INTRODUCTION
Pediatric brain tumors occur at an incidence rate of 5.5 per 100,000 children in the United States (Ostrom et al., Reference Ostrom, Gittleman, Truitt, Boscia, Kruchko and Barnholtz-Sloan2018), and between 45% and 60% of those tumors occur within the posterior fossa (Pollack, Reference Pollack1999). Survivors of embryonal tumors of the posterior fossa, such as medulloblastomas, and astrocytomas, such as pilocytic astrocytomas, demonstrate poorer intelligence quotient (IQ) scores and adaptive functioning compared to healthy peers (Mulhern & Butler, Reference Mulhern and Butler2004; Netson et al., Reference Netson, Conklin, Wu, Xiong and Merchant2012).
With the increasing likelihood of survival over recent decades (Ostrom et al., Reference Ostrom, Gittleman, Liao, Vecchione-Koval, Wolinsky, Kruchko and Barnholtz-Sloan2017), there is now an emphasis on maximizing cognitive outcomes and improving the long-term functioning of brain tumor survivors. Neurosurgical resection has been found to yield cognitive impairments of lesser severity than chemotherapy and radiation therapy (RT); several studies have shown that attention (Jayakar et al., Reference Jayakar, King, Morris and Na2015; Papazoglou et al., Reference Papazoglou, King, Morris and Krawiecki2008), working memory (King et al., Reference King, Na and Mao2015), IQ (Mulhern & Butler, Reference Mulhern and Butler2004), processing speed (Palmer et al., Reference Palmer, Armstrong, Onar-Thomas, Wu, Wallace, Bonner, Schreiber, Swain, Chapieski, Mabbott, Knight, Boyle and Gajjar2013), and independent living (Hoskinson et al., Reference Hoskinson, Wolfe, Yeates, Mahone, Cecil and Ris2018) are most negatively impacted by RT, but are also affected by the use of chemotherapy agents in long-term survivors. Across both theoretical and empirical models, processing speed in particular has been identified as a possible central cognitive skill such that impairments disrupt working memory and attention in brain tumor survivors as well as their IQ and achievement (King et al., Reference King, Ailion, Fox and Hufstetler2019). Deficits in attention following RT are also further exacerbated over time (Kiehna et al., Reference Kiehna, Mulhern, Li, Xiong and Merchant2006). Furthermore, attentional deficits appear to lead to deficits in working memory, affecting the manipulation of information and in turn impacting higher-order cognitive tasks and skills (King et al., Reference King, Ailion, Fox and Hufstetler2019; Raghubar et al., Reference Raghubar, Mahone, Yeates, Cecil, Makola and Ris2017). Thus, impacts on attention and working memory are increasingly important to assess within the scope of cognitive and functional late effects of brain tumor treatment (Hanzlik et al., Reference Hanzlik, Woodrome, Abdel-Baki, Geller and Elbabaa2015; Louis et al., Reference Louis, Perry, Reifenberger, von Deimling, Figarella-Branger, Cavenee, Ohgaki, Wiestler, Kleihues and Ellison2016) and have been an area of focus in analyzing the interacting nature between various cognitive abilities and treatment-related effects within many conceptual and empirical models (Fry & Hale, Reference Fry and Hale2000; Palmer, Reference Palmer2008).
Previous studies have considered these domains of neurocognitive functioning to assess impairments in academic performance and vocational skills due to treatment-related factors (Mulhern & Butler, Reference Mulhern and Butler2004; Palmer et al., Reference Palmer, Armstrong, Onar-Thomas, Wu, Wallace, Bonner, Schreiber, Swain, Chapieski, Mabbott, Knight, Boyle and Gajjar2013); however, the effects of RT on cognition have not been widely considered for their impact on other facets of daily living. Adaptive functioning describes one’s ability to engage in activities of daily living and function independently at an age-appropriate level (Schalock et al., Reference Schalock, Borthwick-Duffy, Bradley, Buntinx, Coulter, Craig, Gomez, Lachapelle, Luckasson, Reeve, Shrogen, Snell, Spreat, Tassé, Thompson, Verdugo-Alonso, Wehmeyer and Yeager2010). The Scales of Independent Behavior-Revised (SIB-R) has been previously used as a measure of adaptive functioning in other clinical populations including autism spectrum disorder (Shattuck et al., Reference Shattuck, Seltzer, Greenberg, Orsmond, Bolt, Kring, Lounds and Lord2007), intellectual disability (Kraemer & Blacher, Reference Kraemer and Blacher2001), traumatic brain injury (Keenan et al., Reference Keenan, Hooper, Wetherington, Nocera and Runyan2007), and epilepsy (Kerr & Fayed, Reference Kerr and Fayed2017), as these central nervous system pathologies often result in deficits in activities of daily living. Similarly, independent living skills are a key area of interest when evaluating cognitive deficits in long-term survivors of brain tumors since previous studies have noted a longitudinal decline in adaptive behavior (Netson et al., Reference Netson, Conklin, Wu, Xiong and Merchant2013). The broad skills encompassed within adaptive functioning are a result of many basic, underlying cognitive processes (i.e., attention span, working memory, and processing speed) that are often impaired in these survivors and are further impacted by other complicating factors such as age at RT administration, treatment complications, and neurological comorbidities (King & Na, Reference King and Na2016; Taiwo et al., Reference Taiwo, Na and King2017; Wolfe et al., Reference Wolfe, Madan-Swain and Kana2012). Neurocognitive impairments are exacerbated by increased doses of RT as well as increased frequency of RT administration, and RT is the most reliable contributor to poor cognitive outcomes (Armstrong et al., Reference Armstrong, Liu, Yasui, Huang, Ness, Leisenring, Hudson, Donaldson, King, Stovall, Krull, Robison and Packer2009, Reference Armstrong, Jain, Liu, Merchant, Stovall, Srivastava, Gurney, Packer and Krull2010). In addition to treatment-related factors, sensory and motor impairments can also contribute to impairments in adaptive functioning and cognition, as can posttreatment complications such as hydrocephalus, hearing loss, seizures, and posterior fossa syndrome (PFS) (King et al., Reference King, Seidel, Di, Leisenring, Perkins, Krull, Sklar, Green, Armstrong, Zeltzer, Wells, Stovall, Ullrich, Oeffinger, Robison and Packer2017; Lanier & Abrams, Reference Law, Bouffet, Laughlin, Laperriere, Brière, Strother, McConnell, Hukin, Fryer, Rockel, Dickson and Mabbott2017).
Recent research on pediatric cancer populations has aimed to evaluate the cumulative impact of both clinical and demographic factors on impairments in neuropsychological performance, with the influence of sex differences on cognition gaining increased consideration. Due to the large amount of variability in samples across other confounding factors, the evidence to suggest the influence of sex differences on cognition posttreatment is equivocal. However, female survivors of brain tumors and other pediatric cancers have been shown to be at greater risk for cognitive deficits than males, most frequently determined by lower IQ (Armstrong et al., Reference Armstrong, Sklar, Hudson and Robison2007; Ris et al., Reference Ris, Packer, Goldwein, Jones-Wallace and Boyett2001). While a global metric such as IQ is informative, assessments of adaptive functioning may more readily predict behavior in daily life, yet there is currently minimal research assessing the influence of sex differences on adaptive functioning in long-term survivors of pediatric posterior fossa tumors.
The present study, therefore, sought to evaluate the interaction between sex and RT on basic elements of cognition such as attention span, working memory, and processing speed as well as adaptive functioning scores on the SIB-R (Bruininks et al., Reference Bruininks, Woodcock, Weatherman and Hill1984). Specifically, this study examined potential sex differences in treatment effects on adaptive functioning skills in long-term survivors of cerebellar brain tumors. We hypothesized that individuals who received RT would have lower IQ, attention span, working memory, processing speed, and adaptive functioning, and that females who received RT would exhibit lower scores across all cognitive and adaptive functioning domains relative to males who had RT.
METHODS
This study was approved by the local institutional review board. Survivors of childhood brain tumors were recruited using mailings from three sources: (1) a previous longitudinal childhood brain tumor study, (2) a large hospital system database, and (3) an advertisement published in an annual newsletter circulated by the state Brain Tumor Foundation. Survivors were excluded if English was not their native language, they met criteria for a pervasive developmental disorder, or they had a diagnosis of neurofibromatosis. All participants were at least 18 years old, at least 4.5 years past their tumor diagnosis, and able to pass standard visual acuity testing, ensuring their ability to view test stimuli. Forty-five participants (24 females) met the criteria and completed the following measures as part of a larger study. Available medical information was extracted from participants’ medical records and included demographics, treatment regimen, comorbidity, complications, and tumor-related information. Further demographic, tumor, and treatment-related data are presented in Table 1.
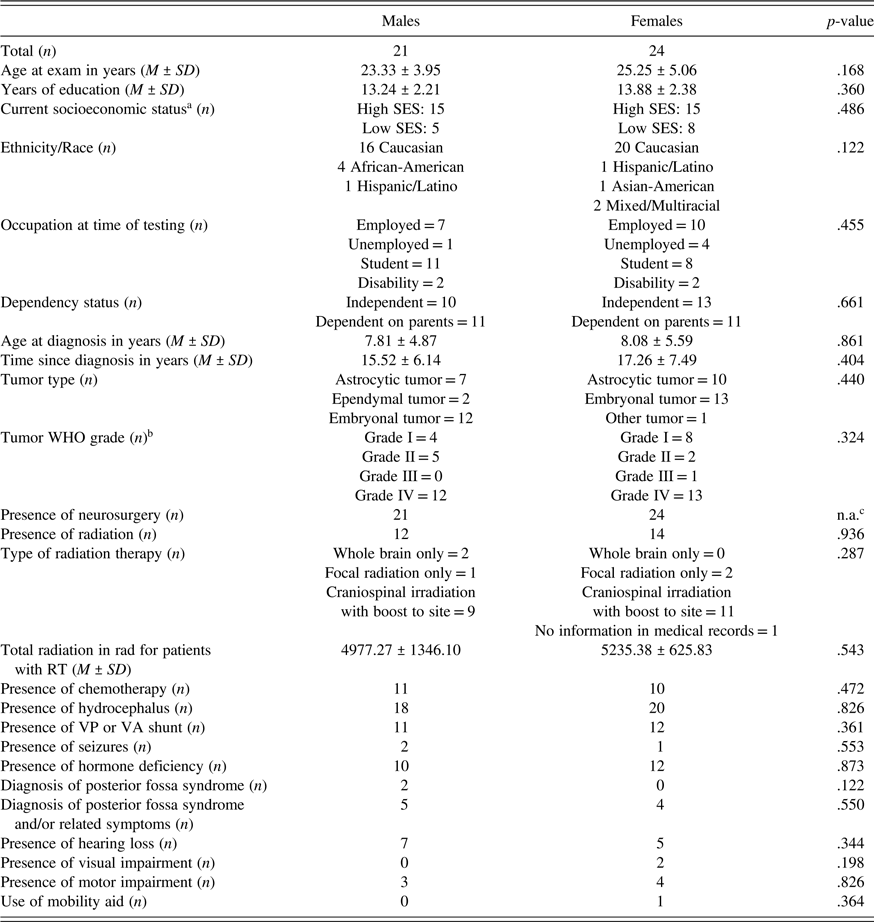
Table 1. Participant demographic and neurologic data

a Two participants (one male and one female) did not have current SES indicated.
b One male participant did not have medical record data indicating specific WHO grade but tumor was described in records as low grade.
c No statistics can be computed because all males and females received neurosurgery; therefore, there is no difference between sexes.
Wechsler Abbreviated Scale of Intelligence, Second Edition
The Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II; Wechsler, Reference Wechsler2011) was administered to participants to evaluate intelligence (IQ). The measure is comprised of four subtests assessing verbal and performance IQ, which combine to form a full-scale IQ (FSIQ).
Wechsler Memory Scale III Digit Span
The Wechsler Memory Scale, Third Edition (WMS-III; Wechsler, Reference Wechsler1997) Digit Span subtest was used to assess attention and working memory. Digit Span Forward (DSF) assesses the ability to attend to information, while the Digit Span Backward (DSB) task assesses the patient’s ability to attend to and manipulate information by repeating the digits backward. Raw scores of the longest span are converted to a z-score according to age-based norms.
Oral Symbol Digit Modalities Test
The Oral Symbol Digit Modalities Test (OSDMT; Smith, Reference Smith1982) measures processing speed. Participants are provided with symbols that correspond to a number at the top of the page. Below the key are the symbols with blank boxes underneath them. Participants are asked to orally identify the number that corresponds with the symbol presented and are given 90 s to complete as much of the measure as they can. The OSDMT was selected over more common writing-based measures of processing speed in order to avoid the potential confound of fine motor abilities, a common area of impairment following cerebellar insult (King et al., Reference King, Ailion, Fox and Hufstetler2019).
Scales of Independent Behavior-Revised
The SIB-R (Bruininks et al., Reference Bruininks, Woodcock, Weatherman and Hill1984) is a structured interview that was completed by an informant. This was generally participants’ parents but also included spouses/significant others, grandparents, siblings, or friends who had lived with the participant for at least 6 months. The SIB-R evaluates daily living skills and is subdivided into four subscales, Motor Skills, Social Communication, Personal Living Skills, and Community Living Skills, and also generates a Broad Independent Living Skills (ILS) score.
Hollingshead Four-Factor Index of Social Status
The Hollingshead Four-Factor Index of Social Status (Hollingshead, Reference Hollingshead1975) quantifies socioeconomic status (SES) on a scale of 1–5 with a score of 1–2 being considered high SES and a score of 3–5 being considered medium to low SES. These scores take into consideration the type of occupation and years of education of the participants if they reported independent tax filing status or of their parents if they reported dependent tax filing status.
Statistical Analyses
Statistical analyses were conducted using IBM SPSS Statistics Version 25.0. Descriptive analyses were used to determine means and frequencies for variables of interest. T-tests and chi-square analyses were conducted to assess the differences between male and female participants for clinical and demographic variables. Preliminary correlational analyses were run to evaluate potential relationships between the criterion variables (SIB-R scores) and cognitive scores (IQ, DSF, DSB, and OSDMT). SIB-R scores were subjected to 2 × 2 ANOVAs (sex × radiation). Simple effects analyses were conducted on significant interactions.
RESULTS
In the present study, 45 participants (24 females) were included. FSIQ data include only 43 participants (24 females) due to missing data for two male participants and OSDMT data includes 44 participants (23 females) due to missing data for one female participant. Twenty-six participants (14 females) had undergone RT and 19 participants (10 females) had not undergone RT. There was no significant difference between males and females who underwent RT, χ2 (1, N = 45) = 0.07, p = .936. No significant demographic or clinical differences between males and females were present, nor were any significant demographic differences between RT and no RT groups present (see Table 1). Of note, PFS diagnosis was not an exclusionary criterion; as such, two patients’ medical records documented a formal diagnosis of PFS, both of whom were male. It is possible that further undiagnosed cases of PFS are present as there were seven additional patients (3 males) with a diagnosis of ataxia in the medical record, a hallmark symptom of PFS. As noted in Table 1, frequencies of PFS diagnoses and/or symptoms did not differ by sex. Additionally, 1 of 19 participants with low-grade tumors had undergone RT, and 25 of 26 participants with high-grade tumors had undergone RT. SIB-R informants were most frequently parents across both sexes regardless of RT status; however, participants who had undergone RT had only family member informants (parent, grandparent, and sibling), whereas some participants who had not undergone RT had spouse/significant other or friend informants.
A Bonferroni-corrected p ≤ .01 was used as a significance threshold for correlations between FSIQ, DSF, DSB, and OSDMT and SIB-R measures, given the five subdomains of the SIB-R (see Table 2). FSIQ was positively correlated with all SIB-R domains except for Personal Living Skills (p = .013), which trended in the same positive direction but was not significant at the corrected threshold. DSF and OSDMT were positively correlated with all SIB-R domains. DSB was positively correlated with all SIB-R domains except for Personal Living (p = .052) and Motor Skills (p = .014).
Table 2. Pearson correlations between FSIQ, DSF, DSB, and OSDMT and SIB-R subscales across all participants

* p < .01; **p < .001.
FSIQ, Full Scale Intelligence Quotient; DSF, Wechsler Memory Scale-Third Edition Digit Span Forward; DSB, Wechsler Memory Scale-Third Edition Digit Span Backward; SIB-R, Scales of Independent Behavior-Revised; OSDMT, Oral Symbol Digit Modality Test.
A 2 × 2 ANOVA assessing FSIQ across sex and RT groups did not meet the assumption of homoscedasticity of error variance and therefore is interpreted with caution. Only a main effect of RT was observed (F(1,42) = 4.48, p = .041, ηp2 = .10). Assumptions were met for all other univariate analyses described below. See Figures 1 and 2 for graphical displays of ANOVA findings.
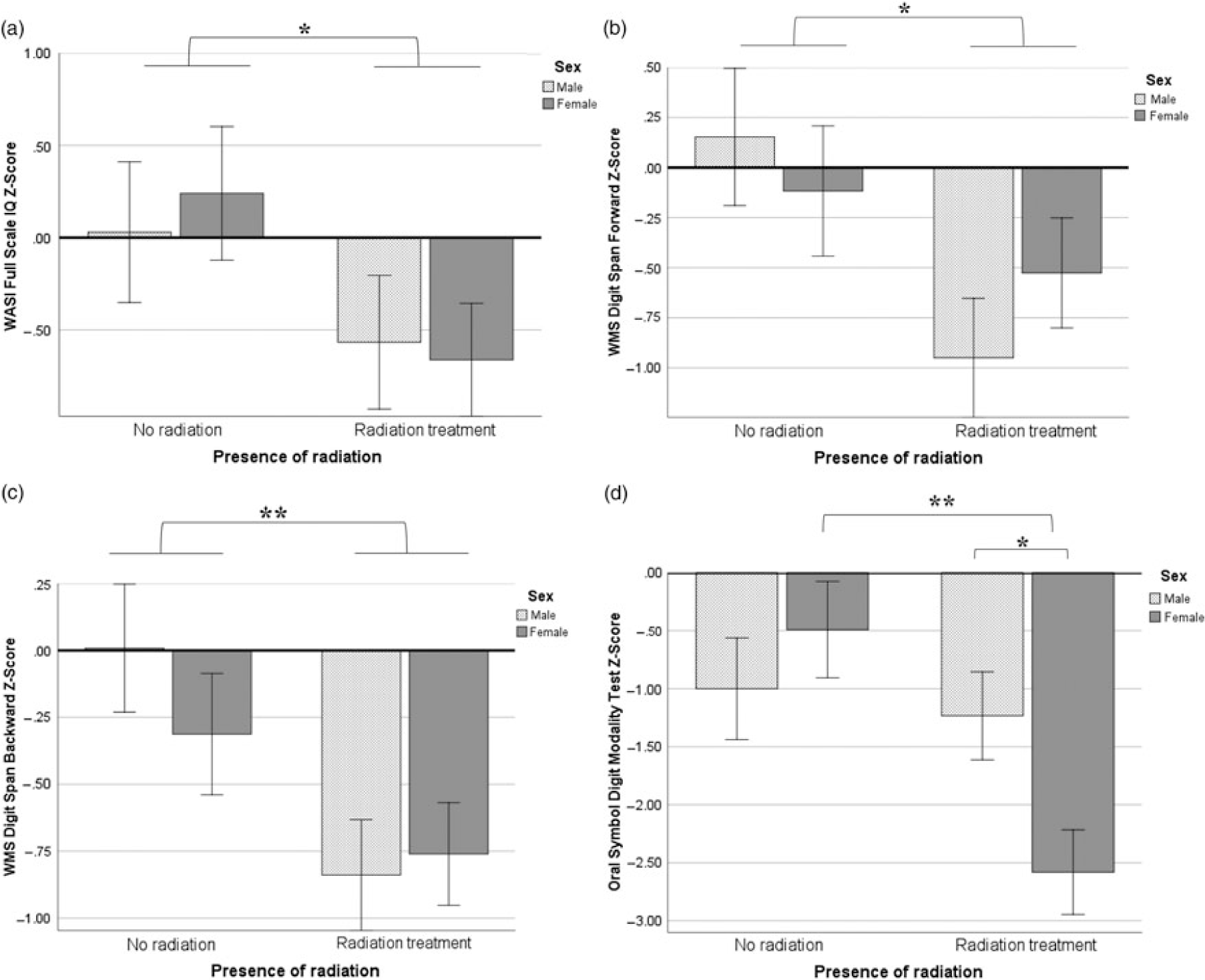
Fig. 1. (a–d) Performance on cognitive measures by sex and radiation treatment. A main effect of RT was seen on FSIQ, DSF, and DSB, and a significant interaction between sex and RT on OSDMT. *p < .05; **p < .01.

Fig. 2. (a–e) Adaptive functioning across SIB-R domains by sex and radiation treatment. (2a–d) A main effect of radiation was shown for motor skills, social communication, broad independent living skills, and personal living skills, and (2e) a significant interaction was shown between sex and radiation in females for community living skills. *p < .05; **p < .01.
Only a main effect of RT was observed for univariate analyses predicting DSF (F(1,44) = 5.91, p = .020, ηp2 = .13) or DSB (F(1,44) = 8.91, p = .005, ηp2 = .18). However, an interaction was observed between sex and RT for OSDMT scores (F(1,43) = 5.37, p = .026, ηp2 = .12) such that females were more impacted by RT than males (Figure 1d).
Within specific SIB-R domains, only a main effect of RT was observed in univariate analyses of Motor Skills (F(1,44) = 15.88, p < .001, ηp2 = .28), Social Communication (F(1,44) = 13.98, p = .001, ηp2 = .25), Broad ILS (F(1,44) = 14.15, p = .001, ηp2 = .26), and Personal Living Skills (F(1,44) = 6.66, p = .014, ηp2 = .14). An interaction was observed between sex and RT in predicting Community Living (Figure 2e), (F(1,44) = 4.62, p = .037, ηp2 = .10) such that no difference was seen in males regardless of radiation status (p = .454), whereas females who did not undergo RT (M = 1.42, SD = 1.13) had significantly greater SIB-R Community Living scores than females who underwent RT (M = −1.01, SD = 1.64) p < .001. Additionally, though they did not reach statistical significance, interactions between sex and RT predicting Personal Living Skills (F(1,44) = 3.31, p = .076, ηp2 = .08) and predicting Broad ILS (F(1,44) = 3.20, p = .081, ηp2 = .07) had similar medium effect sizes as those SIB-R measures that were significant. To further explore the pattern of these trend level interactions, simple effects analyses were run that revealed no difference in males regardless of radiation status for Broad ILS (p = .172) and Personal Living Skills (p = .585). However, similar to Community Living Skills, females who did not undergo RT had significantly greater Broad ILS (M = 1.50, SD = 1.44) and Personal Living Skills (M = 1.22, SD = 1.16) than females who underwent RT (Broad ILS: M = −1.36, SD = 1.78; p < .001), (Personal Living Skills: M = −0.60, SD = 1.36; p = .003).
DISCUSSION
This study aimed to evaluate interactions between sex and RT on basic elements of cognition as well as adaptive functioning skills in long-term survivors of pediatric brain tumors. Findings demonstrated significant negative effects of RT on adaptive functioning, with sex playing an important role in determining the degree of impairment in some domains. Our findings are partially consistent with our hypothesis in that overall, RT consistently negatively impacted cognition and adaptive function. Further supporting our hypotheses, an interaction of medium effect size was observed between sex and RT for community living skills, while trend level interactions were observed for personal living skills and broad independent living skills.
Females’ processing speed was negatively impacted by RT while males’ was not. This aligns with the neurodevelopmental model empirically tested by King and colleagues (Reference King, Ailion, Fox and Hufstetler2019). This model also implicates processing speed deficits as contributing to both higher-order deficits but also other core cognitive processes such as working memory and attention that ultimately feed into higher-order processes. Other studies have also found processing speed to be most sensitive to treatment, age at treatment administration, and location of tumor, suggesting that the origin of processing speed deficits is multifactorial (Jacobson et al., Reference Jacobson, Mahone, Yeates and Ris2018). Of note, when categorized as impaired (z ≤ −1.5) versus within normal limits, 9 of our 21 male participants (3 of the 9 who did not undergo RT and 6 of the 12 who underwent RT) had performed within the impaired range on the OSDMT, indicating that there are likely various tumor and treatment factors that are involved in performance. Furthermore, our findings of many positive correlations between the core cognitive processes and adaptive functioning domains are consistent with King and colleagues’ suggestion that processing speed deficits are significant risk factors for higher-order deficits due to RT (King et al., Reference King, Ailion, Fox and Hufstetler2019).
Across elements of adaptive functioning, in the domain of community living skills, females who underwent RT demonstrated poorer functioning than females who did not undergo RT, whereas males’ functioning was not dramatically impacted by RT status. This same pattern of interaction was observed at trend level in the personal living and broad independent living domains. Community living skills encompass more complex tasks ranging from work skills to time and finance management, all of which are dependent upon basic cognitive processes. Thus, these abilities may be a result of the interactions between various cognitive abilities such as processing speed, attention, and working memory. The similar interaction between RT and sex on processing speed suggests that the disruption of processing speed in particular may ultimately impact these adaptive outcomes negatively. Given the many trend level statistical findings, it can be inferred that with a greater sample size, the relationships between sex and RT for broad independent living skills and personal living skills might emerge as statistically significant and would follow a similar pattern as community living skills.
These findings add nuance to previous studies citing a female disadvantage in long-term cognitive outcomes that have explored the possibility of hormonal differences and changes in DNA repair enzymes as hypotheses for explaining why females are more impacted by RT status than males (Armstrong et al., Reference Armstrong, Sklar, Hudson and Robison2007; Kiehna et al., Reference Kiehna, Mulhern, Li, Xiong and Merchant2006). Furthermore, estrogen has been found to influence synaptic transmission, cerebellar development, and neuroplastic mechanisms in both the hippocampus and the cerebellum, the site of our participants’ tumors, and, in some instances, the target of radiation (Hedges et al., Reference Hedges, Ebner, Meisel and Mermelstein2012). Considering the differences between males and females in estrogen levels, it is possible that hormonal changes posttreatment play a significant role in cognition with estrogen influencing cerebellar brain tumor pathology (Argyriou et al., Reference Argyriou, Assimakopoulos, Iconomou, Giannakopoulou and Kalofonos2011). Neuroanatomical development of the brain with age also varies between sexes with increased white matter tracts and volume in the left hemisphere in males (Schmithorst et al., Reference Schmithorst, Holland and Dardzinski2007). It is possible that our findings may reflect sex differences in brain maturation due to variations in neuronal myelination and synaptic pruning; this may be exacerbated by RT due to variations in neurobiological changes between the sexes during brain development (De Bellis et al., Reference De Bellis, Keshavan, Beers, Hall, Frustaci, Masalehdan and Boring2001).
Despite previous studies implicating the female sex as a risk factor for increased core cognitive deficits post-RT in long-term survivors (Carroll et al., Reference Carroll, Clare, Watson, Hawkins, Spoudeas, Walker, Holland and Ring2013), there was no effect of sex on several of our variables. Specifically, there were no significant sex differences across adaptive functioning domains of motor skills, social communication, personal living skills, and broad independent living skills or across IQ, attention span, or working memory. However, this finding aligns with some previous studies that cite a lack of difference in long-term cognitive outcomes between sexes (Kao et al., Reference Kao, Goldwein, Schultz, Radcliffe, Sutton and Lange1994). Nonetheless, the observed main effects of RT across these cognitive and adaptive function domains demonstrate the deleterious effects of RT, which is consistent with that piece of our hypothesis and with previous studies on late effects of treatment (Wolfe et al., Reference Wolfe, Madan-Swain and Kana2012).
A lack of difference between males and females in IQ, working memory, or attention span also implied that there would be no moderation by sex on the relationship between these cognitive functions and adaptive functioning. However, these three basic processes may contribute to activities of daily living, as exemplified by the significant positive correlations among core cognitive skills, FSIQ, and adaptive function. These findings are consistent with the previous literature citing a relationship between basic cognitive processes and higher-order processes (Beebe et al., Reference Beebe, Ris, Armstrong, Fontanesi, Mulhern, Holmes and Wisoff2005). The closeness of these relationships may explain why some previous studies have found mixed results regarding sex differences in these basic cognitive processes despite the present study’s findings (Kao et al., Reference Kao, Goldwein, Schultz, Radcliffe, Sutton and Lange1994; Reimers et al., Reference Reimers, Ehrenfels, Mortensen, Schmiegelow, Sønderkaer, Carstensen and Müller2003). Our findings add a broader, holistic understanding of the influence of sex across various domains of cognition with varying levels of complexities.
Through the interpretation of these results, it is important to note the effect sizes as measured by partial eta squared (ηp2) (Cohen, Reference Cohen1988). Several of the interactions between RT and sex on adaptive functioning domains had medium effect sizes (ηp2 = .06–.14), whereas main effects of RT yielded large effect sizes (ηp2 > .14). It is clear that this treatment continues to be the most significant contributor to cognitive and functional deficits. This effect was particularly strong with respect to motor skills, with research citing deficits across various motor skills due to RT (Amidei & Kushner, Reference Amidei and Kushner2015). Furthermore, our sample consisted of survivors of posterior fossa tumors and thus received RT focal to the cerebellum or RT to the whole brain, often with a boost to the cerebellar region. Therefore, it is possible that this effect of treatment on motor skills is a result of the damage to areas of the cerebellum that are specific to motor function, whereas interactions between sex and RT for other adaptive functioning domains may be a result of the impact on regions of the cerebellum associated with higher-order cognitive processes. That adaptive motor outcomes did not differ by sex in this sample further supports the specific effect of the interaction of RT and sex on more cognitively demanding elements of daily living.
Outside its historically understood role in motor function, the cerebellum has also been implicated in higher-order cognitive processes (Koziol et al., Reference Koziol, Budding, Andreasen, D’Arrigo, Bulgheroni, Imamizu, Ito, Manto, Marvel, Parker, Pezzulo, Ramnani, Riva, Schmahmann, Vandervert and Yamazaki2014) along with the frontal lobe systems of the brain (Miyake et al., Reference Miyake, Friedman, Emerson, Witzki, Howerter and Wager2000). The fronto-cerebellar network encompasses connections to and from the cerebellum and frontal lobe which allow for basic processes to influence the execution of higher-order cognitive processes, and in survivors, these connections may be altered (Chen et al., Reference Chen, Wang, King and Mao2016). The significant correlations between processing speed and higher-order adaptive functioning suggest that this important core cognitive skill may be a basis for these more advanced, integrated abilities, as has been previously suggested by studies citing white matter loss underlying processing speed deficits (Scantlebury et al., Reference Scantlebury, Bouffet, Laughlin, Strother, McConnell, Hukin, Fryer, Laperriere, Montour-Proulx, Keene, Fleming, Jabado, Liu, Riggs, Law and Mabbott2016). Other clinical factors commonly associated with cognitive deficits such as age at diagnosis and location of tumor have previously been found to be associated with processing speed deficits and white matter loss as well (Palmer et al., Reference Palmer, Glass, Li, Ogg, Qaddoumi, Armstrong, Wright, Wetmore, Broniscer, Gajjar and Reddick2012). This relationship within the fronto-cerebellar network sheds light on the possible neurobiological mechanisms of cognitive impairment due to RT such as white matter loss (Law et al., Reference Lanier and Abrams2011).
The results of the present study should be interpreted in light of the fact that this sample’s treatment may not reflect the most recent advanced treatment approaches. Our participants were, on average, 16 years past diagnosis, and significant strides have been made in providing more targeted and less diffuse and damaging brain tumor treatments. However, this significant time span between the time of diagnosis and time of testing adds merit to the notion that individuals may experience long-term, deleterious late effects several years after treatment.
Furthermore, cognitive and functional data from pre- and immediately posttreatment were not available for these participants; while cross-sectional findings of long-term survivors are valuable, longitudinal data would provide greater information about potentially causal relationships. Additional data from around the time of diagnosis would be useful for characterizing treatment-related impairments, most notably PFS. The average incidence rate of PFS is approximately 20–25% (Lanier & Abrams, Reference Law, Bouffet, Laughlin, Laperriere, Brière, Strother, McConnell, Hukin, Fryer, Rockel, Dickson and Mabbott2017), whereas only two of our 45 participants received a formal diagnosis. However, there were seven additional participants with ataxia among other comorbidities that may be reflective of potentially undiagnosed PFS. Reliance on archival medical records limits the reliability of early PFS diagnosis, as several of our participants were diagnosed with brain tumors in the 1980s and 1990s, not long after PFS was first described by Hirsch and colleagues in 1979 (Hirsch et al., Reference Hirsch, Renier, Czernichow, Benveniste and Pierre-Kahn1979). Thus, it is possible that our lower incidence rate of PFS was a result of a decreased rate in clinician identification of PFS. Additionally, early descriptions of PFS encompassed behavioral deficits and mutism; however, it is now known that PFS incorporates key motor, behavioral, emotional, and sensory impairments (Lanier & Abrams, Reference Law, Bouffet, Laughlin, Laperriere, Brière, Strother, McConnell, Hukin, Fryer, Rockel, Dickson and Mabbott2017).
The multifactorial nature of a clinically complex patient population such as posterior fossa brain tumor survivors leads to possible confounds posed by interacting clinical and functional factors (e.g., motor impairments, sensory and functional deficits, age at diagnosis, and age at treatment administration). Our available sample size limited the use of covariates in our analyses, though analyses of impairment frequency did not demonstrate differences in occurrence by sex in any pattern that would suggest that such factors would be driving the observed interactions. Future studies are encouraged to further investigate how these factors potentially impact adaptive functioning in relation to RT status and sex. However, although it is small, our sample size is respectable for a population that is difficult to track as they transition to adult care, particularly those participants who did not receive ongoing treatment and care following surgical resection. That all participants’ tumors were located within the posterior fossa also lends credence to the findings that emerged and improves interpretability in the context of that which is known about the cerebellum and fronto-cerebellar connections.
Finally, informant-reported data as was obtained by the SIB-R carries inherent risk; although items are meant to be objective, gender biases may have led to differences in reporting. For example, informants’ expectations of young women’s personal living skills may be higher than men’s, leading to underreporting of women’s actual abilities, or informants’ may assume that males are performing better, leading to endorsement of greater abilities on items where they are uncertain. As the SIB-R is normed by age but not by sex, it carries the inherent assumption that these skills do not differ by sex in healthy populations; however, it limits the interpretability in clinical populations where the sexes may be differentially affected by disease or treatment.
Additionally, it is possible that a reporting bias may be found between sexes of SIB-R informants. As informants were categorized as parents, siblings, friends, or spouses/significant others during data collection, demographic data regarding the sex of the informant were not collected, and unfortunately potential differences by informant sex cannot by analyzed. Therefore, inherent sex differences in the informants’ rating of the participants may be an area of further assessment to further explore the possibility of gender bias. The gap in the literature on sex differences in adaptive functioning of healthy individuals must be addressed to better understand this.
With respect to the general utility of the SIB-R, previous literature evaluating adaptive functioning in this brain tumor population have used other adaptive behavior measures that utilize a parent rating assessment in conjunction with parent interview measures and have found both of these methods consistently assess adaptive behavior reliably (Ashford et al., Reference Ashford, Netson, Clark, Merchant, Santana, Wu and Conklin2014). However, the utilization of both neuropsychological performance and informant-report measures of daily functioning is a strength of the current study in that it captures both abilities and actual daily functioning skills of this survivor population. These findings provide important insights into the literature exploring quality of life and every day functioning in this specific population.
Overall, this study demonstrates the contributory role of sex and RT status in predicting deficits in posterior fossa brain tumor survivors. To our knowledge, this study is one of the first to not only examine sex in the context of adaptive functioning alongside key core cognitive processes but also interpret these findings into the broader framework of cognition in the cerebellum. The findings from this study highlight the multifactorial nature of cognitive deficits and how the intersection between sex and late effects of RT are significant indicators of some measures of independent living skills. This suggests a new aspect of interpreting sex differences by differentiating between the different levels of cognitive processes to fully understand the role of sex and treatment on various cognitive processes. Further, these findings increase understanding of deficits in adaptive functioning and basic elements of cognition due to sex and RT for its application into everyday living.
By identifying factors that affect cognition in these survivors, clinicians and researchers can begin to develop interventions to improve or maintain neurocognitive outcomes after patients are in remission. Studies such as one by Reddick et al. (Reference Reddick, Russell, Glass, Xiong, Mulhern, Langston and Gajjar2000) have already begun to experiment with varying reduced doses of RT in an effort to alleviate the dramatic differences in cognition and white matter loss caused by RT while also maintaining similar survival rates. Additionally, a summer cognitive remediation program for pediatric populations with attention, executive functioning, and adaptive functioning difficulties (Murdaugh et al., Reference Murdaugh, King and O’toole2017) focuses on developing these foundational skills in preparation for transition to adult care. Recent focus on computer-based cognitive remediation of executive function has demonstrated long-term efficacy in improving various cognitive skills (Conklin et al., Reference Conklin, Ogg, Ashford, Scoggins, Zou, Clark, Hardy, Merchant, Jeha, Huang and Zhang2015, Reference Conklin, Ashford, Clark, Martin-Elbahesh, Hardy, Merchant, Ogg, Jeha, Huang and Zhang2017; Stavinoha et al., Reference Stavinoha, Askins, Powell, Pillay Smiley and Robert2018), and a similar model can be applied to adaptive skills training as it has to other clinical populations. The techniques and strategies derived from these cognitive interventions for improving deficits may be tailored towards skills such as processing speed and attention in females (Olson & Sands, Reference Olson and Sands2016).
Findings such as those from the present study may inform treatment plans in these settings, allowing clinicians to tailor both initial and long-term treatment by sex and adjuvant treatment factors. For example, following further relevant studies, results such as these may ultimately lead physicians to consider using different types or dosages of RT based on sex given the greater number of methodologies today (Waber et al., Reference Waber, Urion, Tarbell, Niemeyer, Gelber and Sallan1990). These newly developing methodologies would be best informed by understanding the biological mechanisms underlying cognitive deficits. The culmination of these results adds merit to previous research pertaining to optimizing survivors’ everyday function, which may be further explored by understanding the neurobiological, hormonal, and genetic mechanisms (Siegel et al., Reference Siegel, King, Rupji, Dwivedi, Carter, Kowalski and MacDonaldin press) behind the multifactorial nature of cognitive outcomes and independent living.
ACKNOWLEDGMENTS
We are indebted to the participants who willingly gave their time to make this research possible. We also thank the King Developmental Neuropsychology Research Team for helping with data acquisition and management. This research was supported in part by grants from the American Cancer Society (Research Scholar Grant, Principal Investigator: T.Z. King, (#RSGPB-CPPB-114044)); Health Resources and Service Administration (Graduate Psychology Education Program Grant, #2 D40HP19643, Graduate Research Assistant: MEF); the Georgia State University Brains and Behavior Initiative (Graduate Student Fellowship Program: MEF; Summer Undergraduate Scholars Program: TFP); and the Georgia State University Honors Assistantship Program (TFP and TDT).
CONFLICTS OF INTEREST
The authors have nothing to disclose.