculated.
Statistical Analyses
Statistical analyses were performed using R version 3.1.3 statistical package (http://www.r-project.org/). Age correlations with reaction time, recognition performance, and white matter measures were assessed. In addition, regression analyses were done to investigate relationships between recognition performance and white matter measures. Analyses included sex and education as covariates.
One-sample t tests at the group level identified voxels where connectivity with a given seed was higher during correct responses to previously viewed images versus novel images. In addition, using FA and d’, regression analyses were performed to investigate the influence of white matter microstructure and behavior on task-related functional connectivity within the recognition network. Sex and education were covaried. Statistical images were thresholded at p<.001 uncorrected, and were masked inclusively by the group-level univariate recognition contrast. To exclude small clusters and increase the anatomical plausibility of the results, a cluster size threshold of 25 contiguous voxels was used and analyses were restricted to gray matter using a binary mask.
Results
Behavioral Results
Demographics and neuropsychological test performance are in Table 1. The participants’ performance, as measured by d’, ranged from 0.79 to 4.07 (mean±standard deviation [SD]=2.76±0.68) and was negatively correlated with age (t281=−5.65; r2=0.12; p<.05). Hit rates (z-transformed) ranged from 0.13 to 2.03 (mean±SD=1.49±0.4) and false alarm rates (z-transformed) ranged from −2.03 to 0.37 (mean±SD=−1.27±0.48). On average, participants responded significantly more quickly to a previously viewed face (mean±SD=1145.71 ms±284.32) compared to a novel face (mean±SD=1313.11 ms±308.63) (paired t test; t281=−16.31; p<.05). In addition, participants who responded faster had significantly better performance (t281=−8.55; r2=0.22; p<.05), and higher age was correlated with slower reaction time (t281=5.76; r2=0.11; p<.05).
Correlations between Age, Performance, and White Matter Measures
Task behavior (d’, hit rates, and false alarm rates) by decade of age is presented in Table 2. Table 3 lists white matter ROI mean FA values and their zero order correlations (r) with age and performance. There were negative correlations between age and FA in several ROIs: the cingulum subjacent to the posterior cingulate (t281=−2.93; r=−0.172; p=.003), fornix (t281=−5.41; r=−0.307; p<.001), genu (t281=−6.99; r=−0.385; p<.01), splenium (t281=−1.99; r=−0.118; p=.046), and SLF (t281=−2.64; r=−0.155; p=.008). In addition, fornix FA was positively correlated with performance (t281=2.15; r=0.127; p=.03). Given that there were significant effects of age on fornix FA and performance, we assessed whether the relationship between fornix FA and performance remained significant after controlling for age. When we control age on the relationship between fornix FA and performance, we find the following partial correlation r=−0.09, p=.099. In addition, we conducted a mediation analysis to assess whether fornix FA mediated the relationship between age and d’ using bootstrap procedures. Unstandardized indirect effects were computed for each of the 10,000 bootstrapped samples, and the 95% confidence interval was computed by determining the indirect effects at the 2.5th and 97.5th percentiles. The bootstrapped unstandardized indirect effect was −0.00133, and the confidence interval ranged from −0.00689 to 0.004. Thus, fornix FA did not significantly mediate the effect of age on recognition performance.
Table 2 Mean task performance by age group

Table 3 Zero order correlations between FA and age or performance (d’)

*p<0.05.
**p<0.01.
***p<0.001.
FA=fractional anisotropy; SLF=superior longitudinal fasciculus.
gPPI Results
Using each of the a priori ROIs as seed regions, we then performed gPPI analyses. Since the fornix was the only white matter ROI to significantly correlate with performance, we chose to investigate whether task-related function connectivity during recognition was influenced by fornix FA, together with d’.
The imaging regression analysis revealed that fornix FA was positively correlated with dynamic connectivity of the right hippocampus seed and the right caudate during the recognition task (difference between viewing responses to previously viewed images versus novel images) (peak: 8 16 6; t281=3.94; k=41); of the left precuneus seed and the left precuneus (peak: −14 −52 42; t281=4.37; k=80), bilateral middle occipital gyrus (left peak: −22 −76 4; t281=4.02; k=25; right peak: 22 −72 2; t281=3.98; k=80), and the left middle frontal gyrus (peak: −40 30 28; t281=3.87; k=32) (Table 4, Figure 3). Data are not shown for one outlier with a parameter estimate greater than 3 standard deviations from the mean in the left middle frontal gyrus seed PPI connectivity difference with the left precuneus seed (Figure 3A).

Fig. 3 Regions of increased dynamic connectivity during the recognition task associated with greater fornix FA (p<.001, uncorrected; k>25). Linear regressions of parameter estimates of cluster (in magenta) gPPI connectivity with seed regions (in red) and fornix FA are plotted (p<.05). The statistical map is overlaid on a structural template. FA=fractional anisotropy; L=left; R=right; MFG=middle frontal gyrus; MOG=middle occipital gyrus; gPPI=generalized psychophysiological interaction.
Table 4 Regions of dynamic connectivity (p<.001, uncorrected, k>25)

Note. Cluster size is expressed in number of voxels.
L=Left, R=Right.
d’ was positively correlated with dynamic connectivity of the left posterior cingulate seed and left middle frontal gyrus during recognition memory (peak: −30 20 54; t281=3.52; k=44); and of the right cuneus seed with the left hippocampus (peak: −24 −36 −6; t281=3.86; k=62) (Table 4, Figure 4). Data are not shown for one outlier with a parameter estimate greater than 3 standard deviations from the mean in the left middle frontal gyrus PPI connectivity difference with the left posterior cingulate seed (Figure 4B).

Fig. 4 Regions of increased dynamic connectivity during the recognition task associated with greater performance (d’) (p<.001, uncorrected; k>25). Linear regressions of parameter estimates of cluster (in magenta) gPPI connectivity with seed regions (in red) and performance (d’) are plotted (p<.05). The statistical map is overlaid on a structural template. L=left; R=right; MFG=middle frontal gyrus; PCC=posterior cingulate cortex; gPPI=generalized psychophysiological interaction.
One-sample t tests at the group level (p<.001, uncorrected; k>25 voxels) revealed no other region in which there was dynamic connectivity with each of the six seed regions during the recognition task.
Discussion
Age-related variation in episodic recognition performance in middle-to-late aged individuals was found to correlate specifically with the microstructure of the fornix, a crucial connection between the medial temporal lobe and both prefrontal and subcortical regions involved in memory. In addition, the magnitude of dynamic connectivity between recognition-elicited regions covaried across subjects with performance and fornix microstructure. Consistent with previous work in our group (Adluru et al., Reference Adluru, Destiche, Lu, Doran, Birdsill, Melah and Bendlin2014), there were aging effects on fractional anisotropy of the fornix, genu, and splenium of the corpus callosum, the cingulum subjacent to the posterior cingulate, and the SLF. Our results complement previous literature pointing to the relationship between higher connectivity in the context of successful episodic memory retrieval (King, de Chastelaine, Elward, Wang, & Rugg, Reference King, de Chastelaine, Elward, Wang and Rugg2015; Schedlbauer, Copara, Watrous, & Ekstrom, Reference Schedlbauer, Copara, Watrous and Ekstrom2014; Staresina, Cooper, & Henson, Reference Staresina, Cooper and Henson2013; Watrous, Tandon, Conner, Pieters, & Ekstrom, Reference Watrous, Tandon, Conner, Pieters and Ekstrom2013); however, to the authors’ knowledge, this is the first study to determine that recognition-related connectivity is influenced by white matter microstructure.
Mean fornix fractional anisotropy values positively correlated with recognition performance. This finding is consistent with the view that the fornix, together with the hippocampus, is critically and specifically involved in specific facets of episodic memory (Aggleton & Brown, Reference Aggleton and Brown2006; Lockhart et al., Reference Lockhart, Mayda, Roach, Fletcher, Carmichael, Maillard and DeCarli2012; Metzler-Baddeley et al., Reference Metzler-Baddeley, Jones, Belaroussi, Aggleton and O’Sullivan2011; Rudebeck et al., Reference Rudebeck, Scholz, Millington, Rohenkohl, Johansen-Berg and Lee2009). Aggleton and Brown (Reference Aggleton and Brown2006) propose a model that places emphasis on hippocampal interactions with the mammillary bodies and thalamus, also via the fornix, which provides an indirect, alternative route to influence the prefrontal cortex. Across studies of young and older adults, higher fornix FA was found to be associated with better working memory (Zahr, Rohlfing, Pfefferbaum, & Sullivan, Reference Zahr, Rohlfing, Pfefferbaum and Sullivan2009), episodic memory (Rudebeck et al., Reference Rudebeck, Scholz, Millington, Rohenkohl, Johansen-Berg and Lee2009), and with both verbal and visual recall tasks (Rudebeck et al., Reference Rudebeck, Scholz, Millington, Rohenkohl, Johansen-Berg and Lee2009; Zahr et al., Reference Zahr, Rohlfing, Pfefferbaum and Sullivan2009). Moreover, recent diffusion MRI tractography studies (Lockhart et al., Reference Lockhart, Mayda, Roach, Fletcher, Carmichael, Maillard and DeCarli2012; Metzler-Baddeley et al., Reference Metzler-Baddeley, Jones, Belaroussi, Aggleton and O’Sullivan2011) have found age-related decline in recall to be associated with microstructural degradation in the fornix.
We observed that dynamic connectivity between posterior regions (posterior cingulate cortex and cuneus) and frontal (middle frontal gyrus) and the MTL (hippocampus) during the recognition task was associated with better memory performance. This finding supports a recent study (King et al., Reference King, de Chastelaine, Elward, Wang and Rugg2015) that found across three experiments that the magnitude of recollection-related increases in connectivity predicted recollection accuracy. Task-related functional connectivity studies have shown that during episodic encoding higher performing older adults have greater functional connectivity between hippocampal activity and activity in the dorsolateral prefrontal and parietal cortex (Grady et al., Reference Grady, McIntosh and Craik2003). Daselaar and colleagues (Reference Daselaar, Fleck, Dobbins, Madden and Cabeza2006) found that age-related decline in recollection-elicited activity in the hippocampus was associated with decreased functional coupling between the hippocampus and the retrosplenial and parietotemporal cortices. Moreover, greater dynamic connectivity with the middle frontal gyrus supports compensation models that posit older adults may be exhibiting greater top-down modulation from the frontal cortex to account for task demands and diminishing neural function (Cabeza & Dennis, Reference Cabeza and Dennis2012). Our present findings suggest that recognition-related modulation of connectivity can also serve as a predictor of individual differences in recognition accuracy.
Although prior studies have reported relationships between fornix fractional anisotropy and low-frequency fluctuations in the BOLD signal during sustained periods of task engagement or rest (Kehoe et al., Reference Kehoe, Farrell, Metzler-Baddeley, Lawlor, Kenny, Lyons and Bokde2015; Salami, Pudas, & Nyberg, Reference Salami, Pudas and Nyberg2014), we present novel findings indicating that inter-individual variation in fornix microstructure is related to dynamic connectivity between regions within the recognition network. We observed that more preserved fornix white matter microstructure predicted dynamic connectivity between hippocampus and the caudate during a recognition task. The fornix is a major efferent and afferent white matter conduit from the hippocampi with precommissural projections to subcortical structures such as the striatum, which consists of the caudate and putamen. Although most studies examining the role of the striatum have focused on its involvement in motor response learning in anticipation of action-outcome contingencies (Faure, Haberland, Condé, & El Massioui, Reference Faure, Haberland, Condé and El Massioui2005; Williams & Eskandar, Reference Williams and Eskandar2006; Yin, Ostlund, Knowlton, & Balleine, Reference Yin, Ostlund, Knowlton and Balleine2005), it has been argued that the striatum functions are not exclusively motoric, and that they are important for episodic memory behavior. Specifically, it has been suggested that the dorsal striatum supports processes in which certain stimuli need to be filtered out, whereas others need to be actively maintained in working memory (McNab & Klingberg, Reference McNab and Klingberg2008). Of interest, a neuroimaging study found that successful memory was associated with greater activity in both the hippocampus and the striatum during encoding and that this activity correlated for items that were later remembered, but not for items that were forgotten (Sadeh, Shohamy, Levy, Reggev, & Maril, Reference Sadeh, Shohamy, Levy, Reggev and Maril2011). Moreover, episodic memory impairments are found in patients with focal lesions to the striatum, mainly the caudate (Vakil, Blachstein, & Soroker, Reference Vakil, Blachstein and Soroker2004).
In addition, we observed that higher fornix fractional anisotropy predicted dynamic connectivity of the precuneus with the bilateral middle occipital gyrus and left middle frontal gyrus during the recognition task. Although the fornix does not directly project to the precuneus, Zhuang et al. (Reference Zhuang, Sachdev, Trollor, Kochan, Reppermund, Brodaty and Wen2012) found that lower FA values within the fornix coupled with altered white matter microstructure in other brain regions critical to memory including the precuneus, predicting conversion to amnestic mild cognitive impairment. Additionally, the fornix carries direct projections to the medial prefrontal cortex (Poletti & Creswell, Reference Poletti and Creswell1977); although we should caution that our results cannot inform the directionality of dynamic connectivity between the precuneus and the middle frontal gyrus in our study. Altered connectivity as predicted by fornix FA in our study may also reflect common underlying changes due to age, given that during normal aging, the earliest morphological changes occur within medial temporal lobe gray matter, specifically the hippocampus and entorhinal structures with secondary effect to the efferent outflow tract through the fornix and mammillary bodies (Aggleton & Brown, Reference Aggleton and Brown1999; Braak & Braak, Reference Braak and Braak1995; Cassel, Duconseille, Jeltsch, & Will, Reference Cassel, Duconseille, Jeltsch and Will1997). Needless to say, the underlying age-related processes affect an interconnected network of regions rather than single regions.
This functional connectivity study and others shed light on how networks interact to subserve numerous cognitive processes. Anatomical and resting-state connectivity research suggest that brain regions are not part of single networks but are part of multiple networks that have preferred connections or “coordinated states” (Hellyer et al., Reference Hellyer, Shanahan, Scott, Wise, Sharp and Leech2014; McLaren, Sperling, & Atri, Reference McLaren, Sperling and Atri2014). Importantly, the brain can rapidly switch between these coordinated states to perform different cognitive functions. The transition from one state to another enables the brain to integrate information from a multitude of brain regions. FA is sensitive to multiple microstructural properties of white matter, including axon density, myelination, and possibly diameter (Beaulieu, Reference Beaulieu2002; Takahashi et al., Reference Takahashi, Yonezawa, Takahashi, Kudo, Inoue and Tohgi2002). Dynamic connectivity-FA correlations might be caused by variation in axon density or diameter. Electromyography (EMG) amplitude and area both increase with fiber density in physiological studies of peripheral nerves; likewise, EMG amplitude and area increase with fiber density and axon diameter in computer simulations (Finsterer & Fuglsang-Frederiksen, Reference Finsterer and Fuglsang-Frederiksen2000; Nandedkar & Stalberg, Reference Nandedkar and Stalberg1983). It is possible that increased myelination or axon diameter across individuals led to more-synchronized axonal conduction velocities, affecting the degree of interregional modulation observed.
These findings join a body of literature suggesting that microstructural white matter differences influence both cognitive performance and functional connectivity in aging. Microstructural white matter alterations have been associated with slower processing speed, declines in executive function, and lower memory performance (Antonenko et al., Reference Antonenko, Meinzer, Lindenberg, Witte and Flöel2012; Davis, Kragel, Madden, & Cabeza, Reference Davis, Kragel, Madden and Cabeza2012; Steffener et al., Reference Steffener, Habeck and Stern2012). With regard to functional connectivity, older adults with higher corpus callosum fractional anisotropy show stronger functional connectivity during lateralized word matching, a task requiring cross-hemispheric communication (Davis et al., Reference Davis, Kragel, Madden and Cabeza2012). Life experiences, such as bilingualism, have been shown to be associated with higher white matter microstructure as well as with more distributed patterns of functional connectivity in older adults (Luk, Bialystok, Craik, & Grady, Reference Luk, Bialystok, Craik and Grady2011). Moreover, compared to monolinguals, the bilingual older adults show greater cognitive control and display increased functional connectivity in a frontal posterior network. While age itself has a robust effect on white matter microstructure, white matter development over the lifespan is influenced by several factors, both environmental and genetic.
There are a few limitations to the current study. Although the sample was enriched for Alzheimer’s disease risk factors, we did not find an effect of parental family history or APOE ε4 genotype on demographic characteristics, behavior, or any of the imaging measures. Given that the participants in the study were screened for memory impairment, and the close association between our findings and other findings in the field, it is more likely that the results represent age-related changes rather than pathological changes. The participants in this study are followed longitudinally, eventually permitting the separation of participants experiencing pathological aging from the group. In our study, the strength of the relationship between white matter measures with age and performance is smaller than what has been reported in the literature. However, our results are consistent with other studies reporting links between fornix white matter, age, and memory performance (Lockhart et al., Reference Lockhart, Mayda, Roach, Fletcher, Carmichael, Maillard and DeCarli2012; Metzler-Baddeley et al., Reference Metzler-Baddeley, Jones, Belaroussi, Aggleton and O’Sullivan2011) and are reported from a much larger sample size, which may provide a more precise estimate.
We should also note that in addition to age, functional connectivity, and white matter microstructure, other factors not measured in the current study may influence face recognition performance, including the perceived age of presented faces in relation to the participants’ age (Lamont, Stewart-Williams, & Podd, Reference Lamont, Stewart-Williams and Podd2005). Moreover, given that age-related gray matter atrophy has also been associated with memory performance (Du et al., Reference Du, Schuff, Zhu, Jagust, Miller, Reed and Weiner2003; Head, Kennedy, Rodrigue, & Raz, Reference Head, Kennedy, Rodrigue and Raz2009; Persson et al., Reference Persson, Nyberg, Lind, Larsson, Nilsson, Ingvar and Buckner2006; Rajah, Languay, & Grady, Reference Rajah, Languay and Grady2011; Raz et al., Reference Raz, Rodrigue, Kennedy, Dahle, Head and Acker2003; Rodrigue & Raz, Reference Rodrigue and Raz2004; Yonelinas et al., Reference Yonelinas, Widaman, Mungas, Reed, Weiner and Chui2007), future work will aim at investigating the contribution of gray matter decline to changes in functional connectivity of memory networks. Lastly, although the gPPI results are uncorrected, which could result in Type I error, these findings are still informative and useful for the aging literature, especially in light of the dearth of studies with large sample sizes investigating dynamic connectivity and their relationship to structural connectivity and behavior.
In conclusion, the findings suggest that variation in white matter microstructure is associated with memory performance, and functional connectivity among cortical regions in the recognition memory network. While age had a significant impact on microstructure across several white matter regions, the differences in fornix microstructure had the greatest impact on both functional connectivity and memory performance. The results provide further evidence that age-related cognitive differences can be better understood by incorporating measures reflecting white matter tract connectivity.
Acknowledgments
This work was supported by the National Institutes of Health (B.B.B., R01 AG037639), (S.C.J., R01 AG027161), (ADRC P50 AG033514), (S.C.J., R01 AG021155), (T32 GM007507), (T32 AG000213); Veterans Administration Merit Review Grant (S.C.J., I01CX000165); and the Waisman Center Core Grant (P30 HD003352-45) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The project was also facilitated by the facilities and resources at the Geriatric Research, Education, and Clinical Center (GRECC) of the William S. Middleton Memorial Veterans Hospital, Madison, WI. The authors gratefully acknowledge Nancy Davenport, Amy Hawley, Sandra Harding, Caitlin Cleary, Jay Fruehling, Chuck Illingworth, and the support of researchers and staff at the Waisman Center, University of Wisconsin, Madison, where imaging data were collected. Above all, we thank our dedicated volunteers for their participation in this research. Ly, Adluru, Destiche, Lu, Oh, Hoscheidt, Okonkwo, Sager, Johnson, and Bendlin report no conflicts of interest. Rowley has provided consultation to and/or received honoraria from GE Healthcare, Bracco, Lundbeck, HL Gore, and Eli Lilly. Alexander is part owner and Chief Operating Officer of inSERT, Inc.