INTRODUCTION
Multiple sclerosis is a chronic inflammatory demyelinating and degenerative disease of the central nervous system that is increasingly diagnosed in childhood and adolescence (Bigi & Banwell, Reference Bigi and Banwell2012; Waubant & Chabas, Reference Waubant and Chabas2009; Yeh et al., Reference Yeh, Chitnis, Krupp, Ness, Chabas, Kuntz and Waubant2009). Cognitive impairment is typically observed in 30% of youth with MS. Domains of cognition most commonly affected include processing speed, language, visuospatial skills, and memory (Amato et al., Reference Amato, Goretti, Ghezzi, Hakiki, Niccolai, Lori and Cilia2014; Banwell & Anderson, Reference Banwell and Anderson2005; MacAllister, Christodoulou, Milazzo, & Krupp, Reference MacAllister, Christodoulou, Milazzo and Krupp2007; Till et al., Reference Till, Deotto, Tipu, Sled, Bethune, Narayanan and Banwell2011). Executive functions, such as working memory, seem to be particularly vulnerable to MS pathology (Banwell & Anderson, Reference Banwell and Anderson2005; Till et al., Reference Till, Ho, Dudani, Garcia-Lorenzo, Collins and Banwell2012), perhaps as a consequence of disruption to actively maturing white matter (WM) pathways which underlie these functions (Casey, Tottenham, Liston, & Durston, Reference Casey, Tottenham, Liston and Durston2005; Dennis, Reference Dennis2000; Dennis et al., Reference Dennis, Spiegler, Simic, Sinopoli, Wilkinson, Yeates and Fletcher2014).
Magnetic resonance imaging (MRI) measures of WM lesion volume, as quantified by T2- and T1-weighted scan analyses, have correlated only modestly with cognitive function (Till et al., Reference Till, Deotto, Tipu, Sled, Bethune, Narayanan and Banwell2011, Reference Till, Ho, Dudani, Garcia-Lorenzo, Collins and Banwell2012). Moreover, WM and cortical lesion volumes have not been shown to differ between cognitively impaired and preserved pediatric-onset MS patients, whereas lower whole-brain WM volumes have been observed in cognitively impaired patients (Rocca et al., Reference Rocca, De Meo, Amato, Copetti, Moiola, Ghezzi and Pera2015). Smaller whole-brain and thalamic volumes, as compared to age- and sex-normative data, correlate with global IQ, processing speed, and expressive vocabulary; however, these MRI metrics only account for up to 40% of the variability in cognitive performance (Till et al., Reference Till, Deotto, Tipu, Sled, Bethune, Narayanan and Banwell2011).
It is plausible that differences in brain activation patterns or processing efficiency, in addition to, or as a result of, these structural changes may account for variability in cognitive performance in pediatric-onset MS. Changes in activity patterns on functional MRI (fMRI) among spatially distributed brain regions implicated in a task have been hypothesized to indicate disruption of brain function in the context of neurological deficits, or compensatory neuroplasticity in response to disorders such as MS (Reuter-Lorenz & Cappell, Reference Reuter-Lorenz and Cappell2008; Schoonheim, Geurts, & Barkhof, Reference Schoonheim, Geurts and Barkhof2010). The compensation-related utilization of neural circuits hypothesis (CRUNCH) posits that enhanced activation and/or activation of a larger neural network may occur to facilitate adaptation to neuropathology. Cognitive impairment is proposed to occur as structural damage accumulates over time and compensatory mechanisms become insufficient to sustain performance on a task, leading to decreases in activation (Reuter-Lorenz & Cappell, Reference Reuter-Lorenz and Cappell2008; Schoonheim et al., Reference Schoonheim, Geurts and Barkhof2010).
In line with the CRUNCH hypothesis, adult MS patients with intact working memory performance demonstrate greater and more extensive brain activation compared to healthy controls during working memory tasks, with particular recruitment of the lateral and inferior frontal cortex, inferior parietal cortex, and cerebellum (Amann et al., Reference Amann, Dössegger, Penner, Hirsch, Raselli, Calabrese and Gass2011; Audoin et al., Reference Audoin, Ibarrola, Ranjeva, Confort‐Gouny, Malikova, Ali‐Chérif and Cozzone2003; Forn et al., Reference Forn, Barros-Loscertales, Escudero, Benlloch, Campos, Parcet and Avila2006, Reference Forn, Barros-Loscertales, Escudero, Benlloch, Campos, Parcet and Avila2007; Mainero et al., Reference Mainero, Caramia, Pozzilli, Pisani, Pestalozza, Borriello and Pantano2004; Staffen et al., Reference Staffen, Mair, Zauner, Unterrainer, Niederhofer, Kutzelnigg and Ladurner2002; Sweet, Rao, Primeau, Durgerian, & Cohen, Reference Sweet, Rao, Primeau, Durgerian and Cohen2006). Compensatory activity is most apparent in low- versus high-executive demand (ED) conditions (Amann et al., Reference Amann, Dössegger, Penner, Hirsch, Raselli, Calabrese and Gass2011; Sweet et al., Reference Sweet, Rao, Primeau, Durgerian and Cohen2006) and appears to vary as a function of structural damage. MS patients with larger lesion volumes or more significant gray matter (GM) atrophy demonstrate larger magnitudes of task activation (Mainero et al., Reference Mainero, Caramia, Pozzilli, Pisani, Pestalozza, Borriello and Pantano2004; Morgen et al., Reference Morgen, Sammer, Courtney, Wolters, Melchior, Blecker and Vaitl2007; Sweet et al., Reference Sweet, Rao, Primeau, Durgerian and Cohen2006).
Notably, patients demonstrating working memory impairment show less activation than those whose performance is intact, in the bilateral prefrontal and inferior parietal cortices, as well as in the right temporal, supplementary motor, and anterior cingulate cortices (Mainero et al., Reference Mainero, Caramia, Pozzilli, Pisani, Pestalozza, Borriello and Pantano2004). In our prior work with youth and young adults with pediatric-onset MS, patients demonstrated lower activation than controls in the right middle frontal gyrus during a processing speed task; however, increased task-related activation was associated with faster response times in the MS patients but not controls (Akbar et al., Reference Akbar, Banwell, Sled, Binns, Doesburg, Rypma and Till2016).
We now expand our prior observations by evaluating whether cognitively intact youth and young adults with pediatric-onset MS demonstrate heightened activation relative to controls during an executive control (working memory) task. Given prior evidence for differential activation patterns in individuals with and without impairment (Mainero et al., Reference Mainero, Caramia, Pozzilli, Pisani, Pestalozza, Borriello and Pantano2004), we focused our investigation on cognitively preserved youth to increase our signal in detecting compensatory recruitment. Our MS group was thus expected not to differ cognitively from our control sample, despite possible differences in structural MRI (smaller GM, WM, and thalamic volumes). Supplementary analyses were conducted to evaluate relationships between fMRI, structural MRI and neuropsychological outcomes. We anticipated that better performance on the fMRI task would correlate positively with neuropsychological outcomes, as well as with measures of brain health in MS patients (larger brain and smaller lesion volumes). Increased recruitment on the fMRI task was expected to be associated with poorer outcomes on structural MRI in MS patients.
METHODS
Participants
Participants with relapsing-remitting pediatric-onset MS were primarily recruited by approaching patients at the MS clinic at the Hospital for Sick Children. In addition, all eligible patients in the database of the Canadian Pediatric Demyelinating Disease Study, currently or previously cared for at the Hospital for Sick Children, were contacted via telephone or email. Participants were also recruited via online advertisement on the MS Society of Canada Research Portal and the SickKids research4kids Web site, as well as through the Undergraduate Participant Pool at York University. Participants were between the ages of 13 and 25 years, and were recruited in 2011–2015.
Pediatric-onset MS patients had to be younger than 18 at the time of first MS attack and meet revised McDonald 2010 diagnostic criteria for relapsing-remitting MS (Polman et al., Reference Polman, Reingold, Banwell, Clanet, Cohen, Filippi and Lublin2011). Patients were evaluated at least 4 weeks after their most recent clinical relapse or completion of corticosteroid treatment. All participants were screened using a structured interview at the time of recruitment. MS or control participants with a prior medical diagnosis pertinent to neurological and/or cognitive function (e.g., epilepsy, cancer, stroke), history of head trauma, prematurity, alcohol and/or illicit drug abuse, or learning disorder were excluded. Non-MS individuals were also screened for current or past psychiatric diagnosis and/or treatment. The research protocol was reviewed and approved by the Research Ethics Boards at both hospitals, as well as the site of MRI scanning (York University). Written informed consent was obtained from all participants and/or a parent or legal guardian.
The present cohort was selected on the basis of intact cognitive performance, as measured by a neuropsychological battery (details on measures and criteria for impairment below). Of the 24 MS patients and 27 healthy controls initially recruited, 2 patients and 3 controls were excluded after meeting criteria for cognitive impairment. An additional MS patient and four controls were excluded due to excessive motion artefact. Twenty MS patients and 20 age- and sex-matched controls were included in the final sample; note that one of the recruited MS patients was also excluded due to the lack of availability of an age- and sex-matched healthy control.
Measures
Demographic and disease-related information were obtained from patient health records, including: age at disease onset (from first attack), disease duration, number of relapses, most recent Expanded Disability Status Scale (EDSS) score, and current medications. EDSS score was determined by the patient’s MS neurologist.
All participants completed a 45-min neuropsychological battery assessing: IQ, processing speed, attention, verbal learning/memory, and executive function. Specifically, this battery included the following tasks: Rey Auditory Verbal Learning Test (RAVLT; Schmidt, Reference Schmidt1996), 2-subscale form of the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, Reference Weschler1999), Decision Speed and Auditory Working Memory tests of the Woodcock-Johnson III (WJ-III; Woodcock, McGrew, & Mather, Reference Woodcock, McGrew and Mather2001), oral administration of the Symbol Digits Modalities Test (SDMT; Smith, Reference Smith1982), and Trail Making Tests (TMT; Reitan, Reference Reitan1992). For the purposes of the current study, the Verbal and Performance components of the Wechsler Abbreviated Scale of Intelligence were treated as separate measures. Cognitive impairment was defined as performance 1.5 standard deviations below the normative mean on three or more subtests out of the seven unique cognitive measures (Ingraham & Aiken, Reference Ingraham and Aiken1996)Footnote 1.
The following Z-scores were averaged to create a neuropsychological composite Z-score: RAVLT Total, WASI Verbal Comprehension, WASI Matrix Reasoning, WJ-III Decision Speed, WJ-III Auditory Working Memory, SDMT, and Part B of the TMT. The WJ-III Auditory Working Memory task was also investigated separately from the composite, to allow for an examination of working memory performance on a standardized scale, as well a comparison to performance on the fMRI task. Handedness was assessed via the Dutch Handedness Questionnaire (Van Strien, Reference Van Strien2002), and motor coordination was examined using the Nine Hole Peg Test (9HPT; Mathiowetz, Volland, Kashman, & Weber, Reference Mathiowetz, Volland, Kashman and Weber1992) to investigate the role of motor functioning on task performance and activation patterns.
fMRI task
Participants completed the Alphaspan task while undergoing functional neuroimaging (Craik, Reference Craik1986; Turner & Levine, Reference Turner and Levine2008). During this task, participants were asked to study a set of three or five consonant letter strings, and then to either remember the letter set in forward order (low-ED), or to mentally manipulate the letters into alphabetical order (high-ED). The task begins with a screen that informs the subject of the ED condition (“forward”/low-ED or “alphabetize”/high-ED). Participants are then presented with the letter set (either three or five letters) for 3 s. After a 7-s delay, a probe (such as “F-4”) is presented, consisting of a letter and a position, signaling “Was F the fourth letter in the set?” In the forward condition (low-ED), the probe refers to the ordinal position; in the alphabetize condition (high-ED), the probe refers to the ordinal position after the subject has mentally reorganized the letters into alphabetical order.
Participants were instructed to determine if the probe matches what was presented in the set. The test was arranged such that 50% of the prompts were correct. Participants responded with a “yes/no” button press on an MRI compatible keyboard using their index and middle fingers of their dominant hand. An example of the task time series per stimulus is shown in Figure 1.
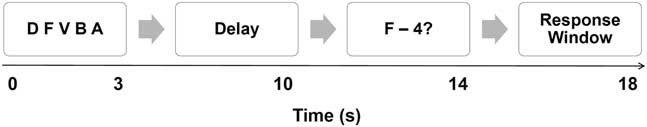
Fig. 1 An example of the Alphaspan time series with a 5-letter set.
Participants completed seven practice trials in each of the four Alphaspan conditions (forward-3 letters; forward-5 letters; alphabetize-3 letters; alphabetize-5 letters) before entering the scanner. During scanning, subjects completed 12 trials of each of the task conditions over a total of 20 min. Four blocks of low-ED trials (containing six stimuli each) were interspersed with four blocks of high-ED trials. Each trial was separated by an inter-stimulus interval of 4–6 s, and each block was separated by an 18-s rest period.
For the current analyses, we focused on the ED variable and collapsed across set size (i.e., three or five letter string) to increase the number of trials per ED condition (i.e., forward [3/5 string] vs. alphabetize [3/5 string]). Task performance was evaluated via measures of accuracy and response time. The percentage of items answered correctly was calculated as a measure of accuracy, and time to respond was averaged across correct trials as a measure of response time, for each of the ED conditions.
MRI protocol
Scans were carried out at the York MRI Facility on a Siemens 3.0 Tesla MAGNETOM TIM Trio scanner. The total time for scanning was 70 min for healthy controls and 90 min for patients. A high-resolution structural volumetric image was acquired from all participants using a T1-weighted three-dimensional (3D) MPRAGE sequence (1 mm isotropic voxel size; repetition time [TR]=2300 ms; echo time [TE]=2.96 ms). Proton density-weighted (TR=2200 ms; TE=10 ms; turbo factor=4) and T2-weighted (TR=4500 ms; TE=83 ms; turbo factor=11) images were acquired from MS patients only for lesion segmentation, using 2D turbo-spin-echo sequences with 1 × 1 × 3 mm3 voxel size, along with a matching 2D turbo FLAIR sequence with TR=9000 ms, TE=88 ms, TI=2407.5 ms.
fMRI images were acquired using a T2-weighted echo planar imaging sequence (TE=30 ms; flip angle 90°; matrix size=86 × 64; field of view=256 × 190.5 mm; TR=2 s). Thirty-four axial slices, parallel to the anterior–posterior commissural plane (thickness=4 mm) and covering the whole brain, were acquired during each measurement.
T2 lesions were segmented using an automated Bayesian classifier (Ghassemi et al., Reference Ghassemi, Nayahanan, Banwell, Sled, Shroff and Arnold2014). This was followed by manual review and correction of the results by a trained specialist. Given their different MRI intensity characteristics, infratentorial T2 lesions were segmented manually. T1 lesions were identified within T2 lesions using an automated threshold technique, as previously described (Ghassemi et al., Reference Ghassemi, Nayahanan, Banwell, Sled, Shroff and Arnold2014). Supratentorial and infratentorial lesion volumes were combined as a measure of total lesion volume.
Brain and thalamic volumes were computed based on the T1-weighted image. First, the brain and skull were extracted from the T1 image (Smith, Reference Smith2002). The brain image was then affine-registered to Montreal Neurological Institute (MNI) 152 space, using the skull image to determine the registration scaling (Jenkinson & Smith, Reference Jenkinson and Smith2001; Jenkinson, Bannister, Brady, & Smith, Reference Jenkinson, Bannister, Brady and Smith2002). Subsequently, tissue-type segmentation with partial volume estimation was carried out (Zhang, 2001) to calculate total volume of brain tissue. Normalized whole-brain GM and WM volumes were estimated using SIENAX (Smith, De Stefano, Jenkinson, & Matthews, Reference Smith, De Stefano, Jenkinson and Matthews2001; Smith et al., Reference Smith, Zhang, Jenkinson, Chen, Matthews, Federico and De Stefano2002).
Thalamic volume was calculated via the following steps: (a) an initial linear (Collins, Neelin, Peters, & Evans, Reference Collins, Neelin, Peters and Evans1994) followed by non-linear registration (Collins, Holmes, Peters, & Evans, 1995) of the raw T1-weighted image to the ICBM152 population template space (Fonov et al., Reference Fonov, Evans, Botteron, Almli, McKinstry and Collins2011), (b) brain extraction using a library of tissue priors that are available in the ICBM152 population template (Eskildsen et al., Reference Eskildsen, Coupe, Fonov, Manjon, Leung and Guizard2012), (c) warping of the thalamus template onto the subject’s raw T1-weighted image using the inverse of the transformation generated in the first step. Thalamic volumes were normalized for head size by dividing by total intracranial volume.
fMRI processing
Skulls were removed using the Brain Extraction Tool (Smith, Reference Smith2002). Preprocessing was conducted using FEAT (fMRI Expert Analysis Tool) from the FMRIB Software Library (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, Reference Jenkinson, Beckmann, Behrens, Woolrich and Smith2012). Images were slice-time corrected, motion corrected using a rigid-body algorithm in MCFLIRT (Motion Correction FMRIB Linear Image Registration Tool; Jenkinson, Reference Jenkinson2003), and temporally smoothed with a high-pass (100 s cutoff) filter. Spatial smoothing was done with an 8mm (Full Width at Half Maximum) 3D Gaussian kernel. Each participant’s functional images were spatially registered to their skull-stripped T1-weighted structural image, using Boundary-Based Registration (Greve & Fischl, Reference Greve and Fischl2009). Subsequently, images were registered nonlinearly to standard MNI space (1 mm), using a 10-mm warp resolution. Participants with excessive motion artifact in the MRI scan were not included in the final analyses.
Statistics
The Shapiro-Wilk and Levene’s tests were used to detect violations of normality and homogeneity of variance within behavioral data sets. A conservative alpha of 0.01 was used across analyses to limit the potential for type I error associated with multiple comparisons. One-tailed tests were used for analysis of directional hypotheses.
Neuropsychological scores (composite, WJ-III Auditory Working Memory, 9HPT) and normalized brain volumes (GM, WM, thalamus) were compared across MS patients and healthy controls by way of an independent samples t test or Mann-Whitney U test, as appropriate, to describe the sample. Outliers were Winsorized to 3 SDs above/below the normative mean where norms were available. Performance on the Alphaspan task was assessed in a 2 (Group) × 2 (Task Demand) mixed design analysis of variance (ANOVA). Given violations to the assumption of normality, the ANOVAs were broken down into three equivalent analyses, with parametric and non-parametric variants, as appropriate.
fMRI analyses
Correct trials on each of the task conditions were modeled in FILM (FMRIB’s Improved Linear Model) using a double-gamma function with temporal derivatives (Jenkinson et al., Reference Jenkinson, Beckmann, Behrens, Woolrich and Smith2012). Six motion correction parameters, error trials, finger taps, and presentation of the set and probes were treated as covariates of no interest in the first level analysis. Voxel-wise parameter estimates were obtained for each participant, reflecting the fit of the model with the underlying time series and a contrast of ED conditions. FILM prewhitening was applied to control for autocorrelation and temporal filtering was applied for low frequencies (Jenkinson, Reference Jenkinson2003).
First-level analyses were then forwarded into a whole-brain mixed effects group analysis, using FLAME (FMRIB’s Local Analysis of Mixed Effects; Greve & Fischl, Reference Greve and Fischl2009). A mixed design ANOVA was conducted with two levels: Group (MS, controls) and Task Demand (low-ED, high-ED), to identify regions in the brain that were differentially activated across these variables. Statistical maps were thresholded using voxels with a Z>4.1 (Prakash et al., Reference Prakash, Snook, Erickson, Colcombe, Voss, Motl and Kramer2007), and a stringent cluster significance level of p<.0001 was set to control for family-wise error, as previously described (Woo, Krishnan, & Wager, Reference Woo, Krishnan and Wager2014). Clusters surviving this thresholding were labeled using the Harvard-Oxford structural atlas on FSL (Jenkinson et al., Reference Jenkinson, Beckmann, Behrens, Woolrich and Smith2012).
Correlational analyses
Spearman correlations were conducted for MS patients, between fMRI task outcomes (activation in recruited regions, task performance) and structural MRI (T1 and T2 lesion, GM, WM, and thalamic volumes). Spearman correlations were also conducted between performance on the Alphaspan task and neuropsychological outcomes (composite, WJ-III Auditory Working Memory, 9HPT). Alpha was set to 0.01 to correct for multiple correlations.
RESULTS
The demographic features of 20 cognitively preserved pediatric-onset MS patients and 20 age- and sex-matched controls are shown in Table 1. Patients did not differ from controls on the neuropsychological composite nor on the WJ-III Auditory Working Memory task, while MS patients trended toward poorer performance on the 9HPT. The MS group demonstrated smaller thalamic volumes; however, whole-brain normalized GM and WM volumes did not differ significantly between groups.
Table 1 Demographic, disease-related, and MRI data for all participants

Note. MS=multiple sclerosis; HC=healthy control; F=female; M=male; EDSS=Expanded Disability Status Scale; WJ-III=Woodcock-Johnson III Test of Cognitive Abilities – 3rd Edition
a Chi-square test.
b Months since first attack.
c Age at first attack.
d Mann-Whitney U test.
e One-tailed test, MS<HC.
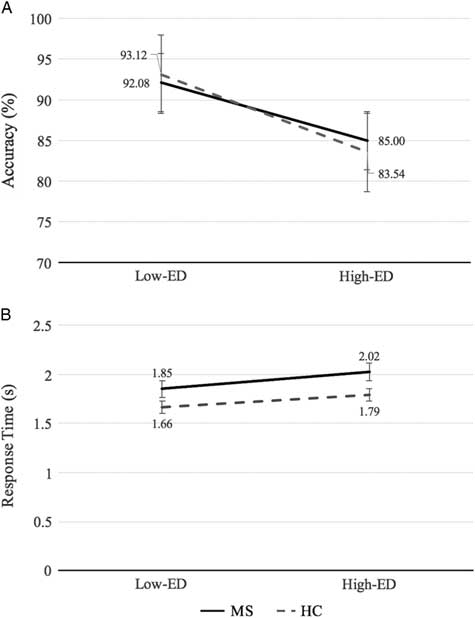
On the Alphaspan task, participants performed less accurately on the high-ED condition relative to the low-ED condition (d=1.85; p<.001), however, response times did not differ significantly between ED conditions. No difference in accuracy was found between groups. MS patients demonstrated an average of 0.21s longer response time than controls on the task (d=0.92; p=.007), with ED condition collapsed. Interactions between group and ED condition were non-significant for both accuracy and response time. MS and control Alphaspan performance data are shown in Figure 2.
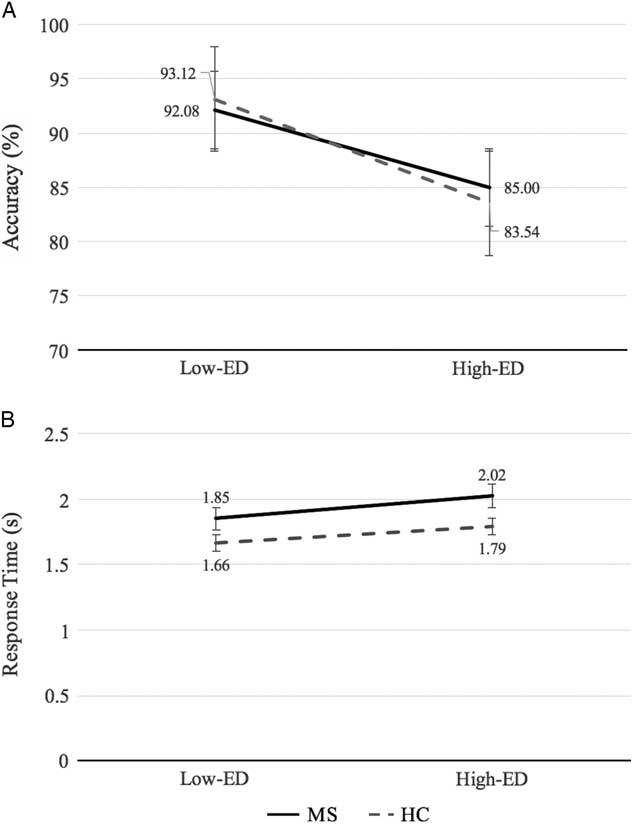
Fig. 2 (A) Percentage accuracy and (B) response time for MS and healthy control groups in the low-executive demand (Low-ED) and high-executive demand (High-ED) conditions of the Alphaspan task. Error bars reflect the standard error.
Accuracy on the Alphaspan task correlated positively with the neuropsychological composite score (r=0.52; p=.010), and trended toward a positive correlation with the WJ-III Auditory Working Memory task (r=0.47; p=.017) in MS patients. Shorter response times correlated with higher scores on the neuropsychological composite (r=–0.59; p=.003) and WJ-III Auditory Working Memory task (r=–0.56; p=.006). Alphaspan performance (accuracy, response time) was not related to scores on the 9HPT. Correlations between Alphaspan performance and structural MRI metrics (T1 and T2 lesion volume, GM, WM, thalamic volume) did not reach significance (not shown).
Functional MRI Results
When considering all participants together, whole-brain analyses revealed a significant effect of task demand in several brain regions, including the bilateral prefrontal, right superior parietal, medial frontal, bilateral limbic, and operculum cortices (shown in Table 2; Figure 3), with greater percent signal change from baseline in the high-ED condition than in the low-ED conditionFootnote 2. No significant activation differences were observed between groups in the whole-brain analysis, and group by task demand interactions were non-significant.

Fig. 3 Areas demonstrating significant change in activation across levels of executive demand, collapsing across groups: (A) High-ED>Low-ED, and (B) Low-ED>High-ED.
Table 2 Clusters demonstrating significant main effect of executive demand in whole-brain analysis, collapsing across groups (N=40)

Note. BA=Brodmann area; MNI=Montreal Neurological Institute; ED=executive demand; L=left; R=right.
Post hoc Analyses
Post hoc orthogonal contrasts were conducted to test for activation differences between groups in regions demonstrating a significant effect of ED. Seventeen unique ROIs were selected based on the cluster peaks identified in the high- vs. low-ED contrast (Table 3). Average percent signal change in the low-ED condition (vs. rest) and high-ED condition (vs. rest) were extracted from spherical ROIs of 10-mm diameter, centered around the MNI coordinates of the cluster peak using FSL featquery (Prakash et al., Reference Prakash, Snook, Erickson, Colcombe, Voss, Motl and Kramer2007). These values were compared across MS patients and controls, using an alpha level of p<.05. For the low-ED condition, MS patients demonstrated greater percent signal change (from rest) than healthy controls in the right middle frontal, left paracingulate, right supramarginal, and left superior parietal gyri. For the high-ED condition, MS patients again demonstrated enhanced activation relative to controls in right supramarginal gyrus. Similar patterns of enhanced activation for MS patients were demonstrated in separate analyses run with left-handers removed and after controlling for response time on the task (data not shown).
Table 3 Comparison of activation across groups per task condition (Low-ED vs. rest; High-ED vs. rest) in cluster peaks identified from the whole-brain analysis

Note. BA=Brodmann area; MNI=Montreal Neurological Institute; ED=executive demand; MS=multiple sclerosis; HC=healthy control; L=left; R=right. Statistically significant comparisons are presented in bold type.
Activation of the left superior parietal gyrus during the low-ED condition was associated with reduced thalamic volume (r=-0.55; p=.006). No other relationships were found between activation during the Alphaspan task and structural MRI metrics (T1 and T2 lesion volume, GM, and WM) in MS patients, p>.01. Similarly, correlations between brain activation during the Alphaspan task and performance did not reach significance, p>.05.
DISCUSSION
We sought out to evaluate whether cognitively intact youth and young adults with pediatric-onset MS demonstrate differential activation patterns, as compared to healthy peers, when engaging in a working memory task. We observed increased activation in these patients in regions implicated in executive control processing. Furthermore, we observed these differences in the context of preserved accuracy on the task, as well as after controlling for response time. Differences in activation levels were observed during performance of high and low executive control demand conditions, suggesting that MS patients draw upon executive control processes to a greater extent than typically developing peers, and particularly when executive control demands are low.
Across all participants, whole-brain analyses revealed enhanced activation patterns during the high-ED condition, as compared to the low-ED condition, in the right superior frontal cortex, as well as bilaterally in the lateral prefrontal cortex (Figure 3). These areas have previously been implicated in executive control processing (Baldo & Dronkers, Reference Baldo and Dronkers2006; Curtis & D’Esposito, Reference Curtis and D’Esposito2003; Deschamps, Baum, & Gracco, Reference Deschamps, Baum and Gracco2014; Smith & Jonides, Reference Smith and Jonides1999; Turner & Spreng, Reference Turner and Spreng2012; Wager & Smith, Reference Wager and Smith2003). Greater deactivation of the medial frontal cortex, limbic cortices and right operculum in the high-ED condition, as compared to the low-ED condition, are believed to reflect a transition away from resting state (Uddin, Kelly, Biswal, Castellanos, & Milham, Reference Uddin, Kelly, Biswal, Castellanos and Milham2009). Alphaspan performance correlated positively with the neuropsychological composite and auditory working memory scores, providing evidence to support the utility of the Alphaspan task as a tool to evaluate MS patients who are otherwise cognitively intact.
Regions that were differentially recruited by our cohort of cognitively preserved pediatric-onset MS patients relative to controls included the right middle frontal, left paracingulate, right supramarginal, and left superior parietal gyri. We replicate the fMRI findings of prior studies using the Alphaspan task, in which participants with diffuse axonal damage but intact performance demonstrated recruitment of these same regions (Turner & Levine, Reference Turner and Levine2008). Our findings also mirror enhanced activation patterns observed in adults with early MS or clinically isolated syndromes (Audoin et al., Reference Audoin, Ibarrola, Ranjeva, Confort‐Gouny, Malikova, Ali‐Chérif and Cozzone2003) and cognitively preserved adults with MS (Forn et al., Reference Forn, Barros-Loscertales, Escudero, Benlloch, Campos, Parcet and Avila2006, Reference Forn, Barros-Loscertales, Escudero, Benlloch, Campos, Parcet and Avila2007; Mainero et al., Reference Mainero, Caramia, Pozzilli, Pisani, Pestalozza, Borriello and Pantano2004) during working memory task processing.
Recruitment in the right middle frontal gyrus was in the premotor cortex (Brodmann Area [BA] 6), which is believed to play a role in the maintenance of visuospatial attention (Owen, McMillan, Laird, & Bullmore, Reference Owen, McMillan, Laird and Bullmore2005). Activation in this region has also been observed to be sensitive to ED (Tsukiura et al., Reference Tsukiura, Fujii, Takahashi, Xiao, Inase, Iijima and Okuda2001; Wager & Smith, Reference Wager and Smith2003). The left paracingulate region in this case represents the frontal eye fields (BA8), which facilitate spatial rehearsal during working memory tasks (Curtis & D’Esposito, Reference Curtis and D’Esposito2003). The parietal regions differentially recruited in MS patients include the precuneus (BA7), which plays a role in visuospatial imagery and episodic memory retrieval (Cavanna & Trimble, Reference Cavanna and Trimble2006), and the supramarginal gyrus (BA40), which has been reported to play a role in phonological processes (Baldo & Dronkers, Reference Baldo and Dronkers2006; Deschamps et al., Reference Deschamps, Baum and Gracco2014). Enhanced activation in these regions are believed to reflect greater resources required for executive control processing or rehearsal in the Alphaspan task.
The CRUNCH hypothesis suggests that differential activation associated with neurological changes may reflect engagement of additional neural resources to support task performance (Reuter-Lorenz & Cappell, Reference Reuter-Lorenz and Cappell2008). As the patients with MS demonstrated comparable levels of task accuracy relative to controls and the regions recruited were in areas anticipated for the task (Amann et al., Reference Amann, Dössegger, Penner, Hirsch, Raselli, Calabrese and Gass2011; Audoin et al., Reference Audoin, Ibarrola, Ranjeva, Confort‐Gouny, Malikova, Ali‐Chérif and Cozzone2003; Forn et al., Reference Forn, Barros-Loscertales, Escudero, Benlloch, Campos, Parcet and Avila2006, Reference Forn, Barros-Loscertales, Escudero, Benlloch, Campos, Parcet and Avila2007; Mainero et al., Reference Mainero, Caramia, Pozzilli, Pisani, Pestalozza, Borriello and Pantano2004; Turner & Levine, Reference Turner and Levine2008), it is proposed that these enhanced activations may reflect changes that are compensatory in this young adult, cognitively intact cohort. This is in line with our previous findings of a positive association between functional activation and performance during a processing speed task in cognitively preserved pediatric-MS patients (Akbar et al., Reference Akbar, Banwell, Sled, Binns, Doesburg, Rypma and Till2016).
Our cohort of cognitively preserved pediatric-onset MS patients demonstrated smaller thalamic volume than their healthy peers. Greater thalamic damage was associated with increased recruitment of the left superior parietal gyrus during the low-ED condition in MS patients, suggesting possible predictive relationships between disease burden and the extent of recruitment required by patients. Mainero et al. (Reference Mainero, Caramia, Pozzilli, Pisani, Pestalozza, Borriello and Pantano2004) similarly found that lesion load was correlated with increased right dorsolateral prefrontal recruitment in adults with MS during a working memory task. Thalamic volume has previously been found to be a robust predictor of cognitive function in pediatric MS (Till et al., Reference Till, Deotto, Tipu, Sled, Bethune, Narayanan and Banwell2011); here, we find it to be the structural neuroimaging measure most sensitive to altered activation patterns in cognitively preserved youth and young adults with MS, relative to lesion volume and whole-brain volume.
As demands increased in the high-ED condition, which involved both working memory maintenance and manipulation processes, we infer that increased activation by control participants in this more difficult condition resulted in reduced between-group differences. Although MS patients demonstrated trends toward greater activation than healthy controls in the high-ED condition, these differences may be comparatively smaller as participants approach a resource ceiling, which corresponds to poorer performance on the task across all participants (Reuter-Lorenz & Cappell, Reference Reuter-Lorenz and Cappell2008). Larger activation differences between groups in the low-ED condition suggest that these pediatric-onset MS patients may recruit executive control processes sooner than their age- and sex-matched peers. This interpretation is consistent with prior findings, showing a pattern of increased activation in adult MS patients during lower levels of ED on a working memory task and reduced activation differences from controls when task demands increased (Amann et al., Reference Amann, Dössegger, Penner, Hirsch, Raselli, Calabrese and Gass2011; Sweet et al., Reference Sweet, Rao, Primeau, Durgerian and Cohen2006).
Our study deliberately evaluated cognitively preserved youth and young adults with pediatric-onset MS, given our goal to explore whether cognitive preservation occurs as a consequence of compensatory activation patterns. Our findings cannot be generalized to the pediatric-onset MS population as a whole, as we would expect approximately a third of patients to demonstrate cognitive impairment. It will be important for future studies to examine how activation patterns evolve as the disease progresses and cognitive deficits arise, to better appreciate the value of serial fMRI as a metric to identify pending cognitive deterioration. Larger sample sizes will allow for greater parameterization of the task with further manipulation of executive control demands, thus facilitating a greater understanding of relationships between activation changes, task demands, and performance.
In summary, cognitively preserved youth and young adults with pediatric-onset MS demonstrate greater activation than healthy peers in regions implicated in executive control during a working memory task. These findings support fMRI as a tool to evaluate the impact of MS on cognitive processing even when cognitive performance remains intact. Implications of increased brain activation in the context of normal cognitive performance require further elucidation, particularly with respect to how increased activation relates to cognitive fatigue and real-world activities, such as academic and career achievements.
ACKNOWLEDGMENTS
Thanks to Dr. Keith Schneider for his comments regarding the fMRI analyses, as well as Joy Williams and Aman Goyal for conducting the scans and providing technical support in processing the functional scans. We thank Mahsa Sadeghi, Austin Sye, Stephanie Grover, and Carmen Yea for their assistance with recruitment and/or testing of research participants. We also thank the children and families who generously contributed their time to this research. B.B. serves as a consultant to Novartis and as an advisor for clinical trials for Biogen Idec, Sanofil and Tevaneuroscience. E.A.Y. performs relapse adjudication for ACI, was on a scientific advisory panel for Juno Therapeutics, has received a speaker’s honorarium from Novartis. All other authors (E.B-K., G.R.T., N.A., M.L., D.L.C., B.A-B., C.T.) report no disclosures. This work was supported by the Scottish Rite Charitable Foundation of Canada (C.T., Research Grant number 2011-2012); as well as by Studentships from the Multiple Sclerosis Society of Canada (E.B-K., MS Society Project Number 2379); Ontario Graduate Scholarship; and Canadian Institute of Health Research (E.B-K, Doctoral Award Application Number 379609).