Published online by Cambridge University Press: 23 January 2006
The present study investigated the utility of the International Classification of Diseases and Related Health Problems, 10th edition (ICD-10) diagnostic criteria for postconcussion syndrome (PCS) symptoms by comparing symptom endorsement rates in a group of patients with mild traumatic brain injury (MTBI) to those of a noninjured control group at one month and three months post-injury. The 110 MTBI patients and 118 control participants were group-matched on age, gender, and education level. Seven of the nine self-reported ICD-10 PCS symptoms differentiated the groups at one month post-injury and two symptoms differentiated the groups at three months post-injury: symptom endorsement rates were higher in the MTBI group at both time periods. Fatiguing quickly and dizziness/vertigo best differentiated the groups at both time periods, while depression and anxiety/tension failed to differentiate the groups at either time period. Collectively, the ICD-10 PCS symptoms accurately classified the MTBI patients at one month post-injury, with the optimal positive test threshold of endorsement of five symptoms coinciding with a sensitivity and specificity of 73% and 61%, respectively. The ICD-10 PCS symptoms were unable to accurately classify the MTBI patients at three months post-injury. (JINS, 2006, 12, 111–118.)
The term postconcussion syndrome (PCS) refers to the cluster of affective, somatic, and cognitive symptoms reported by individuals who have sustained a brain injury. Within the substantial literature on symptom complaints following mild traumatic brain injury (MTBI), the vast majority of studies indicate that postconcussive symptoms are largely resolved within 3-months of injury, with most individuals reporting essentially a full recovery (Alexander, 1995; Binder et al., 1997; Kashluba et al., 2004; Ponsford et al., 2000). Postconcussive symptoms are not reported by all individuals who have sustained a MTBI. Of those who do, a small percentage of individuals complain of symptoms persisting beyond the typical recovery time frame following MTBI, with some reporting difficulties even years later (Alexander, 1995; Kraus & Nourjah, 1988; Ponsford et al., 2000).
A growing number of studies reveal persistent postconcussion symptoms are influenced by factors other than head injury status, suggesting that symptoms commonly associated with PCS may not be specific to MTBI. Similarly, several lines of research have revealed a high rate of postconcussive symptom endorsement in many non-MTBI patient populations including noninjured individuals seeking outpatient psychotherapy (Fox et al., 1995), non–head injured outpatients seen for minor medical treatment (Lees-Haley & Brown, 1993), and non–head injured individuals with chronic pain (Iverson & McCracken, 1997). Moreover, postconcussive symptoms such as headaches, irritability, anxiety, and fatigue have high endorsement rates in the normal population (Gouvier et al., 1988; McClean et al., 1993; Wong et al., 1994). The origin, evolution, and resolution of symptom complaints following MTBI remain important areas for further investigation in light of the small subgroup of individuals who report persistent symptomatology beyond the typical three-month recovery time post-injury.
Despite the relative pervasiveness of MTBI, universally accepted criteria for diagnosing PCS do not currently exist, nor is there a specific symptom complex that has an acceptable predictive value (Gasquoine, 1997; Jagoda & Riggio, 2000). While reference to PCS is frequently made in the literature, in some instances the existence of any one or two self-reported symptoms has been deemed significant (Alves et al., 1993). Consequently, integration of research findings across studies is difficult and the diagnosis of PCS following MTBI continues to be equivocal. There currently exist only two formal sets of guidelines that attempt to standardize diagnostic criteria for PCS: the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2000) and the World Health Organization's (WHO) International Classification of Diseases and Related Health Problems, 10th edition (ICD-10; World Health Organization, 1992). However, the DSM-IV-TR currently includes the diagnostic category only for what it terms Postconcussional Disorder as a “proposed criteria set for further study,” due to a lack of sufficient research to warrant its inclusion as an official diagnostic disorder.
The ICD-10 provides a second set of currently recommended diagnostic criteria for PCS included within the following definition:
The [postconcussional] syndrome occurs following head trauma (usually sufficiently severe to result in loss of consciousness) and includes a number of disparate symptoms such as headache, dizziness (usually lacking the features of true vertigo), fatigue, irritability, difficulty in concentrating and performing mental tasks, impairment of memory, insomnia, and reduced tolerance to stress, emotional excitement, or alcohol. These symptoms may be accompanied by feelings of depression or anxiety, resulting from some loss of self-esteem and fear of permanent brain damage. Such feelings enhance the original symptoms and a vicious circle results. Some patients become hypochondriacal, embark on a search for diagnosis and cure, and may adopt a permanent sick role. The etiology of these symptoms is not always clear, and both organic and psychological factors have been proposed to account them. The nosological status of this condition is thus somewhat uncertain. There is little doubt, however, that this syndrome is common and distressing to the patient. At least three of the features described above should be present for a definite diagnosis. Careful evaluation with laboratory techniques (electroencephalography, brain stem evoked potentials, brain imaging, oculonystagmography) may yield objective evidence to substantiate the symptoms but results are often negative. The complaints are not necessarily associated with compensation motives (WHO, 1992; section F07.2).
Precise interpretation of the ICD-10 criteria for PCS has been criticized as difficult to ascertain, in part due to the ambiguous use of punctuations in the text (Iverson & Lange, 2003). Similarly, the WHO acknowledges limitations with the ICD-10 criteria for PCS by stating that the criteria are difficult to operationalize (Carroll et al., 2004). Operational limitations are particularly evidenced by the definition's lack of a specific set of diagnostic criteria. For example, the statement at least three of the features described above necessitates a subjective interpretation of the definition given that no list of features is included to which this statement refers. Furthermore, the inclusion of statements such as may be accompanied by, may adopt, and some patients indicates that the experience of symptoms following head injury differs among individuals.
The degree to which PCS symptoms are measurable by self-report is important given that symptom complaints are often the primary and/or sole means of obtaining information pertaining to post-injury functioning after MTBI. The ICD-10 criteria for PCS fail to provide a definitive list of symptoms measurable by self-report. However, the following nine ICD-10 PCS symptoms are both determinable by self-report (as compared to more objective laboratory techniques) and commonly found in studies of postconcussion symptom complaints: headache, dizziness, fatigue, irritability, difficulty in concentrating, impairment of memory, insomnia, depression, and anxiety (e.g., Gouvier et al., 1992; Iverson & Lange, 2003; Paniak et al., 2002; Ponsford et al., 2000).
Although additional features are contained within the ICD-10 definition for PCS, they are either not considered typical PCS complaints in the literature or, perhaps more important, are included in the definition in such a manner that it is unclear whether they are intended to be considered a symptom of PCS. For example, the statements some patients become hypochondriacal, may adopt a permanent sick role, loss of self-esteem, and fear of permanent brain damage can all be interpreted as possible outcomes of experiencing PCS symptoms, rather than existing as unique PCS symptoms themselves. Likewise, the three symptoms contained in the statement reduced tolerance to stress, emotional excitement, or alcohol are seldom found in the literature as postconcussive symptoms.
Although the ICD-10 criteria for PCS are one of only two standardized diagnostic systems for postconcussive symptomatology, very few studies of postconcussive symptoms have investigated the incidence of ICD-10 PCS symptoms. Moreover, no study to date has investigated the incidence of ICD-10 PCS symptoms in individuals following MTBI.
The purpose of the present study was to investigate the utility of the ICD-10 diagnostic criteria for PCS symptoms in a MTBI patient sample by comparing their symptom endorsements to those of a noninjured control group at one month and three months post-injury. We evaluated the utility of the diagnostic criteria in several ways. First, we conducted an examination of which ICD-10 symptoms best differentiated the MTBI group from the control group at both time periods, to determine whether specific PCS symptoms were more characteristic of post-MTBI self-reported symptomatology. Considering that symptom complaints are common soon after MTBI and tend to resolve by 3-months post-injury, we expected that indeed most PCS symptoms would differentiate the groups at one month post-injury. Similarly, given that certain PCS symptoms are more commonly reported following MTBI (e.g., headaches, fatigue) we expected that endorsement of ICD-10 symptoms would not be uniform across symptoms. Conversely, we predicted that none of the PCS symptoms would differentiate the groups at the three-month follow-up.
Although the ICD-10 incorporates nine symptoms measurable by self-report, it is unclear whether endorsement of all nine optimally characterizes PCS or whether some combination of the nine is optimal. As such, the present study also investigated whether the ICD-10 PCS symptoms could accurately classify the MTBI patients at both time periods and, if so, how many of the nine symptoms required endorsement for optimum classification. Based on the typical symptom resolution following MTBI, it was expected that the ICD-10 PCS symptoms would correctly classify the MTBI patients at one month post-injury, but would fail to classify MTBI patients accurately at three months post-injury.
Finally, we investigated within-group differences in ICD-10 PCS symptom endorsement, to assess the effect of time from baseline to follow-up, thus providing an indication of whether overall each group improved over time with respect to ICD-10 PCS symptom endorsement. We expected that as a group, the MTBI participants would endorse fewer ICD-10 PCS symptoms by the three-month follow-up and that no changes would be found in the control group's endorsement of symptoms over time.
This study comprised two groups for whom self-report data were available at both time periods of interest: (1) 110 adults with MTBI diagnosed according to the American Congress of Rehabilitation Medicine's (1993) MTBI definition; and (2) 118 uninjured adults who volunteered to participate in a MTBI treatment study. The data used in the current study were collected as part of a larger research project. This study was given Research Ethics Board approval.
The control group participants were recruited from university, hospital, and municipal government offices and included both staff and students. The control participants were approached by a research assistant and asked if they wanted to participate in a study comparing some of the problems experienced by individuals with a concussion to the problems of everyday living experienced by nonconcussed individuals. The control participants did not receive any incentives for participating in the study.
The MTBI participants were drawn from consecutive admissions to two hospital emergency wards and volunteered to participate in a MTBI treatment study. A nurse at each emergency ward reviewed new admissions once or twice per week to identify individuals who may have met the criteria for MTBI. The nurses were instructed to use a liberal interpretation of the American Congress of Rehabilitation Medicine's (1993) MTBI criteria in deciding whom to contact. Potential participants were then contacted by telephone and letter to determine if they would be willing to take part in a study evaluating the efficacy of treatments for concussion. If interested, the participant telephoned the clinical neuropsychologist who conducted a more exacting interview regarding the 1993 MTBI inclusion criteria. If participants met the inclusion and exclusion criteria and consented to participate in the study, they met with the clinical neuropsychologist within three weeks of injury. The MTBI participants sustained their injuries from motor vehicle accidents, sporting activities, falls, bicycle accidents, and assaults.
Exclusionary criteria for both the MTBI and control participants included: (1) a history of inpatient treatment for any psychiatric disorder; (2) a diagnosis of mental retardation; (3) an inability to read fluently in English based on self-report; (4) a history of TBI more severe than MTBI at any point in their life; (5) a MTBI within 1 year prior to participation in the MTBI study; (6) any ongoing central nervous system disorder; or (7) concurrent pregnancy. The control participants were group-matched to MTBI patients on age, sex, and years of education.
MTBI participants were assigned to one of two treatment groups: (1) a one-session education, information, and reassurance group; or (2) a group that received the same treatment with the addition of a brief neuropsychological assessment and physical therapy assessment, followed by treatment as needed (median number of further treatment contacts = 1). As the results from the treatment study indicated that no significant differences in outcome were found between the two treatment groups (Paniak et al., 1998, 2000), the treatment groups were combined to comprise the MTBI group used in the current study.
Participants in both the MTBI and control groups completed the Problem Checklist (PCL; Kay et al., 1995) from the New York Head Injury Family Interview. The PCL consists of 43 items, each reportedly a common TBI complaint. Each PCL item requires a yes/no response and indicates whether the individual currently experiences the problem. Participants in the MTBI group completed the PCL within one month of injury (mean number of days between injury and initial PCL administration 12.09, SD = 5.84, range = 1–27) and again at approximately three months post-injury (mean number of days between injury and follow-up PCL administration 98.28, SD = 11.14, range = 73–164; 66% of MTBI participants were seen for the follow-up PCL administration within 100 days of injury). The control participants also completed the PCL twice, with an approximate three month interval between administrations.
Within the ICD-10 definition for PCS, nine disparate symptoms measurable by self-report are clearly evident. These nine ICD-10 PCS symptoms are included among the list of 43 PCL items administered to the MTBI and control groups: (1) headaches; (2) dizziness/vertigo; (3) fatiguing quickly/getting tired easily; (4) irritability; (5) poor concentration for extended periods of time; (6) being forgetful/difficulty remembering things; (7) sleep disturbance; (8) depression; and (9) anxiety/tension. Symptom endorsement rates were drawn from MTBI and control participants' responses to these nine symptoms from both PCL administrations times of interest.
A logistic regression analysis was performed to determine which individual ICD-10 PCS symptoms best differentiated the MTBI group from the control group at one month and three months post-injury.
Investigation of the accuracy of the ICD-10 PCS criteria in classifying MTBI patients was conducted by a receiver-operating characteristic (ROC) curve analysis and an examination of utility estimates. ROC analysis is used extensively in epidemiological and medical diagnostic research to assess the efficacy of diagnostic systems (Swets, 1996). The overall accuracy of a diagnostic scheme is based on the area under the curve, such that an area of .50 represents classification at chance levels and an area of 1.00 represents perfect classification (qualitatively, the area under an ROC curve can be described using the following ranges: fair: .50–.75; good: .76–.92; very good: .93–.97; and excellent: .98–1.00; Simon, 2005). Precise interpretation of the accuracy of the diagnostic criteria depends on the test threshold used to determine a positive test (i.e., number of symptoms endorsed). Given the exploratory nature of the current study, an examination of the utility statistics at all possible test thresholds (i.e., ranging from zero to nine symptoms endorsed) was conducted.
To investigate whether PCS symptom endorsement rates within each group changed over time, we conducted repeated measures MANOVAs for both the MTBI and control groups.
We found no statistically significant (p < .05) difference between the MTBI and control group on age (M = 33.06, SD = 12.03 vs. M = 30.44, SD = 11.68), years of education (M = 13.83, SD = 2.90 vs. M = 14.18, SD = 2.04), or gender (53% female vs. 60% female).
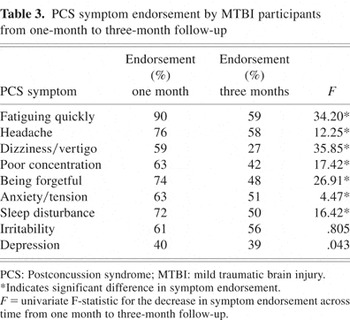
As illustrated in Table 1, the logistic regression revealed that at one month following injury, seven of the nine PCS symptoms differentiated the MTBI group from the control group (p < .05). Only depression and anxiety/tension failed to differentiate the groups at one month post-injury. The MTBI group was more likely than the control group to endorse each of the nine symptoms at one month post-injury, although the probability of symptom endorsement varied and was far greater for some symptoms, such as fatiguing quickly. By the time of the 3-month follow-up, fatiguing quickly and dizziness/vertigo continued to differentiate the MTBI and control groups (p < .05), with higher symptom endorsement in favour of the MTBI group. However, the likelihood of the MTBI group endorsing fatiguing quickly was reduced substantially by three months post-injury, and the odd ratios for the remaining eight PCS symptoms were close to 1:1.
PCS symptom endorsement between groups from one-month to three-month follow-up

ROC curve analysis revealed that the ICD-10 PCS symptoms significantly classified the MTBI and control participants into their respective groups at one month post-injury; the area under the ROC curve was 0.74 (p < .001, SE ± .032; see Figure 1).

ROC curve obtained at one month post-injury.
This measure of the overall ability of the PCS diagnostic criteria to classify MTBI patients and control participants categorizes the criteria as “fair” (Simon, 2005). Precise interpretation of the accuracy of the PCS criteria was achieved by an examination of the utility estimates. For example, as shown in Table 2, if a positive test was determined to be endorsement of all nine PCS symptoms, less than 22% of the MTBI participants would be correctly classified (sensitivity = 21.8%) despite correctly classifying over 96% of control participants (specificity = 96.6%). Thus, taking into consideration the trade-off between true positive and true negative rates, the optimal threshold for a positive test appeared to be endorsement of at least five PCS symptoms. Further support for a test threshold of at least five symptoms endorsed was evidenced by the dramatic decline in participant classification accuracy when a cutoff of less than five PCS symptoms was employed, with sensitivity rates falling below 11%. However, even the optimal positive test threshold of endorsement of five symptoms coincided with a sensitivity of only 73% and specificity of only 61%. Thus, at best, the ICD-10 PCS criteria still incorrectly classified nearly 40% of control participants (false positive rate = 39%) and failed to correctly classify nearly 30% of MTBI participants (false negative rate = 27.3%) at one month following injury.
Utility estimates at one month post-injury in classifying MTBI and control participants

Collectively, the ICD-10 PCS symptoms were not able to accurately classify the MTBI and control participants into their respective groups at 3-months post-injury. The area under the ROC curve at the 3-month follow-up time was 0.55 (p > .05, SE ± .039; not significantly different from an area of 0.50), indicating that endorsement rates for the PCS symptoms were no better than chance alone in classifying the MTBI participants from the control participants. Thus, no further investigation of utility estimates at the three-month follow-up time was conducted. The ROC curve for symptom endorsement rates at three months post-injury is shown in Figure 2.

ROC curve obtained at three months post-injury.
Repeated measures MANOVA on PCS symptom endorsement in the control group found no significant effect of time from baseline to the three-month follow-up time (F(9,109) = 1.17, p = .321). Conversely, the repeated measures MANOVA on symptom endorsement in the MTBI group found a significant effect of time (F(9,101) = 10.30, p < .001) such that PCS symptom endorsement rates were significantly lower at three months post-injury than within one month of injury. Table 3 shows those PCS symptoms that significantly declined from baseline to three-month follow-up in the MTBI group.
PCS symptom endorsement by MTBI participants from one-month to three-month follow-up
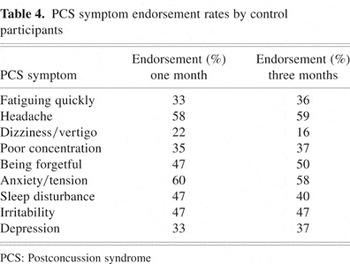
Despite no significant change in ICD-10 PCS symptom endorsement over time in the control group, further investigation revealed consistently high endorsement rates for many PCS symptoms. For example, at the time of the first PCL administration, eight of the nine PCS symptoms were endorsed by at least one third of control participants. Similarly, the same eight PCS symptoms continued to be endorsed by more than one third of control participants at the time of the three-month follow-up, with three symptoms endorsed by at least 50% of the control group (i.e., headaches, anxiety/tension, being forgetful). These findings provide evidence of relatively high, stable base rates of ICD-10 PCS symptom experience in the uninjured control participants. Table 4 lists endorsement rates of the ICD-10 PCS symptoms by the control group at both time periods of interest.
PCS symptom endorsement rates by control participants
The purpose of diagnostic criteria is to facilitate professional communication and the appropriate delivery of health care services, and to ensure that clinical research findings are descriptive of well-defined populations (Slick et al., 1999). As is the case with many clinical disorders or syndromes, universally accepted criteria do not currently exist for the diagnosis of PCS, serving only to further complicate the already confusing evidence surrounding this syndrome. The current study's investigation of the ICD-10 PCS criteria following MTBI provides an important first look at one of only two currently recommended diagnostic systems for PCS.
As expected, most of the ICD-10 PCS symptoms differentiated the MTBI and control groups at one month post-injury, with all symptom endorsements in favor of the MTBI group. However, considerable variability existed in terms of specific symptom endorsement rates in the MTBI group, most notably with respect to relatively high endorsement of fatiguing quickly across time. The variability in symptom endorsement soon after MTBI reveals that not all post-MTBI symptoms carry an equal weight and suggests that a ranking of PCS symptoms following MTBI should be investigated.
Despite higher symptom endorsement rates in the MTBI group at one month post-injury, the results revealed that a relatively high percentage of control group participants endorsed symptoms over time. The finding of high base rates of PCS symptom experience in the control group supports the evidence that symptoms commonly associated with PCS are not specific to MTBI and further highlights the need for symptom assessment following MTBI to focus on pre-injury to post-injury changes rather than merely post-injury symptom occurrence (Gasquoine, 1997; van Zomeren & van den Burg, 1985).
Taking into consideration the relatively high symptom-endorsement rates by control participants, the finding that seven PCS symptoms still differentiated the MTBI and control participants at one month post-injury provides evidence of significant symptomatology in the MTBI group soon after injury and is in keeping with the literature indicating that self-reported symptom complaints are common soon after MTBI (e.g., Alexander, 1995; Carroll et al., 2004; Gasquoine, 1997). However, given the relative dearth of studies investigating the role of psychological distress after injury, medication effects, or pain from associated injuries in the etiology of symptoms in the acute stage of MTBI (Carroll et al., 2004), it cannot be concluded that the MTBI group's symptom complaints at one month post-injury were exclusively neurogenic in nature. According to the WHO, one of the most serious problems in the diagnosis of PCS is linking residual symptoms to the MTBI itself (Carroll et al., 2004). To date, the most consistent predictors of delayed recovery after MTBI in the literature center on compensation and litigation factors (Carroll et al., 2004).
Interestingly, fatiguing quickly and dizziness/vertigo were the same two symptoms that best differentiated the groups at baseline and follow-up suggesting their potential utility as indicators of persistent PCS symptomatology. However, studies investigating post-MTBI symptoms that included more than the nine ICD-10 PCS self-reported symptoms outlined in the current study have found different results. For example, using the Post Concussion Symptom Checklist (Gouvier et al., 1992), Ponsford and colleagues (2000) found subjective reports of dizziness and fatigue (among other symptoms) to differentiate an MTBI group from normal controls at one week post-injury; however, only headaches and concentration difficulties were found to differ between the MTBI and control group at three months post-injury. Thus, with the use of a symptom checklist that includes more than the nine PCS symptoms identified in the ICD-10, the importance of fatiguing quickly and dizziness/vertigo as indicators of persistent symptomatology is seemingly reduced. In this view, one limitation of the ICD-10 criteria for self-reported PCS may lie in its inclusion of too few PCS symptoms.
Symptom endorsement did not differ between the MTBI and control group on depression or anxiety/tension at either time period suggesting that at least these two symptoms may not be useful measures for assessing PCS after MTBI using the ICD-10 criteria. Symptom endorsement rates for depression and anxiety are variable in the literature, with some studies indicating differences in symptom endorsement between MTBI patients and controls (e.g., Lees-Haley & Brown, 1993) and others indicating no differences among similar groups of participants on these two symptoms (e.g., Kashluba et al., 2004; Paniak et al., 2002; Ponsford et al., 2000).
Perhaps most importantly, the findings of the present study revealed that even soon after MTBI a diagnosis of PCS using ICD-10 symptoms as described herein is inadequate and prone to misdiagnosis. Although collectively the PCS symptoms significantly distinguished MTBI from control participants at one month post-injury, the overall accuracy of the diagnostic scheme could only be described as “fair.” As predicted, by the time of the three-month follow-up, the ICD-10 PCS symptoms were unable to classify individual participants into their respective groups accurately, thus providing evidence that the accuracy of the ICD-10 PCS criteria as a diagnostic scheme only decreases as time from injury increases.
Two principal limitations of the ICD-10 criteria for PCS emerged from the current study. First, the ambiguous inclusion of PCS symptoms within the definition of the syndrome itself represents an important operational limitation for clinicians and researchers by imposing a subjective interpretation of the diagnostic criteria. A definitive list of PCS symptoms would prove more beneficial and allow for consistency across studies. Second, the lack of a specified timeline for which a diagnosis of PCS should be considered represents an important diagnostic limitation of the ICD-10 criteria with respect to MTBI. At present, there currently exists no diagnostic differentiation between postconcussive symptoms reported soon after MTBI versus persistent postconcussive symptoms (i.e., symptomatology still present three months post-injury). The present results, together with the well-established time line of symptom resolution following MTBI, suggest that the diagnosis of PCS should be reserved for either those individuals presenting with the common cluster of postconcussive symptoms soon after MTBI or for those individuals with persistent postconcussive symptomatology.
Important limitations of the current study should also be noted. First, the study did not include a non-MTBI patient comparison group (e.g., orthopedic, chronic pain, post-traumatic stress disorder patients). Determining the predictive accuracy of the ICD-10 criteria for PCS in classifying MTBI and non–head injured patients would provide valuable information regarding the sensitivity of the ICD-10 PCS symptoms to brain injury as opposed to clinical abnormality in general. Similarly, inclusion of a non-MTBI clinical sample would allow for comparisons of persisting ICD-10 PCS symptoms between groups, thus providing information regarding the evolution of MTBI symptom complaints as being secondary to brain injury. A second limitation of the current study was that MTBI patients received treatment within a few weeks of injury. Although the treatment received was comparable to standard post-MTBI treatment provided in most medical settings (i.e., brief psycho-educational treatment), it presents as a limitation insomuch that caution should be taken in comparing the results of the current study with those of untreated MTBI patient samples.
Further research utilizing the current MTBI sample should investigate whether the same MTBI patients are endorsing symptoms over time, in order to better explore the nature of persistent symptomatology post-MTBI. Similarly, the role of non-neurological maintaining factors in persisting PCS symptoms should be examined in conjunction with a consideration of pre-injury to post-injury symptom changes following MTBI. Given that the current study is the first of its kind to investigate the ICD-10 PCS symptoms in individuals following MTBI, replication in another MTBI sample is warranted. Finally, the predictive accuracy of the ICD-10 criteria for PCS should be investigated in patient groups following moderate and severe brain injury in order to determine its utility in diagnosing PCS after more serious head injury.

PCS symptom endorsement between groups from one-month to three-month follow-up

ROC curve obtained at one month post-injury.

Utility estimates at one month post-injury in classifying MTBI and control participants

ROC curve obtained at three months post-injury.

PCS symptom endorsement by MTBI participants from one-month to three-month follow-up
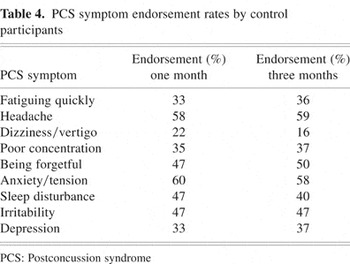
PCS symptom endorsement rates by control participants