INTRODUCTION
The world’s population of people over 65 years of age is growing rapidly and will within 10 years for the first time in history outnumber children under 5 years of age (Kinsella & He, Reference Kinsella, He and Bureau2008). The progressively aging population and the associated increase in patients suffering from neurodegenerative disorders like Alzheimer’s disease (AD) present huge economic and health-related challenges for the near future. Aging is associated with cognitive decline (Salthouse, Reference Salthouse2009), although not uniformly across various cognitive functions (Hedden & Gabrieli, Reference Hedden and Gabrieli2004). Findings of decreased memory among physically and mentally healthy older adults (Chee et al., Reference Chee, Chen, Zheng, Chan, Isaac and Sim2009; Craik, Reference Craik1994) are paralleled by age-related reductions in brain volume, thinning of the cerebral cortex, and expansion of the ventricular system (Allen, Bruss, Brown, & Damasio, Reference Allen, Bruss, Brown and Damasio2005; Blatter et al., Reference Blatter, Bigler, Gale, Johnson, Anderson and Burnett1995; Cardenas et al., Reference Cardenas, Chao, Studholme, Yaffe, Miller and Madison2009; Fjell et al., Reference Fjell, Westlye, Amlien, Espeseth, Reinvang and Raz2009; Fotenos, Snyder, Girton, Morris, & Buckner, Reference Fotenos, Snyder, Girton, Morris and Buckner2005; Jernigan et al., Reference Jernigan, Archibald, Berhow, Sowell, Foster and Hesselink1991, Reference Jernigan, Archibald, Fennema-Notestine, Gamst, Stout and Bonner2001; Kruggel, Reference Kruggel2006; Pfefferbaum et al., Reference Pfefferbaum, Mathalon, Sullivan, Rawles, Zipursky and Lim1994; Raz et al., Reference Raz, Gunning, Head, Dupuis, McQuain and Briggs1997, Reference Raz, Gunning-Dixon, Head, Rodrigue, Williamson and Acker2004; Resnick, Pham, Kraut, Zonderman, & Davatzikos, Reference Resnick, Pham, Kraut, Zonderman and Davatzikos2003; Salat et al., Reference Salat, Buckner, Snyder, Greve, Desikan and Busa2004; Taki et al., Reference Taki, Goto, Evans, Zijdenbos, Neelin and Lerch2004; Walhovd et al., Reference Walhovd, Fjell, Reinvang, Lundervold, Dale and Eilertsen2005; Westlye et al., Reference Westlye, Walhovd, Dale, Bjornerud, Due-Tonnessen and Engvig2010). Moreover, there is evidence of subjectively perceived memory decline in elderly adults, although not necessarily perceived as a threat to daily functioning (Lovelace & Twohig, Reference Lovelace and Twohig1990). The relations between subjectively perceived memory and brain morphology have been studied in individuals with memory complaints (de Groot et al., Reference de Groot, de Leeuw, Oudkerk, Hofman, Jolles and Breteler2001; Minett, Dean, Firbank, English, & O/Brien, Reference Minett, Dean, Firbank, English and O’Brien2005; Miranda et al., Reference Miranda, Madureira, Verdelho, Ferro, Pantoni and Salvadori2008; Saykin et al., Reference Saykin, Wishart, Rabin, Santulli, Flashman and West2006; van der Flier et al., Reference van der Flier, van Buchem, Weverling-Rijnsburger, Mutsaers, Bollen and Admiraal-Behloul2004). However, little is known about the morphometric correlates of everyday subjectively perceived memory in the healthy elderly without worries concerning their cognitive status.
Questions pertaining to the ecological validity of neuropsychological assessments have led to a growing interest in cognition studied outside the laboratory setting (Chaytor & Schmitter-Edgecombe, Reference Chaytor and Schmitter-Edgecombe2003). Studies of subjectively reported everyday memory function may provide a source of knowledge about the human memory system different from standardized tests and can thus be a valuable supplement to laboratory memory research. One widely used inventory to investigate perceived memory failures in everyday settings is the Everyday Memory Questionnaire (EMQ), originally developed to investigate subjective memory impairments in patients with severe brain injuries (Sunderland, Harris, & Baddeley, Reference Sunderland, Harris and Baddeley1983), but also used in nonclinical samples. The EMQ relies on self-report and does not reflect memory processes, per se, but is rather a measure of subjectively perceived memory in the daily life. There is modest support of a relationship between EMQ self-ratings and objective memory performance in patient groups (Boake, Freeland, Ringholz, Nance, & Edwards, Reference Boake, Freeland, Ringholz, Nance and Edwards1995; Lincoln & Tinson, Reference Lincoln and Tinson1989; Sunderland et al., Reference Sunderland, Harris and Baddeley1983; Sunderland, Stewart, & Sluman, Reference Sunderland, Stewart and Sluman1996). Findings of low or modest validity are not necessarily a result of weak questionnaire design, but could reflect that people hold inaccurate beliefs about their own memory performance and that the content of the questionnaire differs a great deal from laboratory tasks (Herrmann, Reference Herrmann1982; Hickox & Sunderland, Reference Hickox, Sunderland and Crawford1992). Moreover, normal subjects seem to give more reliable responses to questionnaires of everyday performance (Hickox & Sunderland, Reference Hickox, Sunderland and Crawford1992), and the EMQ does distinguish various clinical from nonclinical groups (Drysdale, Shores, & Levick, Reference Drysdale, Shores and Levick2004; Montgomery & Fisk, Reference Montgomery and Fisk2007; Royle & Lincoln, Reference Royle and Lincoln2008; Schwartz & McMillan, Reference Schwartz and McMillan1989; Sunderland et al., Reference Sunderland, Harris and Baddeley1983). Some attempts have been made to examine the factor structure of the EMQ (Cornish, Reference Cornish2000; Richardson & Chan, Reference Richardson and Chan1995; Royle & Lincoln, Reference Royle and Lincoln2008) with different sample populations. The factors extracted seem to reflect underlying memory processes and are reasonably consistent across studies with some differences that might reflect the different sample populations used.
What has been learned about the relationship among subjectively perceived memory, cognitive performance, and brain morphometry has been derived from studies of elderly persons referred to the clinic with worries about their memory function. Subjective memory complaints do not necessarily reflect objective measures of cognitive performance (Derouesne et al., Reference Derouesne, Alperovitch, Arvay, Migeon, Moulin and Vollant1989; Hanninen et al., Reference Hanninen, Reinikainen, Helkala, Koivisto, Mykkanen and Laakso1994; McDougall, Becker, & Arheart, Reference McDougall, Becker and Arheart2006; O’Connor, Pollitt, Roth, Brook, & Reiss, Reference O’Connor, Pollitt, Roth, Brook and Reiss1990). Memory complaints in individuals without cognitive impairment have been associated with fronto-temporal and medial temporal lobe (MTL) atrophy (Saykin et al., Reference Saykin, Wishart, Rabin, Santulli, Flashman and West2006; van der Flier et al., Reference van der Flier, van Buchem, Weverling-Rijnsburger, Mutsaers, Bollen and Admiraal-Behloul2004). These regions are part of an episodic memory network comprising MTL structures, medial and lateral parietal, as well as prefrontal cortices (Buckner, Reference Buckner2004; Buckner & Carroll, Reference Buckner and Carroll2007; Buckner & Wheeler, Reference Buckner and Wheeler2001). Cortical thickness in these areas has also been associated with verbal memory performance in healthy participants (Walhovd et al., Reference Walhovd, Fjell, Dale, Fischl, Quinn and Makris2006; Yonelinas et al., Reference Yonelinas, Widaman, Mungas, Reed, Weiner and Chui2007), as well as in patients with mild cognitive impairment (Rossi et al., Reference Rossi, Geroldi, Bresciani, Testa, Binetti and Zanetti2007; Walhovd et al., Reference Walhovd, Fjell, Amlien, Grambaite, Stenset and Bjørnerud2009) and AD (Dickerson et al., Reference Dickerson, Feczko, Augustinack, Pacheco, Morris and Fischl2009; Walhovd et al., Reference Walhovd, Fjell, Amlien, Grambaite, Stenset and Bjørnerud2009). In the present study, we measured thickness in chosen regions within this network, i.e., medial and lateral temporal lobe, medial and lateral parietal lobe, as well as superior and inferior frontal lobe. However, as EMQ covers various aspects of memory, other neural structures are also likely implicated. The hippocampus is essential in various aspects of memory, including transfer and consolidation of information from short-term memory to long-term memory (Squire & Zola-Morgan, Reference Squire and Zola-Morgan1991). Basal ganglia structures are implicated in procedural or implicit memory such as motor memory and operant conditioning (McDonald & White, Reference McDonald and White1993; Packard & White, Reference Packard and White1991), as well as being part of a frontostriatal memory system, including working memory, reasoning, and strategic memory processes (Alexander, DeLong, & Strick, Reference Alexander, DeLong and Strick1986; Gabrieli, Singh, Stebbins, & Goetz, Reference Gabrieli, Singh, Stebbins and Goetz1996).
The purpose of the present study was to investigate the relationships among subjectively reported daily life memory, cortical thickness, and volumes of subcortical structures in the above described memory network in healthy individuals without specific worries concerning their own memory function. In order to investigate if the EMQ is sensitive to information beyond that captured by objective testing in healthy elderly, EMQ data are compared to a standardized test on objective memory performance, the California Verbal Learning Test (CVLT) of verbal learning and memory function, and its relation to morphometry. Because the EMQ consists of several items likely related to a few underlying functions, a principal component analysis (PCA) was performed to identify superordinate structures of subjectively perceived memory function, which again could be related to interindividual variations in brain structure. The purpose of performing PCA, instead of analyzing each EMQ item separately, was that we believe the extracted components may yield more reliable and more valid results, i.e., they will not depend as much upon the specific formulations given in each item of the EMQ. In addition, the number of comparisons is drastically reduced by this approach. As several of the EMQ items are expected to correlate to different degrees, analysis of each item in isolation may also hinder interpretation of the correlations with brain structural measure. The analyses were performed on participants above 60 years of age because age-related changes in cognition are commonly reported in this age range (Beason-Held, Kraut, & Resnick, Reference Beason-Held, Kraut and Resnick2008a, Reference Beason-Held, Kraut and Resnick2008b).
METHOD
Participants
The sample was drawn from a large longitudinal research project called Cognition and Plasticity Through the Life-Span (Fjell et al., Reference Fjell, Westlye, Greve, Fischl, Benner and van der Kouwe2008; Westlye, Walhovd, Bjørnerud, Due-Tønnessen, & Fjell, Reference Westlye, Walhovd, Bjørnerud, Due-Tønnessen and Fjell2009) administrated from the Center for the Study of Human Cognition, University of Oslo. Recruited by newspaper advertisements, volunteers underwent a standardized health screening interview prior to inclusion. The participants were required to be right-handed, be native Norwegian speakers above 60 years, have normal or corrected-to-normal vision and hearing, feel healthy, be free from medications known to affect central nervous system (CNS) functioning, including psychoactive drugs, not receive psychiatric treatment, and be free from worries for own memory abilities and injuries or diseases known to affect CNS functioning, including neurological or psychiatric illness, serious head injury, or history of stroke. All subjects’ MRI scans were examined by a specialist in neuroradiology and deemed free of significant anomalies. Ninety-one subjects satisfied these criteria. Two participants were excluded as a result of lack of MRI data, and 6 as a result of missing EMQ data, reducing the sample to 83 (45 female) subjects ranging 60–85 years of age (mean = 69.3 years, SD = 6.8 years). All participants scored >26 on Mini Mental State Examination (Folstein, Folstein, & McHugh, Reference Folstein, Folstein and McHugh1975) and <16 on Beck Depression Inventory (Beck, Steer, & Brown, Reference Beck, Steer and Brown1987). Mean full-scale IQ as measured by Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, Reference Wechsler1999) was 116.1 (range: 92–145, SD = 11.4).
The study has been approved by the Regional Ethical Committee of South Norway (REK-Sør), and written informed consent was obtained from all participants prior to the examinations. The research was completed in accordance with the Helsinki Declaration.
The Everyday Memory Questionnaire
A Norwegian translation of the EMQ (Sunderland, Harris, & Baddeley, Reference Sunderland, Harris and Gleave1984) was sent to participants by mail prior to the neuropsychological examination. The inventory comprises 28 items, each describing everyday activities where forgetting might be involved. Participants indicated on a scale from 1 (not at all in the last six months) to 9 (more than once a day) how frequently they experienced each event. The questionnaire includes two items indicative of memory impairments usually related to severe brain injuries. These were regarded irrelevant for the current sample and thus excluded from analysis, in accordance with a previous study (Royle & Lincoln, Reference Royle and Lincoln2008).
In order to extract relevant information from the data collected by the EMQ, and to elucidate various aspects of subjectively perceived everyday memory functions covered by the EMQ, a principal component analysis (PCA) was performed. A more theory-driven approach could have been used by separating items based on semantics. However, because we had limited a priori knowledge about the underlying correlation structure of the data, we decided to let the data drive the grouping of items by performing PCA.
Neuropsychological Assessment
Full-scale IQ was estimated from all four subscales of the WASI (Wechsler, Reference Wechsler1999). To evaluate verbal learning and memory functioning, a Norwegian translation of the California Verbal Learning Test-II (CVLT) (Delis, Kramer, Kaplan, & Ober, Reference Delis, Kramer, Kaplan and Ober1987; Lundervold & Sundet, Reference Lundervold and Sundet2004) was administered. Measuring the amount of material learned, recalled, and recognized, the CVLT yields qualitative data of how the verbal learning occurs or fails. A list of 16 words is read five times, and the participant is immediately asked to list the words he or she could recall after each presentation. After the fifth trial, the participant is read a new 16-item list and asked to recall as many items as possible. Then a delayed recall test is presented where the participant again is asked to recall the items in the first list. After a delay of approximately 30 minutes, during which the participant has been working with nonverbal tasks, a long delayed recall test of the first list is presented. Finally, a “yes-no” recognition test is presented, including the 16 items of the first list, 8 from the second list, and 20 random distracter items. In the present study, we report one verbal learning measure (i.e., total learning across trial 1–5) and a delayed recall measure after a 30-minute delay (total correctly recalled words).
MRI Acquisition
MRI data were collected using a 12-channel head coil on a 1.5T Siemens Avanto scanner (Siemens Medical Solutions, Erlangen, Germany). The pulse sequence used for morphometric analysis was a 3D T1-weighted Magnetization Prepared Rapid Gradient Echo (MP-RAGE) with the following parameters: repetition time (TR)/echo time (TE)/time to inversion/flip angle (FA) = 2400 ms/3.61 ms/1000 ms/8°, matrix 192 × 192, field of view = 240. Each volume consisted of 160 sagittal slices with voxel size 1.25 × 1.25 × 1.20 mm. Scan time was 7 min, 42 sec. Two repeated acquisitions were averaged to increase signal-to-noise-ratio (SNR). Raw datasets were transferred to Linux workstations for processing and analyses at the Neuroimaging Analysis Lab, Center for the Study of Human Cognition, with additional use of computing resources from the Titan High Performance Computing facilities (http://hpc.uio.no/index.php/Titan) at the University of Oslo.
The average time between completion of the EMQ and MRI acquisition was 19 days (range: 0–90, SD = 17.4).
Morphometric Analysis
Regional cortical thickness and subcortical volumes were estimated using FreeSurfer (http://surfer.nmr.mgh.harvard.edu/). The cortical surfaces were reconstructed to measure thickness at each vertex using a semiautomated approach described elsewhere (Dale, Fischl, & Sereno, Reference Dale, Fischl and Sereno1999; Dale & Sereno, Reference Dale and Sereno1993; Fischl & Dale, Reference Fischl and Dale2000; Fischl, Liu, & Dale, Reference Fischl, Liu and Dale2001; Fischl, Sereno, & Dale, Reference Fischl, Sereno and Dale1999; Fischl, Sereno, Tootell, & Dale, Reference Fischl, Sereno, Tootell and Dale1999; Segonne et al., Reference Segonne, Dale, Busa, Glessner, Salat and Hahn2004; Segonne, Grimson, & Fischl, Reference Segonne, Grimson and Fischl2005). Briefly, a representation of the gray/white matter boundary was reconstructed (Dale et al., Reference Dale, Fischl and Sereno1999; Dale & Sereno, Reference Dale and Sereno1993), using both intensity and continuity information from the entire 3D MR volume in segmentation and deformation procedures. Maps were created using spatial intensity gradients across tissue classes and were therefore not simply reliant on absolute signal intensity. Next, by using an automated labeling system (Desikan et al., Reference Desikan, Segonne, Fischl, Quinn, Dickerson and Blacker2006; Fischl et al., Reference Fischl, van der Kouwe, Destrieux, Halgren, Segonne and Salat2004), the cortical surface was parcellated into 33 bilateral cortical areas.
To limit the number of comparisons, we selected candidate composite regions of interest (ROIs): MTL (parahippocampal gyrus and entorhinal cortex), lateral temporal lobe (superior temporal, middle temporal, inferior temporal, banks of the superior temporal sulci and temporal pole), medial parietal lobe (precuneus, isthmus cingulate [retrospinal cortex], and posterior cingulate), lateral parietal lobe (superior parietal, supramarginal, and inferior parietal cortices), superior frontal (superior frontal, caudal middle frontal, and rostral middle frontal cortices), and inferior frontal (medial orbitofrontal cortices, pars orbitalis, pars triangularis, pars opercularis, rostral anterior and caudal anterior cingulate cortices, and the frontal pole). The area of each surface label was weighted to maintain its relative size in the composite ROIs. The cortical parcellation is shown in Figure 1.

Fig. 1. Cortical thickness was analyzed in the following regions: superior frontal area, inferior frontal area, lateral temporal lobe, medial temporal lobe, lateral parietal lobe, and medial parietal lobe. Cortical regions of interest with subregions are illustrated with color codes in medial, ventral, and lateral views.
The volume segmentation procedure automatically assigns a neuroanatomical label to each voxel in an MRI volume based on probabilistic information automatically estimated from a manually labeled training set (Fischl et al., Reference Fischl, Salat, Busa, Albert, Dieterich and Haselgrove2002, Reference Fischl, van der Kouwe, Destrieux, Halgren, Segonne and Salat2004). The segmentation uses three pieces of information to disambiguate labels: (1) the prior probability of a given tissue class occurring at a specific atlas location, (2) the likelihood of the image given that tissue class, and (3) the probability of the local spatial configuration of labels given the tissue class. The technique has been shown to be comparable in accuracy to manual labeling (Fischl et al., Reference Fischl, Salat, Busa, Albert, Dieterich and Haselgrove2002). The segmentations were visually inspected for accuracy and none was discarded.
Based on previous studies, the hippocampus and the striatum were selected for statistical analysis. The estimated volumes were regressed on intracranial volume (ICV) and the residuals used in the statistical analyses. ICV was calculated by using an atlas normalization procedure shown to correlate .93 with manually derived ICV (Buckner et al., Reference Buckner, Head, Parker, Fotenos, Marcus and Morris2004). The subcortical ROIs are shown in Figure 2.

Fig. 2. Magnetic resonance images with subcortical segmentation. Regions of interest are illustrated in coronal, horizontal, and sagittal sections through the cerebral hemispheres. Anonymity of the participant is safeguarded through an automated defacing algorithm (seen in sagittal section) (Bischoff-Grethe et al., Reference Bischoff-Grethe, Ozyurt, Busa, Quinn, Fennema-Notestine and Clark2007). Left and right hemispheres are shown in white and green color, respectively.
Statistical Analysis
First, we performed exploratory principal component analysis (PCA) with varimax rotation and Kaiser normalization to investigate composite variables of the EMQ. The factorability of the EMQ scale was inspected by Bartlett’s Test of Sphericity and the Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy. We used Cronbach’s alpha to assess the internal consistency of the extracted factors. The relations among the extracted EMQ components, CVLT test performance, and morphometric measures were investigated. Pearson’s correlations was performed to investigate associations between the EMQ components and test performance on CVLT, as well as for the relations between these measures and cortical thickness; and partial correlations correcting for ICV were performed for subcortical volume relations. Further, the correlations were corrected for the influence of age and sex. To reduce the risk of introducing nonexistent relationships by statistical corrections, only correlations that were significant both with and without corrections for age and sex will be discussed. Post hoc linear regressions using the enter method were performed to further estimate the relative contribution of the significant predictor variables on brain morphology.
RESULTS
Subjectively Perceived Memory Performance
Participants’ total EMQ score, summed over all 28 items (including the two items that were removed prior to other analyses), ranged from 31 to 138.5, with a mean of 63.1 (SD = 24.3). Mean scores for all items are shown in Table 1. EMQ scores, averaged over all 26 items, ranged from 1.11 to 3.84, with a mean of 2.3 (SD = 0.84). Highest mean score was reported on item 13 (Finding that a word is “on the tip of your tongue”), (mean = 4.1, SD = 1.79), and lowest on item 25A (Getting lost where you have been often before) (mean = 1.2, SD = 0.55).
PCA of the EMQ
Inspection of the correlation matrix revealed several coefficients r ≥ .30. The KMO value was .89, exceeding the recommended value of .60, and the Barlett’s Test of Sphericity was significant (p < .0001), suggesting that factor analysis is appropriate (Kaiser, Reference Kaiser1970, Reference Kaiser1974). PCA revealed four components with eigenvalue > 1, explaining 58.6 % of the total variance. This solution included one factor dominated by two items only. Based on inspections of the scree plot, a three-factor solution was chosen. The rotated solution yielded a simple structure (Thurstone, Reference Thurstone1951) with strong loadings on all components (Supplementary Table 1). This solution explained 52.7 % of the total variance, with a contribution of the separate components 1–3 of 18.8 %, 17.6 %, and 16.3 %, respectively. The three-solution component structure with mean scores and factor loadings is shown in Table 1.
Table 1. The EMQ three component solution with mean scores and component loadings

PCA was performed on data from 83 participants aged 60–85 years. The three extracted components with loadings and mean scores are presented. The EMQ material is reproduced from the original article “Memory failures in everyday life following severe head injury” by Sunderland, Harris, & Gleave, published in Journal of Clinical and Experimental Neuropsychology, Jan. 05, 1984, volume 6, issue 2, pp. 127–142, reprinted with permission from the publisher Taylor & Francis Group, http://www.informaworld.com.
Based on the items loadings, the following interpretation was made for the three components:
Component 1
This component contains many of the same items as Royle and Lincoln’s (Reference Royle and Lincoln2008) “retrieval” factor or a combination of Cornish’s “retrieval” and “memories for activities” (Cornish, Reference Cornish2000). Items with high loading within this component concern prospective memories (items 7, 8, 14, and 18) and everyday routines (items 1, 4, and 24). Cronbach’s alpha for this factor was 0.88, suggesting good internal consistency.
Component 2
This was the most diverse component, comprising 10 items. Four items, including the two with highest loadings, concern problems of visual and spatial recognition (2, 23, 25A, and 25B). Three items (6, 15, and 22) involve episodic memory and concerns about forgetting details about recent events. The nature of these items has some resemblance to the contents of component 1 and is consistent with items 15 (0.45) and 22 (0.40) also loading on component 1. Items 9 and 17 concern attention issues when reading. Cronbach’s alpha of 0.85 suggests high internal consistency within component 2.
Component 3
Most items contributing to this component involve executive or attention issues. Items loading within this component concern losing track when watching television or during conversation (3, 10, 21, and 27) and/or repetitive behavior (items 5, 21, 26, and 27), which suggest involvement of implicit procedural memory. A Cronbach’s alpha of 0.84 suggests high internal consistency also within this factor.
Correlations Between EMQ Components and CVLT Test Performance
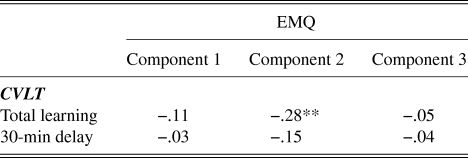
Correlations between the EMQ components and the test performance on CVLT are presented in Table 2. There was a negative correlation between total learning on the CVLT and EMQ component 2 (r = −.28, p < .01), indicating increasing learning scores with decreasing reported everyday memory problems. No other significant associations between EMQ components and CVLT test performance were found.
Table 2. Pearson correlations between EMQ components of everyday memory and CVLT performance
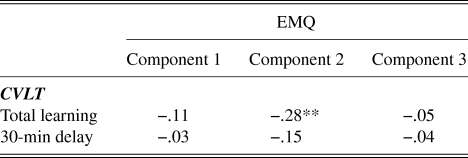
** indicates p ≤ .01.
Correlations Between EMQ, CVLT Test Performance and Cerebral Morphometry
Correlations among components of everyday memory, CVLT test performance, and brain morphometry are presented in Table 3, corrected for age and sex, with uncorrected correlations presented in parenthesis. Investigations with Pearson product-moment correlation indicate negative relations between component 3 and cortical thickness of the MTL (r = −.36, p <. 001), lateral temporal lobe (r = −.25, p < .05), and medial parietal lobe (r = −.25, p < .05). After correcting these correlations for age and sex, only the association with MTL remained significant (r = −.31, p < .01). Post hoc analyses indicated that parahippocampal (r = −.32, p < .01) and entorhinal thickness (r = −.25, p < 0.05) accounted for the effect and that the effect was selectively related to the left hemisphere (r = −.37, p < .001 and r = −.27, p < .01, respectively). No associations were found between the EMQ components and the subcortical volumes investigated.
Table 3. Correlations among EMQ components of everyday memory, CVLT performance, and brain morphometry

Partial correlations among cortical and subcortical ROIs involved in memory processing, self-perceived components of everyday memory, and performance on CVLT. Cortical correlations are corrected for the influence of age and sex; subcortical correlations, for age, sex, and ICV. Uncorrected correlations are presented in parenthesis. Bold indicates p ≤ .05.
Pearson’s correlations indicated a positive relationship between MTL thickness and CVLT total learning performance (r = .27, p < .01), and after a 30-minute delayed recall (r = .42, p < .001). After correcting for age and sex, the association between performance on CVLT 30-minute recall and MTL thickness remained significant (r = .38, p < .001). Post hoc analyses indicated that parahippocampal (r = .34, p < .01) and entorhinal thickness (r = .23, p < .05) accounted for the effect. The effect was selectively related to the left hemisphere (r = .39, p < .001 and r = .23, p < .05, respectively).
The results of the linear regression analyses testing three different models with MTL thickness as a dependent variable are presented in Table 4. Model I with EMQ component 3, age and sex as predictors, yielded a significant effect of EMQ on thickness (B = −.30, p < .001). The full model explained 18% of the variance in MTL thickness (F3,79 = 6.881, p < 0.001. Adjusted R2 = .177). Model 2 that included CVLT 30-minute recall, age, and sex yielded a significant effect of CVLT (B = .36, p < .001) and accounted for 22% of the variance in MTL thickness (F3,78 = 8.805, p < 0.0001, adjusted R2 = .224) in total. Finally, model 3 that included EMQ component 3, CVLT 30-minute recall, age, and sex yielded significant effects of both EMQ (B = −.32, p < .001) and CVLT (B = .36, p < .001) on MTL thickness and increased the amount of explained variance to 31% (F4,77 = 10.284, p < 0.0001, adjusted R2 = .314). Importantly, CVLT, EMQ component 3, and age all provided unique statistical contributions in the prediction of MTL thickness. We found no significant effects of sex in any of the tested models. Inspection of the standardized beta values (see Table 4) shows that the inclusion of CVLT to the model (model 3) slightly increased the impact of EMQ component 3 and reduced the impact of age on MTL thickness.
Table 4. Linear regression analyses testing three different models with MTL thickness as a dependent variable

Model I includes EMQ component 3, age, and sex; model 2 includes CVLT, age, and sex; and model III includes EMQ, component 3, CVLT, age, and sex as predictor variables. Adjusted R2 denotes the full model fit, and the betas are the unique standardized regression slopes for each predictor. F is the F-score for the full model. *p < 0.05, **p < 0.001.
DISCUSSION
Memory performance declines even in healthy aging, and the elderly report increased liability to everyday forgetfulness (Craik, Reference Craik1994; Nyberg, Backman, Erngrund, Olofsson, & Nilsson, Reference Nyberg, Backman, Erngrund, Olofsson and Nilsson1996). To the best of our knowledge, this study is the first to report significant correlations between healthy elderly’s subjectively perceived memory function assessed by EMQ and brain morphometry. Self-perceived memory, potentially tapping into attentional and executive resources, was found to be correlated with cortical thickness of the MTL, a region critically involved in memory functions. Similar relations were found between CVLT 30-minute verbal recall performance and MTL thickness. The CVLT 30-minute verbal recall was not correlated with the relevant EMQ component, and the explanatory values of these cognitive measures on MTL thickness were of similar magnitude. Importantly, including both variables as concurrent predictors for MTL thickness nearly doubled the total amount of explained variance compared to when including only one. Thus, it seems that self-perceived memory could be sensitive to cortical morphometry and might be a valuable supplement to formal psychometric and/or neuropsychological testing.
Although memory processing is dependent upon a large array of distributed cortical and subcortical regions, MTL is assumed to play a critical role in episodic memory (Scoville & Milner, Reference Scoville and Milner1957). Thus, correlations between MTL morphometry and memory could be expected both regarding objective memory performance and subjectively perceived memory function. For verbal memory, there was a relation between MTL thickness and verbal recall after 30 minutes, where higher recall rate was associated with thicker cortex in the parahippocampal and entorhinal regions. Moreover, the effect was selectively related to the left hemisphere. Cortical thickness correlates of verbal recall have been found in distinct areas, including MTL regions, however, mostly pronounced when recall was tested after months (Walhovd et al., Reference Walhovd, Fjell, Dale, Fischl, Quinn and Makris2006). The discrepancy between that and the current study regarding the verbal memory-morphometry relation on the 30-minute recall could possibly be the result of age effects on MTL morphometry and recall (Delis, Kramer, Kaplan, & Ober, Reference Delis, Kramer, Kaplan and Ober1987), given the older sample of the present study as age-related changes of the MTL may not manifest before the sixth and seventh decade (Westlye et al., Reference Westlye, Grydeland, Walhovd and Fjell2010). In sum, our results support a role for MTL thickness in a standardized objective test of verbal memory in healthy elderly participants. The left hemisphere asymmetry may be interpreted in correspondence with the well-known left-hemisphere dominance for verbal functions (Gazzaniga, Reference Gazzaniga1995).
Interestingly, similar relations were found for self-perceived everyday memory, component 3, and morphometry. Higher scores on this component were associated with thinner cortices in the left parahippocampal and entorhinal regions. Seven items loading on this component involve difficulties related to spoken or written language. Thus, again, we found a relation restricted to the left hemisphere that fits with the left-hemisphere dominance for language (Gazzaniga, Reference Gazzaniga1995). Whereas the relations between morphometry and objective memory performance, on the one hand, and subjectively perceived memory function, on the other, were strikingly similar, analyses suggest that the EMQ is picking up information different from that of the CVLT and uniquely contribute to explain the variance in MTL thickness. The relative independence of the measures was also supported by the weak correlations between EMQ components and CVLT performance.
The correlations between EMQ and cortical thickness, and between the CVLT measures and cortical thickness, were not strong. However, as other features of brain structure than what can be detected by MR morphometry is obviously also relevant for cognitive function, one can seldom expect very high correlations between single measures of cognitive functions and single brain measures of brain morphometry in groups of healthy participants. Still, when both EMQ and CVLT were included as predictors, explained variance approached 35%, indicating a structure-function relationship of considerable strength.
Recently, a meta-analysis of voxel-based morphometry studies investigating predictors of AD concluded that the left MTL is the most affected region. In particular, atrophy in hippocampus and parahippocampal gyrus most consistently predicted conversion from amnesic mild cognitive impairment to AD (Ferreira, Diniz, Forlenza, Busatto, & Zanetti, Reference Ferreira, Diniz, Forlenza, Busatto and Zanetti2009). Also, entorhinal atrophy seems to be early implicated in MCI and AD and may provide a marker of incipient AD (de Toledo-Morrell, Goncharova, Dickerson, Wilson, & Bennett, Reference de Toledo-Morrell, Goncharova, Dickerson, Wilson and Bennett2000; Devanand et al., Reference Devanand, Pradhaban, Liu, Khandji, De Santi and Segal2007). It is of interest that whereas the sample of the present study was recruited to be free from worries of their own memory abilities, aspects of the EMQ still seem to be related to structural variations in brain morphology in regions heavily implicated in memory and of major interest for early identification of AD. Nevertheless, the high cognitive functioning of the sample, together with the low correlations with hippocampal volume, indicates that incipient AD is hardly causing the relationships (see below).
As previously shown, the PCA differentiated between memory and attention systems (Royle & Lincoln, Reference Royle and Lincoln2008), where component 3 comprised most items related to attention-related items. Whereas a bigger-is-better relationship was found for this component and MTL thickness, it would not have been surprising if a similar association had been found also for components 1 and 2, which both implicate memory-related items. Some items load on several components, and so one could expect some degree of similarity in the brain structure correlations between these components. However, as the components by definition are not correlated, the combinations of loadings for all items make each component unique. Thus, even though some memory-items load both on components 1 and 2 or 3, only component 3 showed a significant correlation with MTL structure both corrected and uncorrected for age and sex. It is possible that the exclusion criterion of worries for own memory function, which leads to the generally low degree of perceived memory problems among the participants, has contributed to enhance correlations between items less directly related to memory and brain structure compared to the correlations with the typical episodic memory items. Thus, in this sample of healthy elderly, subjective ratings of items related to attention (component 3) are most sensitive to morphometric variations in MTL thickness. Inspection of the uncorrected correlations between components of everyday memory and brain morphometry indicate relations between component 3 and cortical thickness in lateral temporal and medial parietal lobe, suggesting that this measure could be sensitive to morphometric changes in nearby regions as well.
No subjective memory-brain morphometry relations were found for any subcortical volumes. This may indicate that changes in memory processes that are perceived subjectively in cognitively well-functioning elderly are largely supported by higher-order cortical regions, and subjectively perceived memory may in particular depend on cortical integrity. As it has been shown repeatedly that hippocampal volume is an early marker for AD-related pathology, the lack of EMQ-hippocampus correlations in the present study indicate that undetected incipient cognitive decline is likely not the driving cause of the relationships. Importantly, we found no associations between EMQ components or CVLT performance on one side and hippocampal volumes on the other. This indicates that cortical morphometry may be more sensitive to normal variation in perceived memory and test performance in the healthy elderly than what hippocampal volumetry is. This finding supports van Petten’s (2000) neuropsychological perspective positing that hippocampal volumes might be sensitive to memory-related variability in clinical populations but not necessarily in healthy samples.
Another interesting point related to the cortical thickness effect is that a recent study showed that thickness of the temporal lobe was more affected in AD than surface area, whereas the opposite was true in normal aging (Dickerson et al., Reference Dickerson, Feczko, Augustinack, Pacheco, Morris and Fischl2009). Other studies with more statistical power have, however, demonstrated some cortical thinning of the temporal lobe also in normal aging (Fjell et al., Reference Fjell, Westlye, Amlien, Espeseth, Reinvang and Raz2009; Westlye et al., Reference Westlye, Grydeland, Walhovd and Fjell2010). Importantly, individual variabilities in both brain morphometry and memory functions are expected to increase with advancing age (Van Petten, 2004) and might explain larger effect sizes in samples of older adults. In a recent twin study, Panizzon et al. (Reference Panizzon, Fennema-Notestine, Eyler, Jernigan, Prom-Wormley and Neale2009) showed that both cortical thickness and surface area were highly heritable, but that they were essentially unrelated genetically. Taken together, these results demonstrate that cortical volumetric studies confound two sources of information that are genetically independent and may be affected by normal aging and AD in very different ways. In the present study, all cortical effects are caused by interindividual differences in thickness. We have recently demonstrated distinct associations between regional cortical thickness and specific indices of attention functions as measured by the Attention Network Test (ANT) (Westlye, Grydeland, Walhovd, & Fjell, Reference Westlye, Grydeland, Walhovd and Fjell2010), pertaining to the sensitivity of cortical thickness to neurocognitive specificity. Because we have previously found relationships between memory performance and cortical thickness in the MTL (Walhovd et al., Reference Walhovd, Fjell, Dale, Fischl, Quinn and Makris2006), thickness in this and other memory-related areas was chosen as the metric of interest. Although outside the scope of the present study, testing the putative relationships between EMQ and surface area should be a focus for further investigations.
A limitation of our study includes the selection of participants by advertisement in a newspaper with many well-educated readers, in addition to rather strict inclusion criteria for participants with few memory complaints. Whereas these criteria are warranted to make sure we are not measuring pathological processes, they may have biased our sample in favor of increased cognitive abilities and toward nonmemory complaints with a restricted range of subjectively perceived memory experiences. An average IQ of 116 and low mean scores across the EMQ items confirm that these individuals are well-functioning with few memory complaints. Our results thus may not generalize to a random community sample with lower education and a broader range of subjectively perceived memory experiences. However, some cognitive decline associated with changes in neural structures is probably also the case among many healthy elderly with few worries about their memory functions. It is therefore of interest to investigate whether these elderly people’s subjective opinions about their memory have structural correlates that are not necessarily related to pathological processes. Most studies related to subjective memory focus on elderly with cognitive complaints, and so we believe studies of elderly without such worries are needed.
In conclusion, we found that elderly people’s self-perception of memory failures in daily life show distinct associations with regional cortical thickness. Importantly, these associations were not explained by an objective measure of verbal recall performance, although the regional correlations with cortical thickness were similar across measures. Specifically, both self-perceived memory failures related to attentional or executive issues and verbal recall performance were independently associated with cortical thickness in MTL, and including both measures in the statistical model nearly doubled the total amount of explained variance in the thickness of MTL. Thus, even in well-functioning elderly, the EMQ has detectable structural brain correlates in regions critically involved in memory and might be a valuable supplement to standardized tests. However, the results must be interpreted with caution and should be replicated in independent samples and preferably with longitudinal data.
DISCLOSURE/CONFLICT OF INTEREST
None of the authors has any conflict of interest affecting this manuscript.
ACKNOWLEDGMENTS
Financial support: Norwegian Research Council (grants 177404 and 186092 to K. B. W. and grant 175066 to A. M. F, and a student research fellowship to Lars T. Westlye) and University of Oslo to K. B. W. and A. M. F.